Abstract
SLC26A4 mutations cause fluctuating and progressive hearing loss associated with enlargement of the vestibular aqueduct (EVA). SLC26A4 encodes a transmembrane anion exchanger called pendrin expressed in nonsensory epithelial cells of the lateral wall of cochlea, vestibular organs and endolymphatic sac. We previously described a transgenic mouse model of EVA with doxycycline (dox)-inducible expression of Slc26a4 in which administration of dox from conception to embryonic day 17.5 (DE17.5) resulted in hearing fluctuation between 1 and 3 months of age. In the present study, we hypothesized that Slc26a4 is required to stabilize hearing in DE17.5 ears between 1 and 3 months of age. We tested our hypothesis by evaluating the effect of postnatal re-induction of Slc26a4 expression on hearing. Readministration of dox to DE17.5 mice at postnatal day 6 (P6), but not at 1 month of age, resulted in reduced click-evoked auditory brainstem response (ABR) thresholds, less fluctuation of hearing and a higher surface density of pendrin expression in spindle-shaped cells of the stria vascularis. Pendrin expression in spindle-shaped cells was inversely correlated with ABR thresholds. These findings suggest that stabilization of hearing by readministration of dox at P6 is mediated by pendrin expression in spindle-shaped cells. We conclude that early re-induction of Slc26a4 expression can prevent fluctuation of hearing in our Slc26a4-insufficient mouse model. Restoration of SLC26A4 expression and function could reduce or prevent fluctuation of hearing in EVA patients.
Keywords: deafness, DFNB4, EVA, fluctuation, gene therapy, SLC26A4
Introduction
Enlargement of the vestibular aqueduct (EVA; OMIM 600791) is a common malformation in ears of children with sensorineural hearing loss (Morton and Nance, 2006). The onset of hearing loss associated with EVA can be congenital but is frequently postnatal. The severity ranges from slight to profound and can fluctuate with precipitous drops and slower recoveries. Recoveries are often incomplete and the long-term result is overall progression of hearing loss (Jackler and De La Cruz, 1989; Levenson et al., 1989; Griffith and Wangemann, 2011). The fluctuating hearing loss is difficult to rehabilitate, and there are no treatment options of proven benefit.
Mutations of the SLC26A4 gene are the most common detectable cause of EVA (Everett et al., 1997). SLC26A4 encodes a transmembrane anion exchanger called pendrin (Everett et al., 1997). In the mouse inner ear, pendrin is expressed in nonsensory epithelial cells of the lateral wall of cochlea, vestibular end organs and endolymphatic sac (Royaux et al., 2003; Dou et al., 2004; Wangemann et al., 2004). In the cochlea, pendrin is present in root cells of the outer sulcus, cells overlying the spiral prominence, and spindle-shaped cells of the stria vascularis (Wangemann et al., 2004). Spindle-shaped cells are organized in rows, 2-3 cells wide, along both borders of the stria vascularis spanning from the base to the apex of the cochlea. They morphologically differ from the marginal cells of the stria vascularis and the adjacent epithelial cells of the spiral prominence (Katagiri et al., 1968; Anniko, 1976; Luciano et al., 1995). Marginal cells mediate the vectorial secretion of K+ ions from the intrastrial space into the lumen of the scala media (Wangemann et al. 1995; Wangemann et al. 2004). The function of spindle-shaped cells is largely unknown, however, the expression of pendrin and P2RX2 cation channels in the apical membrane suggests a role in pH and ionic homeostasis (Wangemann et al., 2004; Housley et al., 2013).
Mice that are homozygous for a targeted deletion allele of exon 8 (Slc26a4Δ) of Slc26a4 have profound or total loss of auditory and vestibular function, and massively enlarged endolymphatic spaces throughout the inner ear (Everett et al., 2001). These phenotypes of Slc26a4Δ/Δ mice are more severe than those observed in human EVA patients. We thus generated a “tet-on”-based mouse model with two unlinked transgenes, the effector (Tg[E]) and responder (Tg[R]) transgenes, that express pendrin in the presence, but not absence, of doxycycline (dox) administered in the drinking water (Choi et al., 2011). The transgenes are crossed onto the Slc26a4Δ/Δ background so that the only source of functional pendrin is the responder transgene. In this Slc26a4-insufficient model, Slc26a4 expression is required from embryonic day 16.5 (E16.5) to postnatal day 2 (P2) for acquisition of normal auditory brainstem response thresholds at 1 month of age (Choi et al., 2011). Li et al. (Li et al., 2013) used a different transgenic rescue strategy to demonstrate that pendrin must be expressed in the endolymphatic sac, not the cochlea, for the development of normal hearing.
Tg[E];Tg[R];Slc26a4Δ/Δ mice that receive dox from conception until embryonic day 17.5 (“DE17.5”: dox discontinued at E17.5) have partial and asymmetric hearing loss modeling that observed in human EVA patients (Choi et al., 2011). Many of the mice have fluctuations of hearing between 1 and 6 months of age, especially between 1 and 3 months of age, with an overall progressive hearing loss after 6 months of age (Ito et al., 2014). The fluctuation and progressive loss of hearing in Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5 mice is caused by loss of function and degeneration of the stria vascularis (Ito et al., 2015).
In the present study, we hypothesized that re-induction of Slc26a4 expression is required to stabilize hearing in DE17.5 ears. We tested our hypothesis by evaluating the effect of postnatal re-induction of Slc26a4 expression on hearing in Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5 mice. We selected P6 as one time point for re-induction because it is after the critical period (E16.5 to P2) during which pendrin is required for the initial acquisition of normal hearing, but before the maturation of the endocochlear potential (EP). We also evaluated the effect of delaying re-administration until 1 month of age. Our results indicate that restoration of SLC26A4 expression and function could reduce or prevent fluctuation of hearing in EVA patients.
Experimental Procedures
Ethics statement
All animal experiments and procedures were performed according to protocols approved by the Animal Care and Use Committee of the National Institute of Neurological Diseases and Stroke and the National Institute on Deafness and Other Communication Disorders, National Institutes of Health.
