Abstract
Aim
Acute intermittent hypoxia (AIH) promotes persistent increases in ventilation and sympathetic activity, referred as long-term facilitation (LTF). Augmented inspiratory activity is suggested as a major component of respiratory LTF. In the present study, we hypothesized that AIH also elicits a sustained increase in expiratory motor activity. We also investigated whether the expiratory LTF contributes to the development of sympathetic LTF after AIH.
Methods
Rats were exposed to AIH (10 × 6–7 % O2 for 45 s, every 5 min) and the cardiorespiratory parameters were evaluated during 60 min using in vivo and in situ approaches.
Results
In unanesthetized conditions (n=9), AIH elicited a modest but sustained increase in baseline mean arterial pressure (MAP, 104±2 vs 111±3 mmHg, P<0.05) associated with enhanced sympathetic and respiratory-related variabilities. In the in situ preparations (n=9), AIH evoked LTF in phrenic (33±12%), thoracic sympathetic (75±25%) and abdominal nerve activities (69±14%). The sympathetic overactivity after AIH was phase-locked with the emergence of bursts in abdominal activity during the late-expiratory phase. In anesthetized vagus-intact animals, AIH increased baseline MAP (113±3 vs 122±2 mmHg, P<0.05) and abdominal muscle activity (535±94%), which were eliminated after pharmacological inhibition of the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG).
Conclusion
These findings indicate that increased expiratory activity is also an important component of AIH-elicited respiratory LTF. Moreover, the development of sympathetic LTF after AIH is linked to the emergence of active expiratory pattern and depends on the integrity of the neurones of the RTN/pFRG.
Keywords: acute intermittent hypoxia, sympathetic activity, active expiration
INTRODUCTION
Neural plasticity is an important property of the respiratory network and underlies adjustments in the respiratory function (e.g., chemosensitive gain) and motor output (inspiratory and expiratory muscle performance) in response to vigorous stimuli (Mitchell and Johnson, 2003). This plasticity is observed after the exposure to brief episodes of low oxygen interspersed with periods of reoxygenation, or acute intermittent hypoxia (AIH) (Millhorn et al., 1980, Baker and Mitchell, 2000). Experimental evidence obtained in different animal species, including cats, dogs, rats, goats and humans (Hayashi et al., 1993, Millhorn et al., 1980, Turner and Mitchell, 1997, Cao et al., 1992, Chowdhuri et al., 2008, Aboubakr et al., 2001) demonstrates that AIH promotes a compensatory and long-lasting (> 1h) increase in respiratory motor activity, referred as respiratory long-term facilitation (LTF). It has been suggested that the expression of the respiratory LTF is primarily dependent on changes in inspiratory motor output, mainly enhanced phrenic and hypoglossal nerve activities (Bach and Mitchell, 1996, Baker and Mitchell, 2000), which results in increases in both the tidal volume (McGuire et al., 2004, Olson et al., 2001) and, to a lesser extent, in the respiratory frequency (Turner and Mitchell, 1997).
In addition to the respiratory LTF, a persistent increase in the sympathetic nerve activity has been reported after AIH exposure (Leuenberger et al., 2005, Dick et al., 2007, Xing and Pilowsky, 2010). Studies by Dick et al. (2007) described that AIH increased both phrenic and splanchnic sympathetic nerve activities in anesthetized rats. The authors also reported that the elevation in the sympathetic activity after AIH was not tonic, but entrained with the respiratory activity, suggesting that the mechanisms of coupling between sympathetic and respiratory activities underlie the emergence of AIH-induced sympathetic LTF. In contrast, studies by Xing and Pilowsky (2010) demonstrated that anesthetized rats presented a robust increase in the splanchnic sympathetic activity after AIH even in the absence of the phrenic LTF, indicating that changes in the respiratory activity, or at least, in the inspiratory drive, may not be required for the emergence of the sympathetic LTF induced by AIH.
