Abstract
BACKGROUND
Babesiosis is an emerging tick-borne infection in humans. The increasing numbers of reported cases of transfusion-associated babesiosis (TAB), primarily caused by Babesia microti, represents a concern for the safety of the U.S. blood supply.
STUDY DESIGN AND METHODS
This study investigated kinetics of parasitemia, innate immune responses and dynamics of antibody responses during B. microti infection in rhesus macaques using blood smears, quantitative PCR (qPCR), flow cytometry, and indirect fluorescent antibody testing. A total of 6 monkeys were transfused with either hamster or monkey-passaged B. microti infected erythrocytes (2 and 4 monkeys, respectively) simulating TAB.
RESULTS
The prepatent period in monkeys inoculated with hamster-passaged B. microti was 35 days compared with 4 days in monkeys transfused with monkey-passaged B. microti; the latter monkeys also had markedly higher parasitemia levels. The duration of the window period from the first detected parasitemia by qPCR analysis to the first detected antibody response ranged from 10–17 days. Antibody responses fluctuated during the course of the infection. Innate responses assessed by the frequencies of monocytes and activated B cells correlated with the kinetics and magnitude of parasitemia. On day 14, additional activation peaks were noted for CD14+CD16+ and CD14−CD16+ monocytes and for CD11c+ myeloid dendritic cells, but only in animals transfused with monkey-passaged B. microti. Parasitemia persisted in these immunocompetent animals, similar to human infection.
CONCLUSION
The results suggest that transfusion-associated transmission of B. microti leads to rapid onset of parasitemia (day 4) in rhesus macaques, detectable antibody response 14 days later and persistent parasitemia.
Keywords: Antibody, Babesia, Immune, Parasitemia, PCR, Rhesus, Transfusion
INTRODUCTION
Babesiosis is a tick-borne disease caused by intraerythrocytic parasites, including Babesia microti and other Babesia species.1,2 Babesia infection can range from asymptomatic to severe.1,3,4 In severe or persistent cases who require treatment, clindamycin and quinine5 or an alternative regimen using atovaquone and azithromycin have been recommended.6 B. microti infection is not usually clinically silent,7,8 complications of B. microti include hemolytic anemia, acute respiratory distress syndrome, disseminated intravascular coagulopathy, congestive heart failure, hepatic failure, splenic rupture and renal failure. 9–12 In particular, persons who are asplenic, elderly, or otherwise immunocompromised are at increased risk for clinical manifestations and life-threatening infection.1,3,4
Transfusion-associated babesiosis (TAB) is a growing public health concern for the safety of the US blood supply since it may lead to severe morbidity and mortality. Babesiosis became a nationally notifiable disease in January 2011.13 B. microti has been implicated in the majority of US tick-borne and TAB cases, including 159 of the 162 cases of TAB that were identified during the period of 1979–2009; the other 3 cases were caused by B. duncani.13 These figures likely underestimate the actual number of TAB cases, as infections are not always diagnosed nor recognized as transfusion-associated and investigated.13 In the United States, B. microti is endemic in parts of the Northeast and upper Midwest, whereas sporadic cases of infection with B. duncani have been identified in several western states.13 Acute, symptomatic cases of babesiosis typically are diagnosed by light-microscopic identification of organisms on Giemsa-stained thin blood smears.9,14 B. microti elicits both IgM and IgG antibody responses in humans, and serologic testing is commonly used as evidence for transmission in retrospective investigations of donors,15–17 although recent attempts to detect Babesia nucleic acids in blood products have been made to confirm transmission.17–19 In addition, investigational high throughput antibody screening assays including Enzyme immunoassay (EIA) and arrayed fluorescence immunoassay (AFIA) showed promising results for donor screening. 17,20,21 FDA has closely monitored the results of an investigational protocol evaluating B. microti serology and PCR and recently solicited formal input from an advisory committee on appropriate screening methodology and donor deferral, but has yet to define a temporary deferral period for donors who have tested positive for B. microti.21
Nonhuman primates–particularly, rhesus macaques (RMs) are the most widely used animal models of human disease because of their close genetic, physiologic, and metabolic similarity to humans.22 RMs have been used successfully to study the kinetics of B. microti parasitemia.23–25 However, these studies did not document the acute innate immune responses during B. microti parasitemia, as well as before and after seroconversion. Further, immune responses to B. microti infection have been evaluated in a limited number of studies.26,27 Such issues are central to developing effective strategies to prevent TAB, including an automated high-throughput assay for B. microti donor screening. Therefore, we conducted a formal, comprehensive investigation using the RM animal model to fill key gaps and model human TAB with B. microti.
MATERIALS AND METHODS
Ethics statement
All RMs were born at Yerkes National Primate Research Center (YNPRC) and were maintained at the YNPRC of Emory University in accordance with the regulations of the Guide for the Care and Use of Laboratory Animals, 8th edition. YNPRC has been fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International since 1985. The experiments were approved by the Emory Institutional Animal Care and Use Committee as well as biosafety review board. The animals were fed monkey diet (Purina) supplemented daily with fresh fruits or vegetables and water ad libitum. Additional social enrichment, including the delivery of appropriate safe toys, was provided and overseen by the Yerkes enrichment staff, and animal health was monitored daily and recorded by the animal care staff and veterinary personnel, available 24 hours, 7 days a week. Monkeys were caged in socially compatible same-sex pairs to facilitate social enhancement and well-being. Male Golden Syrian Hamsters (Mesocricetus auratus) were used to propagate B. microti Gray strain parasites in accordance with the rules and regulations within CDC animal use protocol “Production of antigen in hamsters and gerbils for the serodiagnosis of babesiosis”.