Animals
The effector (Tg[E]; Tg(RP23-265L9/rtTA2S-M2/NeoR)1Ajg) and responder (Tg[R]; Tg(AcGFP/TRE/Slc26a4)2Ajg) transgenes were crossed with a targeted deletion allele (Slc26a4Δ) of Slc26a4 to generate Tg[E];Tg[R]];Slc26a4Δ/+ and Tg[E];Tg[R]];Slc26a4Δ/Δ mice (n = 32 and 57, respectively) (Choi et al., 2011; Ito et al., 2014; Ito et al., 2015). The genetic background was mixed and included C57BL/6J, SJ/L and 129Sv/Ev.
Genotype analysis
Genomic DNA was isolated and analyzed by polymerase chain reaction (PCR) for the presence of Tg[E], Tg[R], Slc26a4Δ and Slc26a4+ as described (Choi et al., 2011).
Doxycycline administration
Tg[E];Tg[R];Slc26a4Δ/+ and Tg[E];Tg[R];Slc26a4Δ/Δ littermates were administered doxycycline (dox) from conception until embryonic day 17.5 (E17.5) as described (Choi et al., 2011; Ito et al., 2014). Dox was administered in drinking water containing 0.2 g doxycycline hyclate (Sigma-Aldrich) and 5 g sucrose (MP Biomedicals), for palatability, per 100 ml of reagent-grade water. Dox-containing water was provided to the dam from the onset of mating and substituted with dox-free water at E17.5. For the dox readministration paradigm, dox-containing water was substituted for dox-free water at the indicated age.
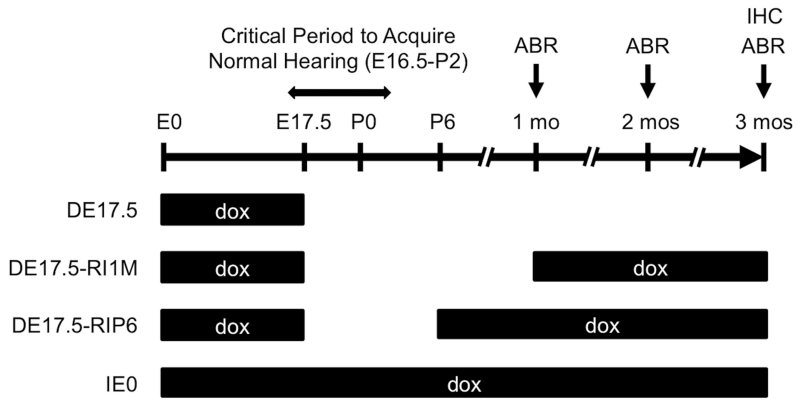
Doxycycline (dox) was readministered to Tg[E];Tg[R];Slc26a4Δ/+ and Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5 mice at postnatal day 6 (“DE17.5-RIP6”: dox reinitiated at P6) or 1 month of age (“DE17.5-RI1M”: dox reinitiated at 1 month of age) (Fig. 1). We also included a control group of DE17.5 mice that did not receive dox after E17.5 as well as a control group (“IE0”: dox initiated at E0) that received dox continuously from conception to determine if dox administration affects hearing in DE17.5 mice.
Figure 1.
Doxycycline Administration.
Tg[E];Tg[R];Slc26a4Δ/+ DE17.5 mice and Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5 mice were readministered doxycycline according to one of three paradigms: (1) no readministration of dox (DE17.5); (2) dox readministered at 1 month of age (DE17.5-RI1M: dox reinitiated at 1 month of age); and (3) dox readministered at postnatal day 6 (DE17.5-RIP6: dox reinitiated at P6). We also included a control group that received dox continuously from conception (IE0: dox initiated at E0). We measured click auditory brainstem response (ABR) thresholds at 1, 2 and 3 months of age, and then euthanized the mice for immunohistochemical (IHC) analysis. For DE17.5-RI1M mice at 1 month of age, we measured ABR thresholds before readministration of dox.
Auditory brainstem response thresholds
Monaural auditory brainstem response (ABR) thresholds for click stimuli were determined for each ear as described (Choi et al., 2011; Ito et al., 2014). If an ear had no detectable waveform in response to the highest intensity level of 110 dB SPL (sound pressure level), the threshold was considered to be 115 dB SPL for subsequent analyses. ABR thresholds were measured at 1, 2, and 3 months of age in DE17.5, DE17.5-RI1M, DE17.5-RIP6 and IE0 groups of Tg[E];Tg[R];Slc26a4Δ/+ ears (n = 30, 14, 14 and 6 ears, respectively) and Tg[E];Tg[R];Slc26a4Δ/Δ ears (n = 44, 30, 28 and 12 ears, respectively). Sample sizes differ because this mouse model has a variable auditory phenotype (Choi et al., 2011; Ito et al., 2014) and we included all of the mice that were generated in order to avoid selection bias. Thresholds values for each ear of each animal were included for subsequent analyses. For DE17.5-RI1M mice, ABR thresholds were measured before readministration of dox. Fluctuation was defined as an ABR threshold difference ≥15 dB between consecutive monthly measurements. The cumulative threshold shift was defined as sum of the absolute values of the ABR threshold differences between consecutive monthly measurements.
Immunohistochemistry
As described (Choi et al., 2011), anti-pendrin antibodies were used to immunostain whole-mounted mouse cochleae and endolymphatic sacs of Tg[E];Tg[R];Slc26a4Δ/+ DE17.5 ears (n = 8 and 6 ears, respectively), Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5 ears (n = 17 ears each), Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5-RI1M ears (n = 12 ears each), Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5-RIP6 ears (n = 10 and 9 ears, respectively) and Tg[E];Tg[R];Slc26a4Δ/Δ IE0 ears (n = 2 ears each) from mice at 3 months of age. Primary antibodies were diluted 1:1000 in blocking solution. The secondary antibody was Alexa Fluor 488–conjugated goat anti-rabbit IgG (#A-11008; Invitrogen) diluted 1:500. Samples were counterstained with rhodamine-phalloidin (Molecular Probes, Eugene, OR) diluted 1:100.