During acute hypoxia (Dick et al., 2004) or chemical activation of peripheral chemoreceptors (Moraes et al., 2012), the vasoconstrictor sympathetic activity increases predominantly during the expiratory period. Moreover, rats exposed to either chronic intermittent hypoxia or sustained hypoxia develop hypertension, baseline sympathetic overactivity and exhibited increased discharge frequency of the ventromedullary pre-sympathetic neurones during expiration (Moraes et al., 2013, Zoccal et al., 2008, Moraes et al., 2014). In conjunction, these data indicate that both acute and chronic peripheral chemoreflex activation enhances sympathetic nerve outflow during the expiratory phase, increasing the vascular resistance and then the arterial pressure levels.
Based on these observations, we hypothesized that the persistent increase in baseline sympathetic activity following AIH exposure depends, at least in part, on changes in the respiratory network and results from the development of LTF in the expiratory motor activity. To check this possibility, in the present study we evaluated the cardiovascular parameters in unanesthetized rats and recorded the activity of phrenic, abdominal and sympathetic nerve activities in the decerebrated arterially-perfused in situ rat preparations before and after AIH. Moreover in anesthetized rats exposed to AIH, we also performed the pharmacological inhibition of the retrotrapezoid nucleus/parafacial respiratory group (RTN/pFRG) – a region critically involved in the generation of active expiratory pattern (Abdala et al., 2009, Pagliardini et al., 2011, Moraes et al., 2012) – and recorded the arterial pressure levels and the diaphragmatic and abdominal activities in order to verify the potential source of excitatory drive to generate the sympathetic and expiratory LTF after AIH.
MATERIAL AND METHODS
Animals and Ethical Approval
Juvenile male Holtzman rats (70–80 g, n=9) and adult male Wistar rats (290–320 g, n=14) were used in the present study. Animals were housed at controlled conditions of temperature (22±1 °C) and humidity (50–60%) under a 12-h light/dark cycle (lights on at 07:00 am) with rat chow and water provided ad libitum. The experimental procedures followed the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85–23 revised 1996) and by the Brazilian National Council for Animal Experimentation Control (CONCEA). All experimental protocols were approved by the Local Ethical Committee in Animal Experimentation of the School of Dentistry of Araraquara (protocol 21/2012) and of the Federal University of Santa Catarina (protocol PP00543).
Measurements of cardiovascular parameters in unanesthetized rats
Twenty-four hours before the experiments, a group of adult animals (290–320 g) were anesthetized with ketamine (60 mg.kg−1, i.p.) and xylazine (10 mg.kg−1, i.p.) and a polyethelene catheter (PE-10 connected to PE-50; Clay Adams, Parsippany, NJ) was inserted into the abdominal aorta through the femoral artery for measurements of pulsatile arterial pressure (PAP, mmHg). The distal end of the catheter was tunnelled subcutaneously, exteriorized through the back of the neck, and sutured to the skin. The rats were then housed singly and their respiratory movements were monitored until they regained consciousness. On the day after the surgery, when the rats had adapted over night to the environment of recording room, the arterial catheter was connected to a pressure transducer (model MLT0380; ADInstruments, Bella Vista, NSW, Australia) and, in turn, to an amplifier (Bridge Amp, ML221; ADInstruments). The PAP signals were acquired by a data acquisition system (PowerLab 4/25, ML845; ADInstruments) and recorded at 2 KHz sampling rate on a hard drive of a computer using an appropriate software (Chart Pro; ADInstruments). Mean arterial pressure (MAP, mmHg) and heart rate (HR, beats per minute - bpm) were derived from PAP signals.