Study design and experimental infections
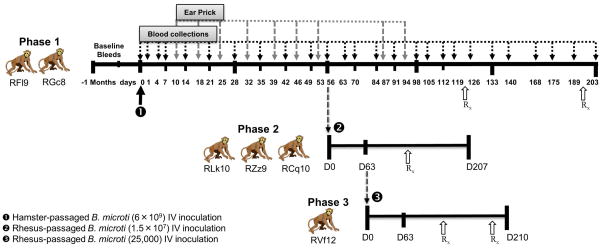
The study was conducted in three different phases as illustrated in Figure 1. A total of 6 adult (7–13 years), female, non-splenectomized, Indian RMs were assigned to this study, specifically 2 monkeys for phase 1 (RFl9, RGc8), 3 monkeys in phase 2 (RLk10, RZz9, RCq10), and 1 animal for phase 3 (RVf12). All the animals were prescreened to rule out prior malaria and/or Babesia infection using blood smears, PCR and IFA test. The animals were pretreated with diphenhydramine prior to exposure to B. microti to prevent potential anaphylaxis. Since B. microti cannot be grown in vitro, freshly collected blood from parasitemic hamsters was utilized as the source of primary inoculation for phase 1. Each of the 2 RMs was inoculated with 2.8 mL of heparinized hamster blood intravenously containing approximately 6×109 hamster-passaged B. microti Gray strain. After inoculation, blood samples were collected aseptically from the femoral vein in EDTA on days 1, 4, 7, 14 and weekly thereafter (Figure 1), for PCR, IFA, flow cytometry, Giemsa-stained thin/thick blood smears, complete blood count and chemistry analyses. Blood smears were also prepared from blood obtained by ear prick procedure to determine the parasitemia at various time points (Figure 1). On day 56, 10 mL blood from each phase 1 monkey was harvested and erythrocytes from each animal were washed in 7.5 mL normal saline and mixed together to a total volume of 15 mL for transfusion into three phase 2 monkeys (Figure 1). Blood smears from pooled blood sample were also prepared to estimate parasitemia. Each phase 2 animal was inoculated intravenously with 5 mL of infected erythrocytes and saline mixture corresponding to approximately 1.5×107 organisms of monkey-passaged B. microti obtained from phase 1. On day 63 of phase 2, blood was collected from RM RCq10, in which B. microti had become undetectable in both thin and thick blood smears but remained positive by IFA and PCR, to test whether transfusion of such blood had the potential to transmit Babesia. Five mL of washed erythrocytes corresponding to a total dose of 25,000 monkey-passaged B. microti (based on qPCR) from phase 2 were prepared as described above and transfused intravenously to RM RVf12 (phase 3). In phase 3, additional blood samples were collected on days 10, 12, and 18. All animals in this study were treated for 10 days with atovaquone (Amneal Pharmaceuticals) @ 20 mg/kg orally twice a day and azithromycin (Greenstone Limited) @ 12 mg/kg orally once a day starting on day 122. The animals were monitored thereafter by blood collections at various time points to document that no parasites were found and to conduct additional analyses as described above. Phase 1 and 3 animals (Figure 1) needed a second course of treatment starting on days 193 and 200, respectively, to achieve parasitic clearance.
Figure 1.
Study design. Phase 1: On day 0, two rhesus macaques were inoculated with erythrocytes containing 6×109 hamster-passaged B. microti. In phase 2, three monkeys were transfused with 1.5×107 monkey-passaged B. microti obtained on day 56 of phase 1. One monkey in phase 3 was transfused with 25,000 monkey-passaged B. microti acquired from a phase 2 monkey. All animals in this study were treated with atovaquone and azithromycin for 10 days starting on day 122. Phase 1 and 3 animals received a second course of treatment starting on days 193 and 200, respectively. Blood samples for PCR, IFA, flow cytometry and ear prick for blood smear examination were collected at multiple time points during the three phases of the study.
Estimation of parasitemia by light microscopy
Peripheral blood thin and thick smears were prepared from EDTA whole blood, stained with Wright-Giemsa stain. Thin blood smears were used for parasite identification and thick blood smears for quantification of organisms detected as described previously.28 Parasitemia was expressed as parasites/μL of blood in a thick blood film by counting parasites until 200 sequential WBCs were enumerated and using the simple mathematical formula:
Nested PCR (nPCR) and Quantitative PCR (qPCR)
DNA isolation was performed on 200 μL of EDTA whole-blood using QIAamp DNA Blood Mini Kit using the QIAcube automated system (QIAGEN, CA). Detection of B. microti DNA was performed in a nPCR assay as described elsewhere,29 with some modifications. For qPCR, a standard curve was created by serially diluting three blood samples from experimentally infected hamsters with known parasitemia levels, corresponding to 220,000, 935,000 and 1,280,000 parasites/μL, respectively (Figure S1). qPCR was performed using a published TaqMan real-time PCR assay targeting the 18S rRNA gene.30 Each sample was run in triplicate with appropriate negative and positive controls. The Ct-values were compared to the standard curve to derive numbers of parasites present in the RM samples. Parasitemia was expressed as parasites/μL of blood.
Indirect fluorescent antibody test
An IFA assay for IgG antibodies to B. microti was used as described earlier.31 Briefly, sera were diluted fourfold starting at 1:4 to 1:4096. 20 μL of sera was added to the appropriate well on the coated B. microti slides and incubated for 30 min at 37°C. Slides were washed for 5 min with PBS and dried followed by addition of 20 μL goat anti-human immunoglobulin cross reacting with rhesus immunoglobulin labeled with fluorescein (FITC, 1 mg/mL) at 1:50 in PBS with 0.1% Evans blue and were incubated for 30 min at 37°C. The fluorescence was determined with an Olympus BX60 microscope equipped with a X-cite Series 120–Q fluorescence illumination source at 400× magnification. A titer of 1:64 with B. microti antigen was considered a positive reaction.
Flow cytometry
Freshly isolated peripheral blood mononuclear cells (PBMC) (~1×106) were stained with three fluorescent antibody panels with predetermined concentrations of antibodies against CD3 (SP34-2), CD4 (L200), CD8 (RPA-T8), CD20 (L27), CD28 (CD28.2), CD95 (DX2), NKG2A (Z199), HLADR (Tu36), CD38 (AT-1), CD14 (M5E2), CD16 (3G8), CD11c (3.9), CD123 (7G3), CD21 (B-Ly4), CD27 (MT271), CD127 (R34.34), CD25 (4E3), CD86 (IT2.2), CD25 (1HT44H32) (Table S2). Cells were incubated with the antibody cocktails for 30 min at 4°C, washed with PBS containing 2% FBS, permeabilized with perm/fix buffer (BD) washed with 2 mL of perm wash buffer. The cells were then additionally stained with anti-Ki67 (B56) or for Foxp3 (206D). Cells were finally fixed in 1% paraformaldehyde, acquired on LSR-II flow cytometer driven by FACS DiVa software. Analysis of the acquired data was performed using FlowJo software (version 9.4.11; TreeStar).
Statistical analyses
Flow cytometry data were analyzed using a 2-tailed paired t-test to compare activation patterns in phase 1 and 2 animals.