Quantification of protein expression
Images were captured with an LSM 780 confocal microscope equipped with a 40×, 1.3 NA oil objective and ZEN 2012 software (Zeiss, Jena, Germany). All the images from the lateral wall of cochlea or the endolymphatic sac were captured with a 488-nm argon laser or 561-nm diode-pumped solid state laser under the same laser power and gain settings, respectively. The laser power and gain settings were determined using Tg[E];Tg[R];Slc26a4Δ/+ and Slc26a4Δ/Δ samples to avoid saturating pixels or high background signal. Z-stack images (0.4 μm) were captured from the apical surface of the lateral wall of the cochlea or the endolymphatic sac to the depths specified below. After the images were captured, ImageJ software (National Institutes of Health) was used to measure the area and anti-pendrin fluorescence intensity of spindle-shaped cells in the stria vascularis and the root cells in the outer sulcus of the middle turn of the cochlea, and endolymphatic sac cells. For spindle-shaped cells, the apical surfaces of 5 cells were analyzed for each ear. For outer sulcus root cells, 10 cells were measured for each ear at three levels: the apical surface, 2.0 μm from the apical surface, and 4.0 μm from the apical surface. For endolymphatic sac cells, 10 cells were measured for each ear at the apical surface, 0.8 μm from the apical surface, and 1.6 μm from the apical surface. Therefore, we included spindle-shaped cells, root cells and endolymphatic sac cells of Tg[E];Tg[R];Slc26a4Δ/+ DE17.5 ears (n = 40, 80 and 60 cells, respectively), Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5 ears (n = 85, 170 and 170 cells, respectively), Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5-RI1M ears (n = 60, 120 and 120 cells, respectively), Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5-RIP6 ears (n = 50, 100 and 90 cells, respectively) and Tg[E];Tg[R];Slc26a4Δ/Δ IE0 ears (n = 10, 20 and 20 cells, respectively) for analyses as independent observations. In the lateral wall of the cochlea, the anti-pendrin fluorescence intensity of marginal cells, which do not express pendrin, was calculated as the level of background intensity. In the endolymphatic sac, the anti-pendrin fluorescence intensity of pendrin-negative cells was considered to be background intensity. These pendrin-negative cells are ribosome-rich cells, which were distinguished from pendrin-positive mitochondria-rich cells based upon rhodamine-phalloidin staining (Royaux et al., 2003; Kim and Wangemann, 2011). Background fluorescence intensity was subtracted from each cell’s measured anti-pendrin fluorescence intensity for further analysis. Since measurements of fluorescence intensity could be influenced by differences in cell size associated with the site(s) of measurement, pathologic changes in cell size (Jabba et al., 2006; Ito et al., 2015), or both, fluorescence intensity per unit area was calculated for each cell. The median fluorescence intensity per unit area for cells in each group of ears was calculated and normalized to that of Tg[E];Tg[R];Slc26a4Δ/+. For correlation analyses, the mean fluorescence intensity per unit area for each ear in spindle-shaped cells, root cells or pendrin-positive endolymphatic sac cells (n = 5, 10 and 10 cells per ear, respectively) in each group of ears was calculated.
Statistics
Statplus:mac software (AnalystSoft, Walnut, CA) was used for statistical analyses. Shapiro-Wilk testing was performed to evaluate normality. Kruskal-Wallis and Mann-Whitney U tests were performed since the data was not normally distributed. Mann-Whitney U tests with a Bonferroni correction were performed as a post hoc test when significant differences were detected by Kruskal-Wallis testing. Fisher’s exact and Pearson correlation tests were also performed. p < 0.05 was considered to be significant.
Results
Fluctuation of hearing
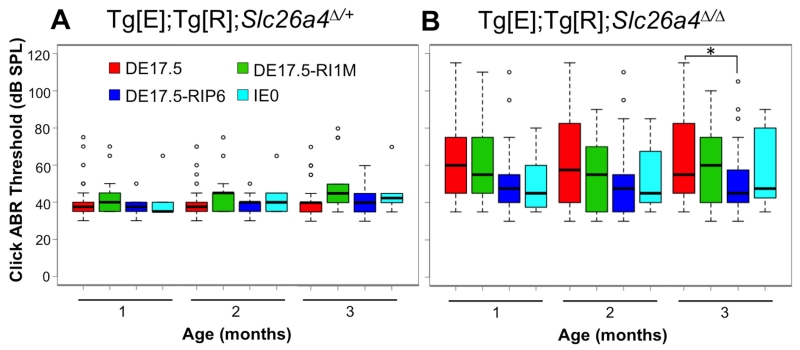
Auditory brainstem response (ABR) thresholds in response to click stimuli were measured in DE17.5, DE17.5-RI1M, DE17.5-RIP6 and IE0 groups of Tg[E];Tg[R];Slc26a4Δ/+ ears and Tg[E];Tg[R];Slc26a4Δ/Δ ears at 1, 2 and 3 months of age (Fig. 2). Tg[E];Tg[R];Slc26a4Δ/+ ears showed no difference in median ABR thresholds among the three dox-administration groups (DE17.5, DE17.5-RI1M, and DE17.5-RIP6) at each time point (Kruskal-Wallis, H(2) = 4.14, 2.83 and 4.19, respectively, all p values > 0.05, Fig. 2A). Tg[E];Tg[R];Slc26a4Δ/Δ ears showed no difference in median thresholds among the same three dox-administration groups at 1 or 2 months of age (Kruskal-Wallis, H(2) = 5.69 and 4.55, respectively, all p values > 0.05). At 3 months of age, readministration of dox to DE17.5 mice at P6 resulted in a lower median threshold than in DE17.5 mice with no readministration of dox (Kruskal-Wallis, H(2) = 6.80, p < 0.05; Mann-Whitney U, p < 0.05, Fig. 2B). There was no difference in median thresholds between Tg[E];Tg[R];Slc26a4Δ/+ IE0 and Tg[E];Tg[R];Slc26a4Δ/Δ IE0 ears at 1, 2 and 3 months of age (Mann-Whitney U, all p values > 0.05).
Figure 2.
Auditory Brainstem Response (ABR) Thresholds.