From PAP signals, beat-by-beat time series of the systolic arterial pressure (SAP) and pulse interval (PI) were extracted from the last 30 minutes of the recordings (Chart Pro, ADInstruments) to determine the autonomic and respiratory modulation of the cardiovascular system through the analysis of variability, as previously described (Moraes et al., 2014, Zoccal et al., 2009a). Briefly, arterial pressure and heart rate oscillations at low-frequency range (LF) are representative of the modulatory effects of the sympathetic activity controlling vascular tonus and the heart activity, while oscillations at high-frequency range (HF) are associated with the respiratory or parasympathetic modulation of blood vessels and the heart, respectively (Malliani et al., 1991, Bernardi et al., 2001). The overall variability of the SAP and PI was assessed in the frequency domain using fast Fourier transform spectral analysis (Cardioseries Software v2.4, available on https://www.sites.google.com/site/cardioseries/home). The power of the oscillatory components obtained from rats of the control and AIH groups was quantified in two frequency bands: LF (0.20–0.75 Hz) and HF (0.75–3.0 Hz) (Cerutti et al., 1991, Malliani et al., 1991, Zoccal et al., 2009a). Oscillations lower than 0.20 Hz was not quantified. In addition to the spectral analysis of arterial pressure and heart rate, we also determined the spontaneous baroreflex gain (SBG), which reflects the cardiac baroreflex function over the physiological range of fluctuations in arterial pressure. To this, we used an appropriated software (Cardioseries Software v2.4) that automatically detected spontaneous ramps of progressive increases and decreases of four or more values of SAP that paralleled with changes in pulse interval with a linear correlation higher than 0.8. The ramp sequences were defined as up sequences when SAP increases were associated with PI lengthening or as down sequences when SAP decreases were associated with PI shortening, and the overall SBG was assessed by the average of the linear regression slopes (in ms/mmHg) between the SAP and the subsequent PI (Zoccal et al., 2009a).
In situ working heart-brainstem preparation
Working heart-brainstem preparations (Paton, 1996) were surgically prepared, as previously described (Zoccal et al., 2008). The juvenile rats (70–80 g) were deeply anesthetized with halothane (AstraZeneca, Cotia, SP, Brazil) until the loss of the paw withdrawal reflex, transected caudal to the diaphragm, submerged in a chilled Ringer solution (in mM: NaCl, 125; NaHCO3, 24; KCl, 3; CaCl2, 2.5; MgSO4, 1.25; KH2PO4, 1.25; dextrose, 10) and decerebrated at the precollicular level. Lungs were removed. Preparations were then transferred to a recording chamber, the descending aorta was cannulated and perfused retrogradely with Ringer solution containing 1.25 % Polyethylene glycol (an oncotic agent, Sigma, St Louis, USA), sodium lactate (2 mM) and a neuromuscular blocker (vecuronium bromide, 3–4 µg.mL−1, Cristália Produtos Químicos Farmacêuticos Ltda., São Paulo, Brazil), using a roller pump (Watson-Marlow 502s, Falmouth, Cornwall, UK) via a double-lumen cannula. The perfusion pressure was maintained in the range of 50–70 mmHg by adjusting the rate flow to 21- 25 ml.min−1 and by adding vasopressin to the perfusate (0.6 – 1.2 nM, Sigma, St. Louis, MO, USA). The perfusate was gassed continuously with 5% CO2-95% O2, warmed to 31–32°C and filtered using a nylon mesh (pore size: 25 µm, Millipore, Billirica, MA, USA). Sympathetic and respiratory nerves were isolated and their activity recorded simultaneously using bipolar glass suction electrodes held in micromanipulators (Narishige, Tokyo, Japan). Left phrenic nerve (PN) discharges were recorded from its central end and its rhythmic ramping activity was used to monitor preparation viability. Right thoracic/lumbar abdominal nerves (AbN; T13-L1) were isolated from abdominal muscles, cut distally and their central activity recorded. Thoracic sympathetic activity was recorded from the left sympathetic chain (tSN) at T8–T12 level. All the signals were amplified, band-pass filtered (0.1–3 kHz; P511, Grass Technologies, Middleton, USA) and acquired in an A/D converter (CED micro 1401, Cambridge Electronic Design, CED, Cambridge, UK) to a computer using Spike 2 software (5 KHz, CED, Cambridge, UK). At the end of the experiments, the perfusion pump was turned off to determine the electrical noise (after the death of the preparations).
All analyses were carried out on rectified and integrated signals (time constant of 50 ms) and performed off-line using Spike 2 software (CED, Cambridge, UK) after noise subtraction. PN activity was evaluated by its burst frequency and amplitude. PN burst frequency was derived from the time interval between consecutive integrated phrenic peak bursts and expressed in bursts per minute (bpm). Integrated PN burst amplitude was determined (in µV) by the value difference between the burst peak and the minimal activity observed during expiratory period. The times of inspiration and expiration (expressed in seconds) were estimated from the measurements of PN burst length and burst interval, respectively. Baseline tSN and AbN activities were calculated as the mean values (in µV) of integrated signals. The changes in the PN amplitude, tSN and AbN mean activities induced by intermittent hypoxia were expressed as percentage values in relation to basal values prior to the hypoxic stimuli (see below). The changes in the other parameters were expressed in their raw units.