RESULTS
Level and duration of parasitemia during B. microti infection
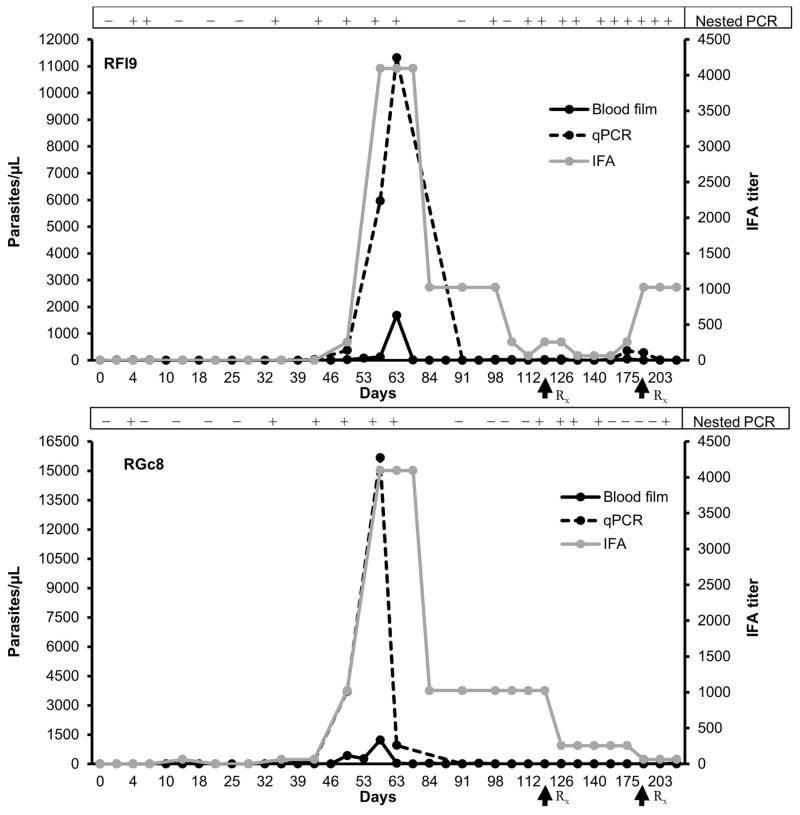
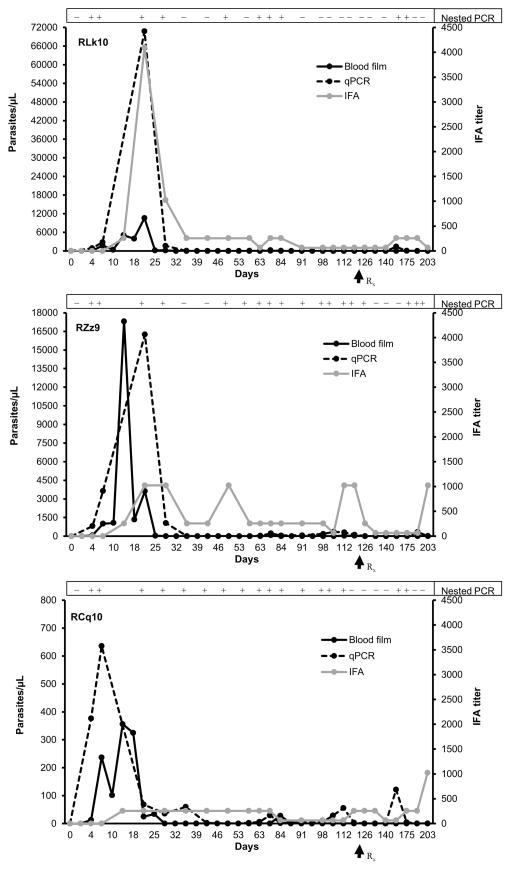
To model TAB in monkeys, we utilized a 3 tiered experimental protocol: The initial phase was to generate monkey-adapted B. microti for direct transfusion of “high acute” parasitemia (phase 2) and low chronic parasitemia (phase 3). For the xenogeneic transfusion (phase 1), patent infection developed in both monkeys inoculated with 6×109 hamster-passaged B. microti (Gray strain) after 35 and 49 days in the 2 monkeys (RFl9, RGc8) based on qPCR and blood smear data, respectively (Figures 2 and S2). The parasitemia peaked on day 56 (RGc8) and 63 (RFl9) after experimental infection, and the peak parasitemia detected with qPCR and blood smears showed good temporal correlation. Peak parasitemia ranged from 1227 to 1679 organisms/μL on thick blood smears or 11,319 to 15,676 organisms/μL blood by qPCR. After peak parasitemia, both animals showed intermittent low level parasitemia (<50 organisms/μL) prior to initiating drug treatment on day 122. This low level parasitemia persisted in RFl9 based on nPCR/qPCR analysis leading to a second round of therapy on day 193. Erythrocytes from both phase 1 monkeys were collected on day 56 and transfused to phase 2 monkeys (corresponding to 1.5×107 organisms). Even though the parasite dose was >100 fold lower than the dose administered to phase 1 monkeys, the prepatent period was markedly reduced (4 days). The peak parasitemia identified (qPCR and blood smears) on day 14 or day 21 post infection was also markedly higher in 2 of the 3 phase 2 monkeys compared to phase 1 monkeys (Figure 3). Phase 2 RM RCq10 surprisingly exhibited markedly lower peak parasitemia, suggesting individual differences in susceptibility. Beyond the acute parasitemia, these monkeys also exhibited intermittently detectable low level chronic parasitemias (<50 organisms/μL) prior to atovaquone/azithromycin treatment. The intermittent chronic low level parasitemia was detected predominantly by nPCR, the most sensitive technique, though confirmed by qPCR followed by measurable transient rebounds in IFA titers (Figures 2–4 and Table S1), while parasites could not be detected in blood smears beyond the acute infection.
Figure 2.
Course of parasitemia in the two phase 1 rhesus macaques (RFl9, RGc8) transfused with hamster-passaged B. microti. The parasitemia values (left y axis) were compared with an IFA test titer (right y axis) and nested PCR. A titer of 1:64 with B. microti antigen was considered a positive reaction. Parasitemia levels determined on thick blood smears and qPCR were expressed as parasites/μL. Nested PCR results are reported as positive or negative. Arrows indicate initiation of atovaquone/azithromycin treatment.
Figure 3.
Parasitemia in the three phase 2 rhesus macaques (RLk10, RZz9, and RCq10) transfused with monkey-passaged B. microti. The parasitemia values (left y axis) were compared with an IFA test titer (right y axis) and nested PCR. A titer of 1:64 with B. microti antigen was considered positive. Parasitemia levels determined on thick blood smears and qPCR were expressed as parasites/μL. Nested PCR results are reported as positive or negative. Arrows indicate initiation of atovaquone/azithromycin treatment.