Box plots of click ABR thresholds measured at 1, 2 and 3 months of age are shown for the DE17.5, DE17.5-RI1M, DE17.5-RIP6 and IE0 groups of (A) Tg[E];Tg[R];Slc26a4Δ/+ ears (n = 30, 14, 14 and 6, respectively) and (B) Tg[E];Tg[R];Slc26a4Δ/Δ ears (n = 44, 30, 28 and 12, respectively). The box includes the median (heavy line) and represents the first and the third quartiles. The whiskers show the range of data points that are within ≤1.5 times the interquartile range from the box. Threshold values outside the range of whiskers are indicated by dots. *p < 0.05.
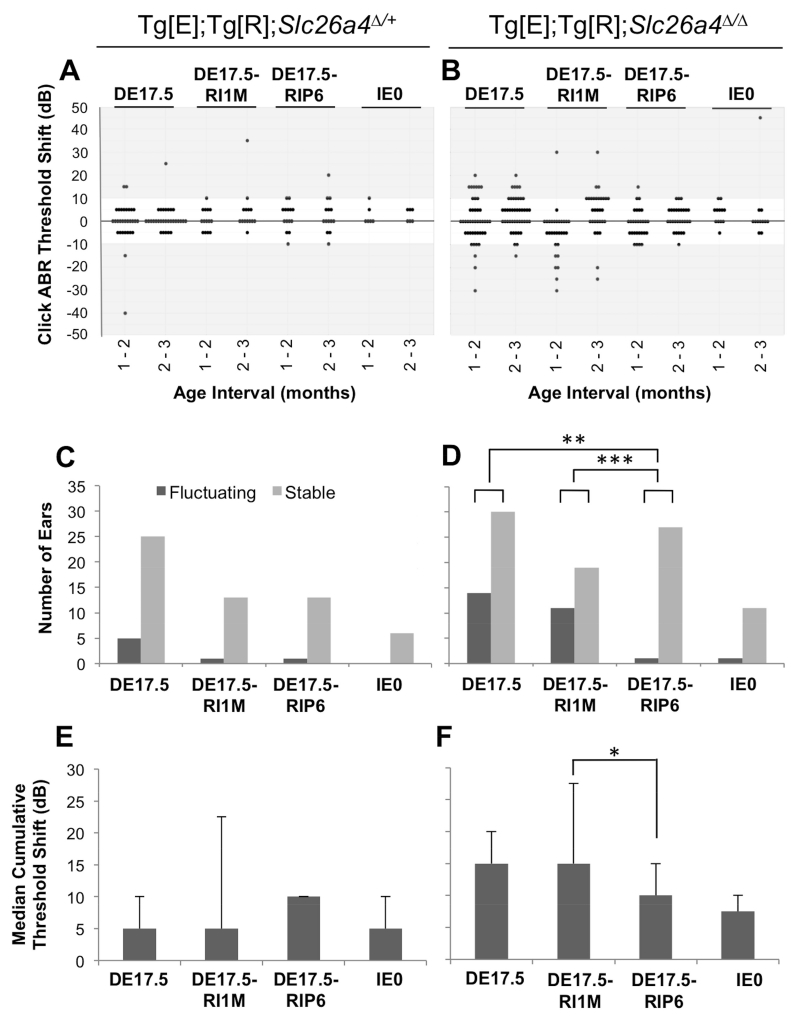
Tg[E];Tg[R];Slc26a4Δ/+ ears showed no difference in prevalence of fluctuation of hearing among the three dox-administration groups (DE17.5, DE17.5-RI1M, and DE17.5-RIP6; Fisher’s exact, all p values > 0.05, Figs. 3A,C). Among Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5 ears, 14 (32%) of 44 ears with no dox readministration showed fluctuation and 1 (4%) of 28 ears with dox readministered at P6 showed fluctuation (Figs. 3B,D). Dox readministration at P6 therefore had a significant effect on the prevalence of fluctuation of hearing (Fisher’s exact, p < 0.01). Readministration of dox at 1 month of age showed no effect on prevalence (37%) of fluctuation of hearing (Fisher’s exact, p > 0.05, Fig. 3D). There was no difference in prevalence of fluctuation of hearing between Tg[E];Tg[R];Slc26a4Δ/+ IE0 and Tg[E];Tg[R];Slc26a4Δ/Δ IE0 ears (0% and 8%, respectively; Fisher’s exact, p > 0.05). We calculated cumulative threshold shifts in both Tg[E];Tg[R];Slc26a4Δ/+ and Tg[E];Tg[R];Slc26a4Δ/Δ ears, and found no differences among the three groups of Tg[E];Tg[R];Slc26a4Δ/+ ears (Kruskal-Wallis, H(2) = 1.36, p > 0.05, Fig. 3E). Among Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5 ears, readministration of dox at P6 resulted in a smaller median cumulative threshold shift than readministration of dox at 1 month of age (Kruskal-Wallis, H(2) = 7.38, p < 0.05; Mann-Whitney U, p < 0.05, Fig. 3F). There was no difference in median cumulative threshold shifts between ears with no dox readministration and ears with readministration at P6 (Mann-Whitney U, p >0.05, Fig. 3F).
Figure 3.
Fluctuation of Hearing.
The difference in click ABR threshold measured at consecutive monthly intervals in individual ears is shown for the DE17.5, DE17.5-RI1M, DE17.5-RIP6 and IE0 groups of (A) Tg[E];Tg[R];Slc26a4Δ/+ ears (n = 30, 14, 14 and 6, respectively) and (B) Tg[E];Tg[R];Slc26a4Δ/Δ ears (n = 44, 30, 28 and 12, respectively). Threshold increases are shown as positive shifts and decreases are displayed as negative shifts. The number of fluctuating or stable ears is shown for each group of (C) Tg[E];Tg[R];Slc26a4Δ/+ ears and (D) Tg[E];Tg[R];Slc26a4Δ/Δ ears. Fluctuation was defined as a click ABR threshold difference > 10 dB between consecutive monthly measurements. The median (± median absolute deviation) cumulative sum of differences in consecutive monthly measurements of click ABR threshold for each individual ear are shown for each group of (E) Tg[E];Tg[R];Slc26a4Δ/+ ears and (F) Tg[E];Tg[R];Slc26a4Δ/Δ ears. Fisher’s exact test (C, D) and Kruskal-Wallis test (E, F), *p < 0.05, **p < 0.01, ***p < 0.005.