Cardiorespiratory evaluation in anaesthetized rats
Surgical procedures and experiments were performed under anesthesia with urethane (1.2 g.kg−1, i.p.), as previously described (Lemes and Zoccal, 2014). The level of anesthesia was constantly assessed by the absence of corneal and toe-pinch withdrawal reflexes. Additional doses of urethane were administrated (10–20% of initial dose) to maintain adequate levels of anesthesia, when necessary. Body temperature was maintained at 36–38 °C. Animals were placed in supine position and a cervical incision was performed. The rats were then tracheotomised to allow administration of gas mixtures. Animals breathed spontaneously and were maintained at 100% O2 during surgery and experimental protocols, except when exposed to hypoxia. Polyethylene catheters (PE-50 connected to PE- 10) were inserted into the right femoral artery and vein for arterial pressure measurements and systemic administration of drugs and fluids, respectively. Bipolar stainless steel electrodes were implanted in the diaphragm (DIA) and in the oblique abdominal muscles (ABD) to perform electromyographic (EMG) recordings of inspiratory and expiratory motor activities, respectively. After the catheter and electrodes implants, the animals were positioned in a stereotaxic apparatus (David Kopf, Tujunga, CA) to perform bilateral microinjections of the GABAA agonist muscimol (1 mM, Sigma, St Louis, USA) in the RTN/pFRG, using a needle (30 gauge) connected to a 1 µL syringe (Hamilton Company, NV, USA) through a P10 catheter. The following stereotaxic coordinates were used to target the RTN/pFRG: −2.6 mm from lambda; 1.8 mm lateral from the longitudinal suture and 10.8–11.0 mm ventral from the dorsal surface (Paxinos & Watson, 1998).
Before starting the experimental protocol, a period of at least 30 min was allowed for recording stabilization. To minimize deviations in blood pH and maintain fluid balance, slow intravenous administration of Ringer´s solution containing lactate (2 mM) was performed (3–4 ml.kg−1.h−1) during the experiments. Values of mean arterial pressure (MAP, mmHg) and heart rate (HR, bpm) were determined from PAP signals, as aforementioned. DIA and ABD signals were amplified (Bioamplifier, Insight, Ribeirão Preto, SP, Brazil), band-pass filtered (0.1–2 KHz) and acquired at a sampling rate of 2 kHz (LabChart, ADInstruments). Cardiovascular parameters and respiratory motor outputs were recorded simultaneously. The EMG signals were rectified and smoothed (50 ms) for analysis. DIA motor activity was evaluated by its burst amplitude (mV), frequency (bursts per minute, bpm) and duration (time of inspiration, s) as well as by the time between consecutive burst (time of expiration, s). ABD motor activity was assessed by its burst amplitude (mV).The changes in DIA and ABD amplitudes were expressed as percentage values (%) in relation to respective basal activity. All analyses were performed offline using LabChart software.
At the end of the experiments, animals were euthanized with intravenous injections of KCl 10%. For histological verification of the microinjection sites in the RTN/pFRG, microinjections of Evans Blue dye (2%, 50 nL; Vetec, Fine Chemicals Ltd., Rio de Janeiro, RJ, Brazil) were performed and the brain tissue was rapidly removed and fixed in formalin solution (10%) for 48–72 hours and then in sucrose solution (30%) overnight. After, 30 µm coronal sections were obtained at the level of RTN/pFRG (Leica, CM1850 UV; Wetzlar, Hesse, Germany), stained using the Nissl method and analysed using a microscope (Leica, DM5500 B; Wetzlar).