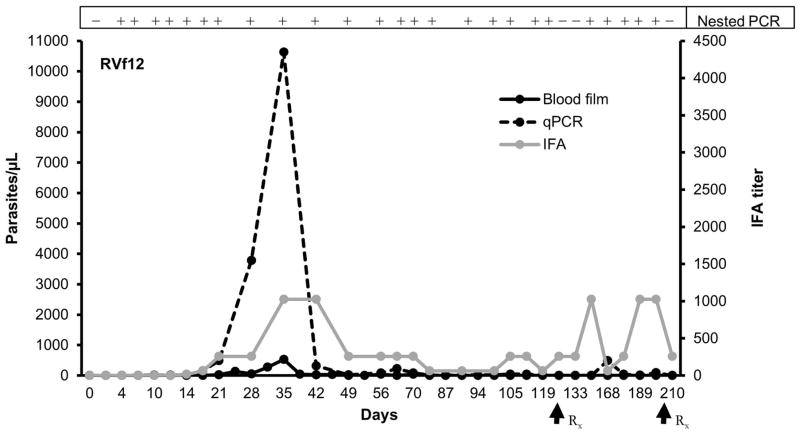
Figure 4.
Parasitemia in the one phase 3 rhesus macaque (RVf12) transfused with monkey-passaged B. microti. The parasitemia values (left y axis) were compared with an IFA test titer (right y axis) and nested PCR. A titer of 1:64 with B. microti antigen was considered positive. Parasitemia levels determined on thick blood smears and qPCR were expressed as parasites/μL. Nested PCR results are reported as positive or negative. Arrows indicate initiation of atovaquone/azithromycin treatment.
To determine the clinical relevance of low chronic parasitemia, blood collected from RM RCq10 at day 63 post transfusion (blood smears with no parasites found and barely positive qPCR signal) was transfused to phase 3 monkey (RVf12), amounting to about 25,000 monkey-passaged B. microti organisms, based on qPCR. However, this low dose exposure also resulted in patent infection. The prepatent period was 4 days based on qPCR/nPCR and blood smears detected B. microti on day 21. Peak parasitemia was detected on day 35 (Figure 4, 528 organisms/μL by blood smears and 10,631 organisms/μL by qPCR). RVf12 also showed intermittently detectable low level parasitemia (<50 organisms/μL) on blood smears until day 70 followed by nPCR/qPCR detection prior to starting treatment on day 122. This animal, similar to phase 1 RFl9 required a 2nd course of treatment to eliminate detectable parasite.
The kinetics and detection of parasitemia have been summarized in Table 1 and Figures 2–4, suggesting clear differences in prepatency depending on the source of the TAB, while peak parasitemia in animals transfused with monkey-passaged B. microti appear to reflect the infectious dose, though phase 3 only comprised 1 animal. There was fluctuation of parasitemia after the acute peak with no pattern in the timing of recurrences of detectable parasitemia during chronic infection.
Table 1.
Kinetics of parasitemia and antibody titers in the rhesus macaques infected with B. microti during the three phases of the study
|
|
|
||
|---|---|---|---|
| Hamster-passaged B. microti
|
Monkey-passaged B. microti
|
||
| Screening Test | Phase 1 | Phase 2 | Phase 3 |
| qPCR parasitemia* | |||
| First detection | 35 | 4 | 4 |
| Peak detection | 56–63 | 14–21 | 35 |
| Blood smear parasitemia* | |||
| First detection | 49 | 4 | 21 |
| Peak detection | 56–63 | 14–21 | 35 |
| IFA titer* | |||
| First response | 35–49 | 14 | 21 |
| Peak response | 56 | 21 | 35 |
Data correspond to respective study day(s) in the particular phase (Phase 1: monkeys RFl9 and RGc8; Phase 2: monkeys RLk10, RZz9, and RCq10; Phase 3: monkey RVf12)
Of note, clinical symptoms, e.g. flu-like illness, and deviations in hematological parameters (hemoglobin, hematocrit, WBC or differential counts) were not observed in these animals.
Antibody responses during and after B. microti infection
The kinetics of parasitemia and antibody titers are summarized in Table 1 and Figures 2–4. During phase 1, the first antibody IFA responses (1:64) were detected on days 35 and 49, on the day of, or 2 weeks after the first qPCR detection for RGc8 and RFl9, respectively. The peak antibody titers (1:4096) in both animals were detected on day 56 coinciding with peak parasitemia. The antibody titers gradually declined to 1:1024 on day 84, and lower levels (1:64) were still observed for RGc8 on day 207. Conversely, RFl9’s titer fluctuated, and a titer of 1:1024 was observed on day 207 despite the absence of detectable parasitemia. In phase 2, the first positive antibody titers (1:256) were detected on day 14 i.e. 10 days after the first detectable parasitemia. Peak antibody titers were identified on day 21 again, coinciding with the peak parasitemia. Of note, peak antibody titers in 2 of the 3 phase 2 RMs and the phase 3 RM were markedly lower than in phase 1 monkeys. The first antibody detection in the phase 3 monkey was on day 21, likely attributable to the comparatively small inoculum transfused. With the exception of RCq10, the peak antibody titers rapidly declined in all animals, though fluctuations in antibody titers were noted for the monkeys transfused with monkey-passaged Babesia but not with hamster-passaged parasites, suggesting occasional parasite replication which is not necessarily detectable in blood.
Innate immune responses to B. microti infection
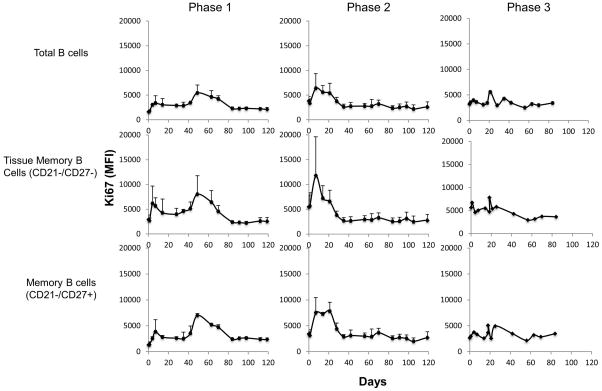
We hypothesized that B. microti infection invokes innate responses in vivo. Therefore, we monitored PBMCs at periodic intervals over the course of the study to address changes and activation of various cell lineages. Evidence of immune activation was most clearly observed in B cells, T cells, monocytes, and myeloid dendritic cells, whereas changes in NK cells (NKG2a+) or T regulatory cells (CD25+/FoxP3+/CD127−) were not observed in blood. As expected for parasite driven responses, the kinetics for immune activation paralleled those of parasite burden. B cell activation/proliferation—assessed by the frequency and density of Ki67 expression on CD20+ cells—showed a biphasic increase on days 7 and 49 in both phase 1 animals (Figure 5). In the phase 2 monkeys, B cell activation peaked sharply on day 7 and decreased thereafter towards baseline values by day 21 or 35. Activation of B cells in the phase 3 animal was weaker, which may reflect the comparatively low inoculum. For all 6 monkeys, the peak activation was essentially reflective of tissue memory (CD21−/CD27−, p=0.0441 on day 7) and of memory (CD21−/CD27+; p=0.0198 on day 7 and p=0.0012 on day 14 relative to baseline) B cell subsets, whereas the naïve B cell subsets (CD21+/CD27−) did not show such change (not shown).