Pendrin expression
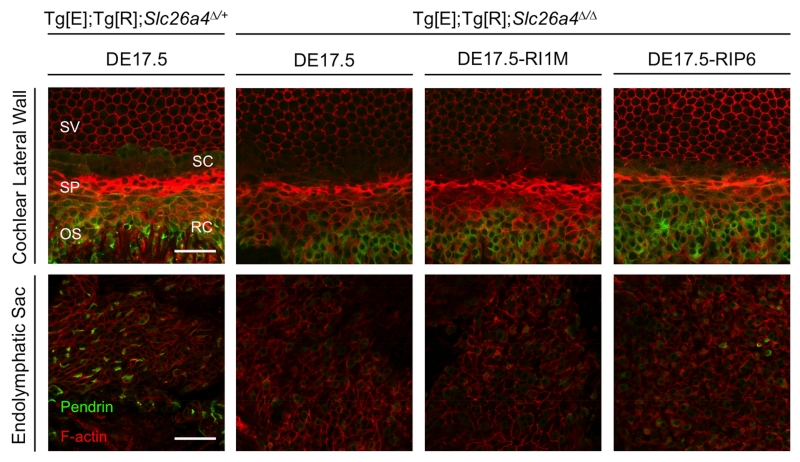
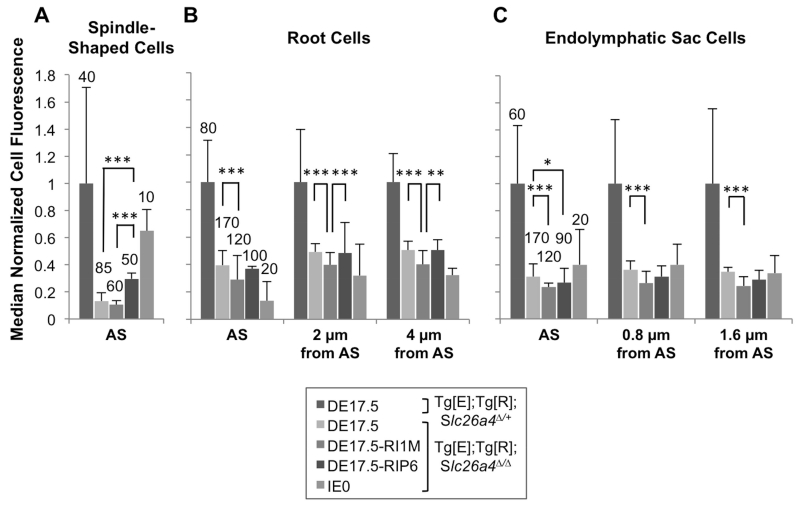
To determine if the stabilizing effect of dox readministration on loss and fluctuation of hearing is correlated with increased expression of pendrin, we measured anti-pendrin antibody binding to spindle-shaped cells of the stria vascularis, root cells of the outer sulcus, and endolymphatic sac cells in the inner ears of Tg[E];Tg[R];Slc26a4Δ/+ DE17.5, Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5, Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5-RI1M, Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5-RIP6 and Tg[E];Tg[R];Slc26a4Δ/Δ IE0 mice. Figure 4 shows representative pendrin expression in the spindle-shaped cells of the stria vascularis in the middle turn of the cochlea, root cells of the outer sulcus in the middle turn of the cochlea, and endolymphatic sac cells. Although we detected pendrin expression in spindle-shaped cells and root cells along the length of the cochlear duct, we had difficulty preparing apical or basal turn specimens with sufficient integrity for quantitative immunohistochemistry. Therefore we focused our quantitative analyses on the middle turn of the cochlea. Since measurements of fluorescence intensity could be influenced by differences in cell size associated with the site(s) of measurement, pathologic changes in cell size (Jabba et al., 2006; Ito et al., 2015), or both, we also measured the surface area. We calculated fluorescence intensity per unit area since the mean sizes of cells differed among the dox-administration groups. In Tg[E];Tg[R];Slc26a4Δ/Δ ears, readministraton of dox at P6 resulted in higher median pendrin expression per unit area in spindle-shaped cells in comparison to ears with no readministration of dox (Kruskal-Wallis, H(2) = 28.3, p < 0.005; Mann-Whitney U, p < 0.005, Fig. 5A), while readministration of dox at 1 month of age showed no effect in spindle-shaped cells (Mann-Whitney U, p > 0.05, Fig. 5A). Readministration of dox at 1 month of age resulted in lower median pendrin expression per unit area in root cells in comparison to ears with no readministration of dox at the levels of the apical surface, 2 μm from the apical surface and 4 μm from the apical surface (Kruskal-Wallis, H(2) = 25.4, 23.1 and 19.5, respectively, all p values < 0.005, Fig. 5B), while readministration of dox at P6 showed no effect on median pendrin expression per unit area in root cells (Mann-Whitney U, all p values > 0.05). No readministration of dox resulted in higher pendrin expression per unit area at the apical surface of endolymphatic sac pendrin-positive cells in comparison to ears with readministration of dox at 1 month of age or P6 (Kruskal-Wallis, H(2) = 23.2, p < 0.05; Mann-Whitney U, p < 0.005 and p < 0.05, respectively, Fig. 5C). No readministration of dox resulted in higher pendrin expression per unit area in endolymphatic sac pendrin-positive cells in comparison to ears with readministration of dox at 1 month of age at 0.8 μm from the apical surface and 1.6 μm from the apical surface (Kruskal-Wallis, H(2) = 25.0 and 24.0, respectively, all p values < 0.05; Mann Whitney U, all p values < 0.005, Fig. 5C).
Figure 4.
Pendrin Expression.
Representative immunostaining is shown for pendrin (green) and F-actin (red) in whole-mounted lateral wall of the middle turn of the cochlea and endolymphatic sac of Tg[E];Tg[R];Slc26a4Δ/+ DE17.5 ears (n = 8 and 6, respectively), Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5 ears (n = 17 each), Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5-RI1M ears (n = 12 each), and Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5-RIP6 ears (n = 10 and 9, respectively). Pendrin expression in spindle-shaped cells was reduced in Tg[E];Tg[R];Slc26a4Δ/Δ ears in comparison to Tg[E];Tg[R];Slc26a4Δ/+ ears. Scale bars: 50 μm. SV, stria vascularis; SP, spiral prominence; OS, outer sulcus; SC, spindle-shaped cell; RC, root cell.