In vivo and in situ acute intermittent hypoxia
For induction of AIH in unanesthetized freely-moving rats, the animals were maintained in an acrylic chamber (5L) that was flushed with gas mixtures using a gas mixer device connected to a gas analyser (AVS Projetos, São Carlos, Brazil) and to cylinders of pure N2 and O2 (White Martins, São Carlos, Brasil). The animals were placed individually in the chamber and then subjected to 10 episodes of hypoxia (6–7% O2 balanced in N2, for 45 seconds) interspersed by 5 minutes of normoxia (21% O2), as previously described (Dick et al., 2007, Xing and Pilowsky, 2010, Rafacho et al., 2013). The gas injections were performed at the upper level of the chamber to avoid direct jets of gas impacting on the animals. In anesthetized animals, the same AIH paradigm was used, with the exception that the episodes of hypoxia were interspersed by periods of hyperoxia (99.9% O2). The hypoxic and hyperoxic gases were initially humidified and then administrated to the animals through the tracheal cannula. In the in situ experiments, the hypoxic episodes were achieved by briefly substituting the carbogen of the perfusion solution by a hypoxic mixture. The preparations were then exposed to 10 episodes of 6–7% O2 (balanced in 5% CO2 and 88–89% N2) for 45 s, followed by 5 min of 95% O2 and 5% CO2. The CO2 content in the perfusate was maintained constant during the hypoxic periods to avoid depression of the respiratory drive consequent to hypocapnia (Molkov et al., 2011). After the AIH exposure in vivo and in situ, the cardiorespiratory parameters were monitored for 60 min.
Statistical analyses