Figure 5.
Activation of total and subpopulations of B cells in rhesus macaques during the course of the three phases of the study of B. microti infection. Lines represent the average Ki67 MFI for each phase with the error bars indicating standard deviation. MFI: mean fluorescence intensity.
Activation of T cells was predominantly seen in the CD8+ central memory T cells (Tcm) (CD28+CD95+) on day 7 post transfusion for animals infected with monkey-passaged B. microti, as measured by the percentages of Ki67+ and HLA-DRhi cells (Figure S3), whereas such increases in percentages were much more modest in phase 1 animals. In contrast to CD8+ cells, CD4+ T cells showed little evidence of activation, except perhaps in a phase 1 monkey (RGc8; data not shown).
For monocytes and myeloid dendritic cells, activation measured by increases in Ki67 and CD86 mean fluorescence intensity demonstrated low and highly variable levels of activation without a clear trend in phase 1 monkeys (data not shown). However, in phase 2 monkeys, acute activation was clearly seen on day 14 for activated CD14+/CD16+ and atypical CD14−/CD16+ monocytes, as well as for myeloid dendritic cells (Figure S4). This activation was highest for RCq10, followed by RLk10 and RZz9, suggesting that activation of monocytic lineages was not proportional to the magnitude of parasitemia. Activation of these subsets was minimal in the phase 3 monkey (data not shown).
DISCUSSION
In the US, almost 5 million patients are recipients of red blood cells transfusion annually, the safety of which remains a constant source of concern.32 Babesiosis is now the most commonly reported transfusion-associated parasitic disease.33,34 Clinical features of TAB cases are usually considered similar or more severe than those observed after arthropod vectored transmission, primarily due to underlying health-related issues and lowered immune competence in blood recipients, promoting the development of clinically manifest babesiosis following blood transfusion with a Babesia-contaminated unit.8
While there is increased interest in developing a high-throughput assay for B. microti blood donor screening, further research is also needed to understand the kinetics of parasitemia and antibody responses. Various experimental animal models have been used to investigate the underlying pathophysiology of Babesia infection, and hamsters are commonly used as a model to study babesiosis and explore drug therapies.35,36 Mouse models are usually less representative because they develop low grade parasitemia.37 Despite the presence of existing rodent models, the pathophysiology of human babesiosis is not completely understood. Therefore, in the present study we investigated clinically relevant aspects of transfusion-associated transmission of B. microti in a primate model that is physiologically closer to man.
Data on duration of B. microti parasitemia or persistence of infection in humans are limited.38,39 In this study, the prepatent period in monkeys inoculated with hamster-passaged Gray stain cells ranged from 35 to 49 days, and the peak parasitemia on blood smears ranged from 1227 to 1679 organisms/μL. Morphologically, the parasites resembled those seen in the hamsters, humans and NHP. In the late 1970s and early 1980s, RMs have been used successfully to study the kinetics of B. microti parasitemia.23–25 These studies reported a prepatent period of 15 to 46 days in monkeys infected with the hamster-passaged Briard strain of B. microti, and peak parasitemia ranged from 496 to 3906 organisms/μL. Parasitemia persisted for at least 90 days in all the animals and parasites were detected 559 days after inoculation in one animal, 23 suggesting variability in the ability of B. microti strains to replicate in vivo. In addition, recurrences of parasitemia were seen following splenectomy in RMs infected intravenously or via B. microti infected nymphal ticks.24,25 The phase 2 and 3 animals in this study revealed a far shorter prepatent period (4 days) than the phase 1 monkeys suggesting a possible host adaptability of B. microti, and a far more representative model of TAB in humans. The basis for such variability in parasite dynamics between hamster-passaged and monkey-passaged B. microti strains may be secondary to parasite genome mutations, or the acquisition of species specific host markers that somehow facilitate replication in vivo. It has been reported that comparative studies of parasite genomes can reveal genetic adaptations to parasitism.40 However, the extent to which the combination of host environment and genetic variation accounts for variability in pathogenicity associated with B. microti strains remains to be investigated.
The possibility of asymptomatic persistence of B. microti in blood donors is supported by numerous TAB cases.13,41,42 A recent study documented that seropositive donors can have protracted low level parasitemia,38 which was corroborated in the present study up to the time that the monkeys received antimicrobial therapy. The possible explanation of recurrent parasitemia in this study could be due to combination of premunition (concomitant immunity) and/or process of “antigenic variation” which has been reported to occur in Babesia spp. including B. microti and is thought to aid Babesia spp. survival by evasion of host adaptive immune responses.43–45
One major challenge posed by this mostly clinically silent infection is the possibility of persistent parasitemia that is below the level of detection of currently available tests. As seen here, detection of parasites in blood smears, the current reference for patient diagnosis in acutely ill patients, including transfusion recipients, is relatively insensitive, and absence of Babesia detection by this method does not exclude the possibility of parasite transmission to transfusion recipients (Figures 3, 4). Although the number of animals available to us did not allow for extensive testing of transmission during chronic Babesia infection, the transmission of B. microti from a phase 2 monkey to the phase 3 monkey readily occurred even though qPCR values and nPCR signals at the time of collection were very low (≤5 organisms/μL of blood), providing proof of concept that such transmission does occur. Further, antibody titers measured by IFA were quite variable post infection, and often barely positive, despite low (intermittently detectable) levels of Babesia nPCR/qPCR signals, suggesting that single nucleic acid based test or IFA assay available to date may not be adequate by itself for adequate donor screening to prevent Babesia transmission, particularly after the acute phase of Babesia infection. This is not secondary to the sensitivity of individual assays but more to the size of the representative samples tested. The findings in the current study underscore that the protracted low levels of parasitemia pose a risk for transmission. Of note, the transfusion used in our study differs from human blood transfusion by the absence of blood storage as in human blood banking conditions. In addition, a single course of prescribed atovaquone and azithromycin failed to clear the parasites in 3 animals suggesting additional courses of treatment are required for complete clearance of B. microti, which requires further investigation. Similar finding has been reported in few B. microti infected humans where the subjects were retreated with atovaquone and azithromycin due to persistent positive B. microti PCR.38
Little is known also regarding the minimal infectious dose of B. microti which can cause infection in naïve hosts. The monkey in phase 3 of this study became infected with a dose of 25,000 B. microti organisms obtained from 5 mL of erythrocytes harvested during the chronic phase of infection even though no parasites could be detected by blood smear. Considering the average volume of a human blood transfusion for adults, this dose represents approximately 1.5×106 B. microti in a blood unit assuming 300 mL of packed RBC/unit. Of note, while the low dose transfused resulted in a delayed peak of parasitemia and lower magnitude measured by blood smear, the qPCR parasitemia loads were similar to those of the other monkeys transfused with higher Babesia doses.