Figure 5.
Pendrin Expression Levels.
Median (± median absolute deviation) normalized cell fluorescence per unit area of (A) spindle-shaped cells, (B) root cells, and (C) pendrin-positive endolymphatic sac cells in Tg[E];Tg[R];Slc26a4Δ/+ DE17.5, Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5, Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5-RI1M, Tg[E];Tg[R];Slc26a4Δ/Δ DE17.5-RIP6, and Tg[E];Tg[R];Slc26a4Δ/Δ IE0 mice. For (B) root cells, measurements were performed at three levels: the apical surface, 2.0 μm from the apical surface, and 4.0 μm from the apical surface. For (C) pendrin-positive endolymphatic sac cells, measurements were performed at three levels: the apical surface, 0.8 μm from the apical surface, and 1.6 μm from the apical surface. The number of cells is shown for each group. The number of ears is given in the legend for Fig. 4. n = 2 ears for the IE0 group. Background fluorescence was subtracted from measured fluorescence as stated in the methods. We normalized fluorescence intensity per unit area to the fluorescence intensity per unit area of the same cell types in Tg[E];Tg[R];Slc26a4Δ/+ control ears. Kruskal-Wallis test, *p < 0.05, **p < 0.01, ***p < 0.005. AS, apical surface.
Correlation of hearing with pendrin expression
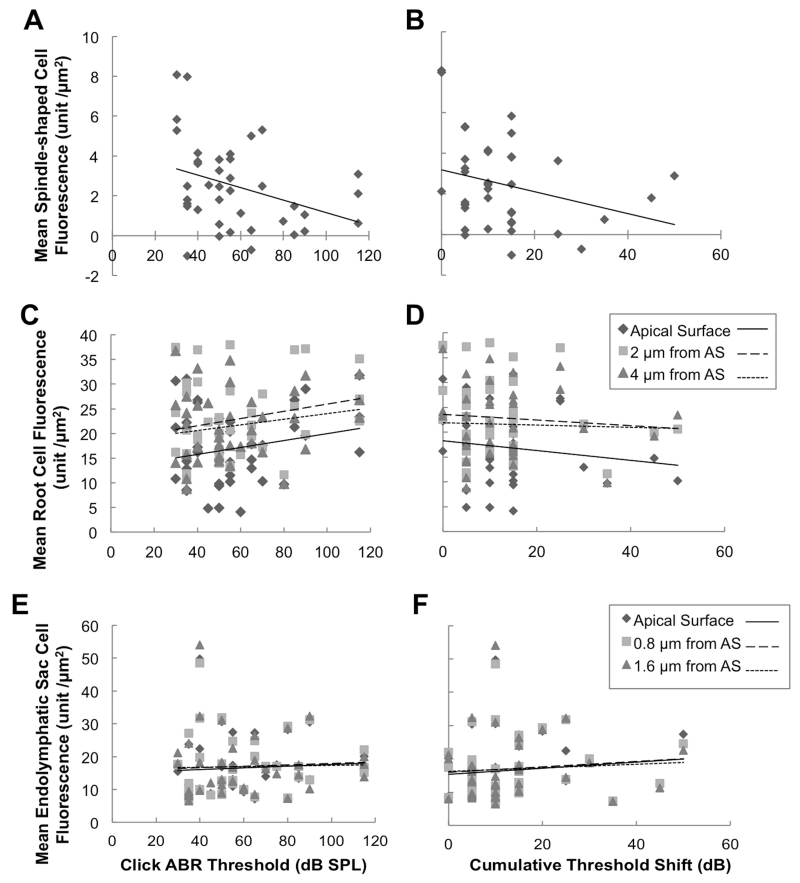
We sought to determine if there was a correlation between hearing loss or fluctuation with the expression of pendrin induced by readministration of dox. In Tg[E];Tg[R];Slc26a4Δ/Δ ears, the click ABR thresholds measured at 3 months of age were inversely correlated with the mean pendrin fluorescence intensity per unit area in spindle-shaped cells (Pearson correlation, r = −0.35, p < 0.05, Fig. 6A). A correlation was not present in root cells at the apical surface, 2 μm from the apical surface or 4 μm from the apical surface (Pearson correlation, r = 0.22, 0.22 and 0.19, respectively, all p values > 0.05, Fig. 6C) or endolymphatic sac pendrin-positive cells at the apical surface, 0.8 μm from the apical surface or 1.6 μm from the apical surface (Pearson correlation, r = 0.072, 0.058 and 0.024, respectively, all p values > 0.05, Fig. 6E). There was no correlation of cumulative threshold shift with fluorescence of mean pendrin fluorescence intensity per unit area in spindle-shaped cells (Pearson correlation, r = −0.28, p > 0.05, Fig. 6B), root cells (Pearson correlation, r = −0.15, −0.080 and −0.037, respectively, all p values > 0.05, Fig. 6D) or endolymphatic sac pendrin-positive cells (Pearson correlation, r = 0.12, 0.10 and 0.065, respectively, all p values > 0.05, Fig. 6F).
Figure 6.
Correlation of Pendrin Expression with Hearing.
Mean pendrin fluorescence intensity for each ear is shown for Tg[E];Tg[R];Slc26a4Δ/Δ spindle-shaped cells (A,B; n = 39 ears, 5 cells per ear), root cells (C,D; n = 39 ears, 10 cells per ear at each measurement level: apical surface, 2 μm from apical surface, and 4 μm from apical surface) or pendrin-positive endolymphatic cells (E,F; n = 38 ears, 10 cells per ear at each measurement level: apical surface, 0.8 μm from apical surface, and 1.6 μm from apical surface) as a function of click ABR threshold measured at 3 months of age (A,C,E) or cumulative ABR threshold shift (B,D,F). Lines represent best-fit linear regression. AS, apical surface.
Discussion
We previously showed that doxycycline (dox) administration (Slc26a4 expression) is required from embryonic day 16.5 (E16.5) to postnatal day 2 (P2) for acquisition of normal hearing at 1 month of age in Tg[E];Tg[R];Slc26a4Δ/Δ mice (Choi et al., 2011). Discontinuation of dox during the critical time period led to the development of fluctuating hearing loss, with overall downward progression, caused by dysfunction and degeneration of the stria vascularis after 1 month of age (Ito et al., 2014; Ito et al., 2015). Although we used a fixed-effect model in this study so the results cannot be assumed to apply to a population of animals, our current results indicate that re-induction of Slc26a4 expression in spindle-shaped cells of the stria vascularis prior to the development of the endocochlear potential and prior to the onset of hearing can prevent this fluctuation of hearing in mice.
At 3 months of age, click ABR thresholds of Tg[E]; Tg[R]; Slc26a4Δ/Δ mice were reduced and the prevalence of fluctuation of thresholds between 1 and 3 months of age was also reduced by readministration of dox at P6. In contrast, readministration of dox at 1 month of age had no effect on click ABR thresholds or fluctuation of hearing (Figs. 2 and 3). We did not observe a difference in cumulative threshold shifts, a measure of the degree of hearing fluctuation, between ears with no dox readministration and ears with readministration of dox at P6 (Fig. 3F). This negative result may be due to the large variability of measurements, the high proportion of ears with no fluctuation that affected the median values for all groups, or our prospective monthly measurements that likely do not detect all of the threshold shifts that are actually occurring. Furthermore, we tested only click-stimulus ABR thresholds so it is possible the results with pure-tone stimuli at specific frequencies could be different.
Pendrin expression in spindle-shaped cells was inversely correlated with click ABR thresholds (Fig. 6A), suggesting that restoration of pendrin expression in these cells may underlie an overall protective effect against loss of hearing. However, we did not observe a correlation of level of pendrin expression with the cumulative threshold shift (Fig. 6B), which is a measure of the degree of hearing fluctuation. This might be due to the limited number of samples, as well as the aforementioned caveats to interpreting cumulative threshold shifts. It is also possible that pendrin fluorescence intensity is not a precise correlate of pendrin expression or function since we observed it in ears without dox readministration (Figs. 4, 5A, 5B and 5C). We cannot rule out leaky expression of the responder transgene or expression of alternative isoforms from Slc26a4Δ. Several truncated C-terminal isoforms of human SLC26A4 mRNA are annotated in the UCSC genome browser (https://genome.ucsc.edu/cgi-bin/hgGateway, accessed on December 13, 2015). No orthologous mouse Slc26a4 mRNA isoforms have been annotated or experimentally demonstrated but they could nevertheless exist and potentially be expressed from the Slc26a4Δ allele. The antibodies we used are directed against the C-terminus of pendrin so they could bind to all isoforms that include this portion of the protein. This could account for our observation that the anti-pendrin antibody fluorescence in ears with dox readministration was not higher than in ears without dox readministration (Figs. 5B and 5C).
Readministration of dox at 1 month of age or at P6 resulted in lower pendrin expression per area in endolymphatic sac cells (Fig. 5C). It suggests that dox readministration might reduce pendrin expression, increase pendrin degradation, or both in these cells. This effect was more pronounced for ears with readministration of dox at 1 month of age than readministration at P6. Furthermore, pendrin levels were not correlated with click ABR thresholds (Fig. 6E) or cumulative threshold shifts (Fig. 6F). Thus the difference of pendrin expression in endolymphatic sac cells is probably not important for stabilization of hearing.
The comparative levels of pendrin expression in root cells (Fig. 5B) were similar to those for endolymphatic cells (Fig. 5C). Measurement of pendrin expression levels was more challenging in root cells due to their morphology and we have less confidence interpreting these findings. We cannot conclude whether root cells are important for stabilization of hearing.
We used mice with a mixed genetic background since we have been unable to generate sufficient numbers of mice from a congenic line for the experiments. We generated CBA/CaJ congenic lines segregating Tg[E], Tg[R], Slc26a4Δ and Slc26a4+ but breeding was difficult. We sought to mitigate effects of a mixed genetic background in our experiments by using littermate controls and studying large numbers of ears and mice.
The effect of dox readministration at P6 on fluctuation of hearing and pendrin expression in spindle-shaped cells of the stria vascularis was strong. These findings suggest that the stabilization of hearing by readministration of dox at P6 is due to increased pendrin expression in spindle-shaped cells. Therefore pendrin expression in spindle-shaped cells may be required to maintain stable hearing, whereas pendrin expression in the endolymphatic sac is required to acquire normal hearing (Li et al., 2013). This is consistent with our observation that fluctuation of hearing is caused by dysfunction of the stria vascularis in the mature DE17.5 ear. Although our earlier results suggested that pendrin is not required to maintain hearing in a normally developed ear (Choi et al., 2011), our current results indicate that it is required to maintain hearing in an abnormally developed ear. This raises the possibility that pendrin may be important for maintaining hearing in other ototoxic conditions, such as noise exposure, that adversely impact the structure and function of the stria vascularis.
Conclusions
We conclude that early re-induction of Slc26a4 expression can prevent fluctuation of hearing in our Slc26a4-insufficient mouse model. Postnatal day 6 in the mouse inner ear is equivalent to a prenatal time point in human ears, but the onset of hearing loss in EVA patients is often postnatal with months or years elapsing before the beginning of loss or fluctuation of hearing (Jackler and De La Cruz, 1989; Levenson et al., 1989; Griffith and Wangemann, 2011). This delayed onset may offer a window of therapeutic opportunity to ameliorate hearing loss and fluctuation in human EVA patients.
Highlights.
SLC26A4 mutations can cause fluctuating hearing loss in human patients.
Slc26a4-insufficient mice have fluctuating hearing loss from 1 to 3 months of age.
Re-induction of Slc26a4 expression stabilizes hearing in Slc26a4-insufficient mice.
Stabilization is mediated by Slc26a4 expression in cells in the stria vascularis.
Restoration of SLC26A4 expression could reduce fluctuation of hearing in patients.