Innate responses such as monocyte activation has been reported upon infection of monkeys with B. microti.46 Another study reported higher frequency of IL-10 producing B cells (Bregs) and CD4+CD25+FoxP3+ T cells during the course of B. microti infection in mice.19 Our study confirmed an early activation of monocyte subsets and myeloid dendritic cells corresponding to the peak parasitemia, suggesting a role for these cell lineages in the early control of parasitemia. The sharp decline in activation following this time point coincides with a drop in parasitemia and it has been demonstrated that in mice, monocyte/macrophages are important for parasite control.47 Although this study did not examine other tissues, it is possible that these monocytes are migrating to tissues such as the spleen where they develop into macrophages and assist in the removal of infected erythrocytes. Conversely, memory B cells activation preceded the peak of parasitemia, suggesting the induction of adaptive responses precedes the peak of parasitemia. Anecdotally, the animal with the lowest peak parasitemia (RCq10, phase 2) exhibited the highest level of immune activation overall, suggesting that perhaps these responses are indeed involved in the control of parasite replication. These responses were transient only, even though low levels of parasitemia persisted in most monkeys, suggesting that perhaps Babesia may have developed strategies to limit anti-parasitic defenses allowing for its survival at low levels.
Taken together, the study has enhanced our understanding of B. microti host invasion and suggested possible evolutionary adaptation of B. microti strains to a specific host and illustrated the complexity of persistence of B. microti. Overall, the antibody response was consistently detected 14 days after the first detection of parasitemia. qPCR detected infections several days before IFA upon homologous monkey infections while results from both techniques were matched for the detection of the hamster derived infections. However, the onset of parasitemia was far more rapid during the homologous transfusion. In addition, the data presented here provide a potential model to study the complexity of host-parasite interaction and the mechanisms of parasite persistence.
Supplementary Material
Figure S1. Generation of a standard curve by serially diluting three blood samples from B. microti infected hamsters with known parasitemia. The Ct-values from the standard samples were plotted against the parasite counts to create a standard curve, which was later used to estimate the number of parasites present in the rhesus macaque specimens. Parasitemia was expressed as parasites/μL of blood.
Figure S2. Photomicrograph of intraerythrocytic B. microti trophozoites (arrowheads) in a Wright-Giemsa-stained peripheral blood smear. 1000 × magnification.
Figure S3. Activation of the CD8+ central memory T cells (Tcm) during B. microti infection in rhesus macaques. Lines represent the average percentage of Ki67+ or HLA-DRhi cells in the CD8+ central memory T cell subset for each phase. The error bars indicate standard deviation. MFI: mean fluorescence intensity.
Figure S4. Activation of monocyte and dendritic cell populations in phase 2 rhesus macaques. A & B) CD14−CD16+ monocytes, C) CD14+/CD16+ monocytes, and D) CD11c+ dendritic cells. Lines represent the average Ki67 of CD86 MFI for each cell population. Error bars indicate standard deviations.
Figure S5. Indirect fluorescent antibody test for B. microti in phase 3 monkey (RVf12) with a titer of 1:64. 100 × magnification.
Table S1. Comparisons between nested and quantitative PCR data for the rhesus macaques infected with B. microti during the three phases of the study. Discrepancies between nested and quantitative PCR data are highlighted.
Table S2. Details of Flow cytometry antibody panels used in the study.
Acknowledgments
Source of support: This work was supported by Abbott Diagnostics and the NIH support to the YNPRC P51OD11132.
We thank Dr. Jennifer S Wood and the animal care staff of the YNPRC for their excellent care of the animals for this study. We also thank Drs. George J Dawson, John R Hackett and Alexandre J Da Silva for feedback on study design. Fernanda S Nascimento was supported by CNPq fellowship (236608/2013-4). The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Conflict-of-interest disclosure
The authors declare no competing financial interests.
References
- 1.Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366:2397–407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 2.Krause PJ. Babesiosis. Med Clin North Am. 2002;86:361–73. doi: 10.1016/s0025-7125(03)00092-0. [DOI] [PubMed] [Google Scholar]
- 3.Rowin KS, Tanowitz HB, Rubinstein A, Kunkel M, Wittner M. Babesiosis in asplenic hosts. Trans R Soc Trop Med Hyg. 1984;78:442–4. doi: 10.1016/0035-9203(84)90054-3. [DOI] [PubMed] [Google Scholar]
- 4.Rosner F, Zarrabi MH, Benach JL, Habicht GS. Babesiosis in splenectomized adults. Review of 22 reported cases. Am J Med. 1984;76:696–701. doi: 10.1016/0002-9343(84)90298-5. [DOI] [PubMed] [Google Scholar]
- 5.Wittner M, Rowin KS, Tanowitz HB, Hobbs JF, Saltzman S, Wenz B, Hirsch R, Chisholm E, Healy GR. Successful chemotherapy of transfusion babesiosis. Ann Intern Med. 1982;96:601–4. doi: 10.7326/0003-4819-96-5-601. [DOI] [PubMed] [Google Scholar]
- 6.Krause PJ, Lepore T, Sikand VK, Gadbaw J, Jr, Burke G, Telford SR, 3rd, Brassard P, Pearl D, Azlanzadeh J, Christianson D, McGrath D, Spielman A. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med. 2000;343:1454–8. doi: 10.1056/NEJM200011163432004. [DOI] [PubMed] [Google Scholar]
- 7.Krause PJ, McKay K, Gadbaw J, Christianson D, Closter L, Lepore T, Telford SR, 3rd, Sikand V, Ryan R, Persing D, Radolf JD, Spielman A. Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg. 2003;68:431–6. [PubMed] [Google Scholar]
- 8.Leiby DA. Transfusion-transmitted Babesia spp: bull’s-eye on Babesia microti. Clin Microbiol Rev. 2011;24:14–28. doi: 10.1128/CMR.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vannier E, Gewurz BE, Krause PJ. Human babesiosis. Infect Dis Clin North Am. 2008;22:469–88. viii–ix. doi: 10.1016/j.idc.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White DJ, Talarico J, Chang HG, Birkhead GS, Heimberger T, Morse DL. Human babesiosis in New York State: Review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med. 1998;158:2149–54. doi: 10.1001/archinte.158.19.2149. [DOI] [PubMed] [Google Scholar]
- 11.Hatcher JC, Greenberg PD, Antique J, Jimenez-Lucho VE. Severe babesiosis in Long Island: review of 34 cases and their complications. Clin Infect Dis. 2001;32:1117–25. doi: 10.1086/319742. [DOI] [PubMed] [Google Scholar]
- 12.Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am. 2015;29:357–70. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herwaldt BL, Linden JV, Bosserman E, Young C, Olkowska D, Wilson M. Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med. 2011;155:509–19. doi: 10.7326/0003-4819-155-8-201110180-00362. [DOI] [PubMed] [Google Scholar]
- 14.Gray J, Zintl A, Hildebrandt A, Hunfeld KP, Weiss L. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010;1:3–10. doi: 10.1016/j.ttbdis.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Krause PJ, Telford S, 3rd, Spielman A, Ryan R, Magera J, Rajan TV, Christianson D, Alberghini TV, Bow L, Persing D. Comparison of PCR with blood smear and inoculation of small animals for diagnosis of Babesia microti parasitemia. J Clin Microbiol. 1996;34:2791–4. doi: 10.1128/jcm.34.11.2791-2794.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause PJ, Telford SR, 3rd, Ryan R, Conrad PA, Wilson M, Thomford JW, Spielman A. Diagnosis of babesiosis: evaluation of a serologic test for the detection of Babesia microti antibody. J Infect Dis. 1994;169:923–6. doi: 10.1093/infdis/169.4.923. [DOI] [PubMed] [Google Scholar]
- 17.Moritz ED, Winton CS, Johnson ST, Krysztof DE, Townsend RL, Foster GA, Devine P, Molloy P, Brissette E, Berardi VP, Stramer SL. Investigational screening for Babesia microti in a large repository of blood donor samples from nonendemic and endemic areas of the United States. Transfusion. 2014;54:2226–36. doi: 10.1111/trf.12693. [DOI] [PubMed] [Google Scholar]
- 18.Bloch EM, Lee TH, Krause PJ, Telford SR, 3rd, Montalvo L, Chafets D, Usmani-Brown S, Lepore TJ, Busch MP. Development of a real-time polymerase chain reaction assay for sensitive detection and quantitation of Babesia microti infection. Transfusion. 2013 doi: 10.1111/trf.12098. [DOI] [PubMed] [Google Scholar]
- 19.Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol. 2012;50:903–8. doi: 10.1128/JCM.05848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levin AE, Williamson PC, Erwin JL, Cyrus S, Bloch EM, Shaz BH, Kessler D, Telford SR, 3rd, Krause PJ, Wormser GP, Ni X, Wang H, Krueger NX, Caglioti S, Busch MP. Determination of Babesia microti seroprevalence in blood donor populations using an investigational enzyme immunoassay. Transfusion. 2014;54:2237–44. doi: 10.1111/trf.12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Food and Drug Administration: Blood Products Advisory Committee Meeting. Strategies for Implementation of Antibody and Nucleic Acid-based testing for Babesia microti in Blood Donors. Silver Spring, MD: 2015. [Accessed July 2, 2015]. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/BloodVaccinesandOtherBiologics/BloodProductsAdvisoryCommittee/ucm441228.htm. [Google Scholar]
- 22.Stewart CB, Disotell TR. Primate evolution - in and out of Africa. Curr Biol. 1998;8:R582–8. doi: 10.1016/s0960-9822(07)00367-3. [DOI] [PubMed] [Google Scholar]
- 23.Ruebush TK, 2nd, Collins WE, Healy GR, Warren M. Experimental Babesia microti infections in non-splenectomized Macaca mulatta. J Parasitol. 1979;65:144–6. [PubMed] [Google Scholar]
- 24.Ruebush TK, 2nd, Collins WE, Warren M. Experimental Babesia microti infections in Macaca mulatta: recurrent parasitemia before and after splenectomy. Am J Trop Med Hyg. 1981;30:304–7. doi: 10.4269/ajtmh.1981.30.304. [DOI] [PubMed] [Google Scholar]
- 25.Ruebush TK, 2nd, Piesman J, Collins WE, Spielman A, Warren M. Tick transmission of Babesia microti to rhesus monkeys (Macaca mulatta) Am J Trop Med Hyg. 1981;30:555–9. doi: 10.4269/ajtmh.1981.30.555. [DOI] [PubMed] [Google Scholar]
- 26.Jeong YI, Hong SH, Cho SH, Lee WJ, Lee SE. Induction of IL-10-producing CD1dhighCD5+ regulatory B cells following Babesia microti-infection. PLoS One. 2012;7:e46553. doi: 10.1371/journal.pone.0046553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Terkawi MA, Nishikawa Y, Aboge GO, Luo Y, Ooka H, Goo YK, Yu L, Cao S, Sun Y, Yamagishi J, Masatani T, Yokoyama N, Igarashi I, Xuan X. Macrophages are critical for cross-protective immunity conferred by Babesia microti against Babesia rodhaini infection in mice. Infect Immun. 2012;80:311–20. doi: 10.1128/IAI.05900-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ameri M. Laboratory diagnosis of malaria in nonhuman primates. Vet Clin Pathol. 2010;39:5–19. doi: 10.1111/j.1939-165X.2010.00217.x. [DOI] [PubMed] [Google Scholar]
- 29.Persing DH, Mathiesen D, Marshall WF, Telford SR, Spielman A, Thomford JW, Conrad PA. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992;30:2097–103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hojgaard A, Lukacik G, Piesman J. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick Borne Dis. 2014;5:349–51. doi: 10.1016/j.ttbdis.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Chisholm ES, Sulzer AJ, Ruebush TK., 2nd Indirect immunofluorescence test for human Babesia microti infection: antigenic specificity. Am J Trop Med Hyg. 1986;35:921–5. doi: 10.4269/ajtmh.1986.35.921. [DOI] [PubMed] [Google Scholar]
- 32.Whitaker BI, Hinkins S. The 2011 National Blood Collection and Utilization Survey Report. U.S. Department of Health and Human Services; 2011. [Accessed August 3, 2015]. Available from: http://www.hhs.gov/ash/ [Google Scholar]
- 33.Food and Drug Administration. Fatalities reported to FDA following blood collection and transfusion: Annual summary for fiscal year 2012. Silver Spring, MD: CBER Office of Communication, Outreach, and Development; 2012. [Accessed May 12, 2015]. Available from: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/ucm346639.htm. [Google Scholar]