Acknowledgements
This work was supported by NIH intramural research funds Z01-DC000060 and Z01-DC000080. A.N. was supported in part by a JSPS Research Fellowship for Japanese Biomedical and Behavioral Researchers at NIH. P.W. was supported by NIH grant R01-DC012151. We thank Wade Chien, Keiji Honda, Meghan Drummond and our NIDCD colleagues for helpful suggestions and advice, Ken Kitamura for support, and Tom Friedman and Doris Wu for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anniko M. Surface structure of stria vascularis in the guinea pig cochlea. Normal morphology and atoxyl-induced pathologic changes. Acta oto-laryngologica. 1976;82:343–353. doi: 10.3109/00016487609120918. [DOI] [PubMed] [Google Scholar]
- Choi BY, Kim HM, Ito T, Lee KY, Li X, Monahan K, Wen Y, Wilson E, Kurima K, Saunders TL, Petralia RS, Wangemann P, Friedman TB, Griffith AJ. Mouse model of enlarged vestibular aqueducts defines temporal requirement of Slc26a4 expression for hearing acquisition. The Journal of clinical investigation. 2011;121:4516–4525. doi: 10.1172/JCI59353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Xu J, Wang Z, Smith AN, Soleimani M, Karet FE, Greinwald JH, Jr., Choo D. Co-expression of pendrin, vacuolar H+-ATPase alpha4-subunit and carbonic anhydrase II in epithelial cells of the murine endolymphatic sac. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2004;52:1377–1384. doi: 10.1177/002215540405201014. [DOI] [PubMed] [Google Scholar]
- Everett LA, Belyantseva IA, Noben-Trauth K, Cantos R, Chen A, Thakkar SI, Hoogstraten-Miller SL, Kachar B, Wu DK, Green ED. Targeted disruption of mouse Pds provides insight about the inner-ear defects encountered in Pendred syndrome. Human molecular genetics. 2001;10:153–161. doi: 10.1093/hmg/10.2.153. [DOI] [PubMed] [Google Scholar]
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED. Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS) Nature genetics. 1997;17:411–422. doi: 10.1038/ng1297-411. [DOI] [PubMed] [Google Scholar]
- Griffith AJ, Wangemann P. Hearing loss associated with enlargement of the vestibular aqueduct: mechanistic insights from clinical phenotypes, genotypes, and mouse models. Hearing research. 2011;281:11–17. doi: 10.1016/j.heares.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Morton-Jones R, Vlajkovic SM, Telang RS, Paramananthasivam V, Tadros SF, Wong AC, Froud KE, Cederholm JM, Sivakumaran Y, Snguanwongchai P, Khakh BS, Cockayne DA, Thorne PR, Ryan AF. ATP-gated ion channels mediate adaptation to elevated sound levels. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7494–7499. doi: 10.1073/pnas.1222295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Li X, Kurima K, Choi BY, Wangemann P, Griffith AJ. Slc26a4-insufficiency causes fluctuating hearing loss and stria vascularis dysfunction. Neurobiology of disease. 2014;66:53–65. doi: 10.1016/j.nbd.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Nishio A, Wangemann P, Griffith AJ. Progressive irreversible hearing loss is caused by stria vascularis degeneration in an Slc26a4-insufficient mouse model of large vestibular aqueduct syndrome. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabba SV, Oelke A, Singh R, Maganti RJ, Fleming S, Wall SM, Everett LA, Green ED, Wangemann P. Macrophage invasion contributes to degeneration of stria vascularis in Pendred syndrome mouse model. BMC medicine. 2006;4:37. doi: 10.1186/1741-7015-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackler RK, De La Cruz A. The large vestibular aqueduct syndrome. The Laryngoscope. 1989;99:1238–1242. doi: 10.1288/00005537-198912000-00006. discussion 1242-1233. [DOI] [PubMed] [Google Scholar]
- Katagiri S, Kawamoto K, Watanuki K. Some surface views of the stria vascularis and its adjacent areas. Acta oto-laryngologica. 1968;66:386–398. doi: 10.3109/00016486809126304. [DOI] [PubMed] [Google Scholar]
- Kim HM, Wangemann P. Epithelial cell stretching and luminal acidification lead to a retarded development of stria vascularis and deafness in mice lacking pendrin. PloS one. 2011;6:e17949. doi: 10.1371/journal.pone.0017949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson MJ, Parisier SC, Jacobs M, Edelstein DR. The large vestibular aqueduct syndrome in children. A review of 12 cases and the description of a new clinical entity. Archives of otolaryngology--head & neck surgery. 1989;115:54–58. doi: 10.1001/archotol.1989.01860250056026. [DOI] [PubMed] [Google Scholar]
- Li X, Sanneman JD, Harbidge DG, Zhou F, Ito T, Nelson R, Picard N, Chambrey R, Eladari D, Miesner T, Griffith AJ, Marcus DC, Wangemann P. SLC26A4 targeted to the endolymphatic sac rescues hearing and balance in Slc26a4 mutant mice. PLoS genetics. 2013;9:e1003641. doi: 10.1371/journal.pgen.1003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano L, Reiss G, Iurato S, Reale E. The junctions of the spindle-shaped cells of the stria vascularis: a link that completes the barrier between perilymph and endolymph. Hearing research. 1995;85:199–209. doi: 10.1016/0378-5955(95)00047-8. [DOI] [PubMed] [Google Scholar]
- Morton CC, Nance WE. Newborn hearing screening--a silent revolution. The New England journal of medicine. 2006;354:2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- Royaux IE, Belyantseva IA, Wu T, Kachar B, Everett LA, Marcus DC, Green ED. Localization and Functional Studies of Pendrin in the Mouse Inner Ear Provide Insight About the Etiology of Deafness in Pendred Syndrome. JARO - Journal of the Association for Research in Otolaryngology. 2003;4:394–404. doi: 10.1007/s10162-002-3052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC medicine. 2004;2:30. doi: 10.1186/1741-7015-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P, Liu J, Marcus DC. Ion transport mechanisms responsible for K+ secretion and the transepithelial voltage across marginal cells of stria vascularis in vitro. Hearing research. 1995;84:19–29. doi: 10.1016/0378-5955(95)00009-s. [DOI] [PubMed] [Google Scholar]