- 34.Stramer SL. Current perspectives in transfusion-transmitted infectious diseases: emerging and re-emerging infections. ISBT Sci Ser. 2014;9:30–6. doi: 10.1111/voxs.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wozniak EJ, Lowenstine LJ, Hemmer R, Robinson T, Conrad PA. Comparative pathogenesis of human WA1 and Babesia microti isolates in a Syrian hamster model. Lab Anim Sci. 1996;46:507–15. [PubMed] [Google Scholar]
- 36.Weiss LM, Wittner M, Wasserman S, Oz HS, Retsema J, Tanowitz HB. Efficacy of azithromycin for treating Babesia microti infection in the hamster model. J Infect Dis. 1993;168:1289–92. doi: 10.1093/infdis/168.5.1289. [DOI] [PubMed] [Google Scholar]
- 37.Matsubara J, Koura M, Kamiyama T. Infection of immunodeficient mice with a mouse-adapted substrain of the gray strain of Babesia microti. J Parasitol. 1993;79:783–6. [PubMed] [Google Scholar]
- 38.Leiby DA, Johnson ST, Won KY, Nace EK, Slemenda SB, Pieniazek NJ, Cable RG, Herwaldt BL. A longitudinal study of Babesia microti infection in seropositive blood donors. Transfusion. 2014;54:2217–25. doi: 10.1111/trf.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krause PJ, Spielman A, Telford SR, 3rd, Sikand VK, McKay K, Christianson D, Pollack RJ, Brassard P, Magera J, Ryan R, Persing DH. Persistent parasitemia after acute babesiosis. N Engl J Med. 1998;339:160–5. doi: 10.1056/NEJM199807163390304. [DOI] [PubMed] [Google Scholar]
- 40.Tsai IJ, Zarowiecki M, Holroyd N, Garciarrubio A, Sanchez-Flores A, Brooks KL, Tracey A, Bobes RJ, Fragoso G, Sciutto E, Aslett M, Beasley H, Bennett HM, Cai J, Camicia F, Clark R, Cucher M, De Silva N, Day TA, Deplazes P, Estrada K, Fernandez C, Holland PW, Hou J, Hu S, Huckvale T, Hung SS, Kamenetzky L, Keane JA, Kiss F, Koziol U, Lambert O, Liu K, Luo X, Luo Y, Macchiaroli N, Nichol S, Paps J, Parkinson J, Pouchkina-Stantcheva N, Riddiford N, Rosenzvit M, Salinas G, Wasmuth JD, Zamanian M, Zheng Y, Cai X, Soberon X, Olson PD, Laclette JP, Brehm K, Berriman M. The genomes of four tapeworm species reveal adaptations to parasitism. Nature. 2013;496:57–63. doi: 10.1038/nature12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gubernot DM, Nakhasi HL, Mied PA, Asher DM, Epstein JS, Kumar S. Transfusion-transmitted babesiosis in the United States: summary of a workshop. Transfusion. 2009;49:2759–71. doi: 10.1111/j.1537-2995.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 42.Tonnetti L, Eder AF, Dy B, Kennedy J, Pisciotto P, Benjamin RJ, Leiby DA. Transfusion-transmitted Babesia microti identified through hemovigilance. Transfusion. 2009;49:2557–63. doi: 10.1111/j.1537-2995.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- 43.Homer MJ, Bruinsma ES, Lodes MJ, Moro MH, Telford S, 3rd, Krause PJ, Reynolds LD, Mohamath R, Benson DR, Houghton RL, Reed SG, Persing DH. A polymorphic multigene family encoding an immunodominant protein from Babesia microti. J Clin Microbiol. 2000;38:362–8. doi: 10.1128/jcm.38.1.362-368.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jackson AP, Otto TD, Darby A, Ramaprasad A, Xia D, Echaide IE, Farber M, Gahlot S, Gamble J, Gupta D, Gupta Y, Jackson L, Malandrin L, Malas TB, Moussa E, Nair M, Reid AJ, Sanders M, Sharma J, Tracey A, Quail MA, Weir W, Wastling JM, Hall N, Willadsen P, Lingelbach K, Shiels B, Tait A, Berriman M, Allred DR, Pain A. The evolutionary dynamics of variant antigen genes in Babesia reveal a history of genomic innovation underlying host-parasite interaction. Nucleic Acids Res. 2014;42:7113–31. doi: 10.1093/nar/gku322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei Q, Tsuji M, Zamoto A, Kohsaki M, Matsui T, Shiota T, Telford SR, 3rd, Ishihara C. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J Clin Microbiol. 2001;39:2178–83. doi: 10.1128/JCM.39.6.2178-2183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Duivenvoorde LM, Voorberg-van der Wel A, van der Werff NM, Braskamp G, Remarque EJ, Kondova I, Kocken CH, Thomas AW. Suppression of Plasmodium cynomolgi in rhesus macaques by coinfection with Babesia microti. Infect Immun. 2010;78:1032–9. doi: 10.1128/IAI.00921-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terkawi MA, Cao S, Herbas MS, Nishimura M, Li Y, Moumouni PF, Pyarokhil AH, Kondoh D, Kitamura N, Nishikawa Y, Kato K, Yokoyama N, Zhou J, Suzuki H, Igarashi I, Xuan X. Macrophages Are the Determinant of Resistance to and Outcome of Nonlethal Babesia microti Infection in Mice. Infect Immun. 2015;83:8–16. doi: 10.1128/IAI.02128-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Generation of a standard curve by serially diluting three blood samples from B. microti infected hamsters with known parasitemia. The Ct-values from the standard samples were plotted against the parasite counts to create a standard curve, which was later used to estimate the number of parasites present in the rhesus macaque specimens. Parasitemia was expressed as parasites/μL of blood.
Figure S2. Photomicrograph of intraerythrocytic B. microti trophozoites (arrowheads) in a Wright-Giemsa-stained peripheral blood smear. 1000 × magnification.
Figure S3. Activation of the CD8+ central memory T cells (Tcm) during B. microti infection in rhesus macaques. Lines represent the average percentage of Ki67+ or HLA-DRhi cells in the CD8+ central memory T cell subset for each phase. The error bars indicate standard deviation. MFI: mean fluorescence intensity.
Figure S4. Activation of monocyte and dendritic cell populations in phase 2 rhesus macaques. A & B) CD14−CD16+ monocytes, C) CD14+/CD16+ monocytes, and D) CD11c+ dendritic cells. Lines represent the average Ki67 of CD86 MFI for each cell population. Error bars indicate standard deviations.
Figure S5. Indirect fluorescent antibody test for B. microti in phase 3 monkey (RVf12) with a titer of 1:64. 100 × magnification.
Table S1. Comparisons between nested and quantitative PCR data for the rhesus macaques infected with B. microti during the three phases of the study. Discrepancies between nested and quantitative PCR data are highlighted.
Table S2. Details of Flow cytometry antibody panels used in the study.