The data were expressed as mean ± standard error of mean (SEM). Before analyses, data distribution was checked using the Shapiro–Wilk normality test. The effects of AIH on the cardiorespiratory parameters were then compared using one-way ANOVA for repeated measurements followed by Newman-Keuls post-test. The comparisons were carried out using GraphPad Prism software (version 5, La Jolla, CA, USA) and differences were considered significant at P < 0.05.
RESULTS
Cardiovascular parameters in conscious rats exposed to AIH
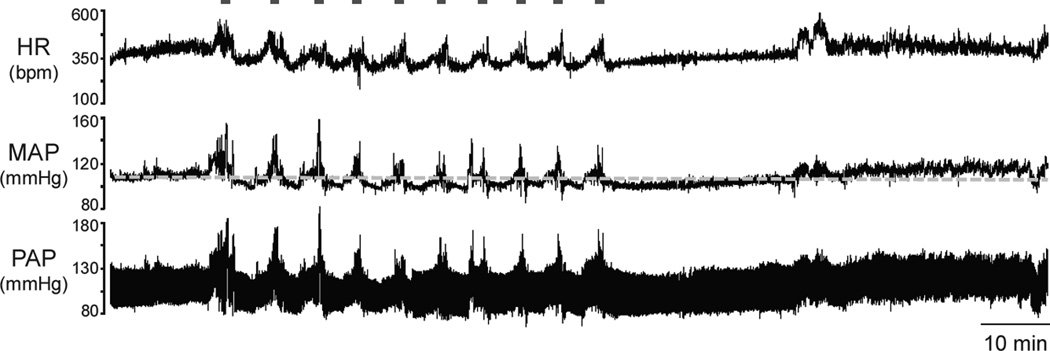
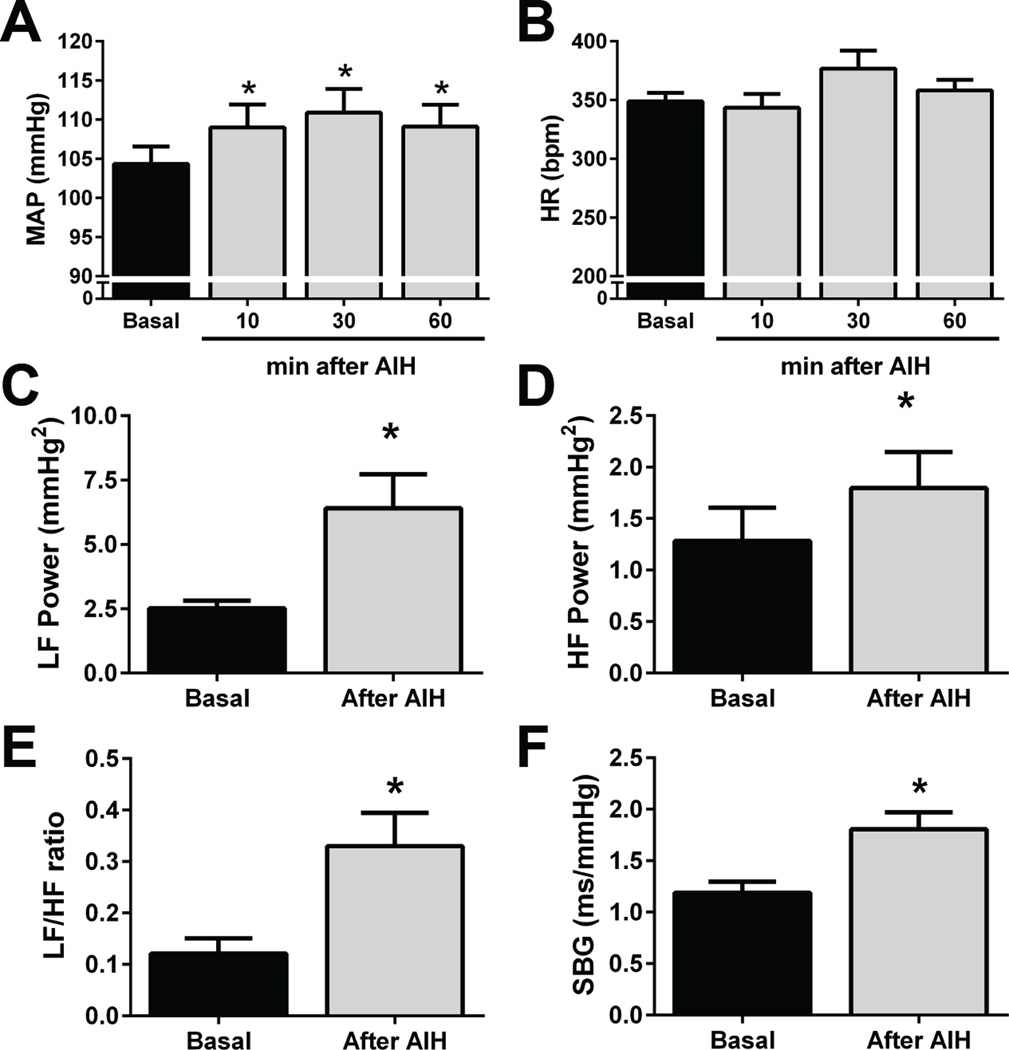
During the exposure to AIH, every hypoxic episode elicited transient behavioural, pressor and tachycardic responses (Figure 1). After the last episode of hypoxia, the rats (n=9) showed a modest, but sustained and significant increase in baseline MAP that persisted during the 60 min of evaluation (104±2 vs 109±3; 111±3; 109±3 mmHg, respectively basal, 10, 30 e 60 min after AIH, P<0.05, Figure 2A). No significant changes were observed in baseline HR after AIH (348±7 vs 344±12; 378±15; 358±9 bpm, respectively basal, 10, 30 e 60 min after the AIH, Figure 2B). The increased arterial pressure levels after AIH was accompanied by an elevation of both LF (2.5±0.3 vs 6.4±1.3 mmHg2, P<0.05, Figure 2C) and HF components of SAP variability (1.3±0.3 vs 1.8±0.3 mmHg2, P<0.05, Figure 2D). With respect to the autonomic modulation to the heart, the increased LF/HF ratio (0.12±0.03 vs 0.33±0.06, P<0.05, Figure 2E) suggests that the sympathetic modulation of the heart rate was enhanced after AIH. AIH also increased the spontaneous baroreflex gain (1.2±0.1 vs 1.8±0.2 ms.mmHg−1, P<0.05, Figure 2E). These findings demonstrate that unanesthetized rats exposed to AIH presented a sustained increase in baseline arterial pressure associated with enhanced sympathetic and respiratory modulation and augmented cardiac baroreflex gain.
Figure 1. Conscious rats exhibit a persistent increase in baseline arterial pressure after AIH.
Recordings of baseline heart rate (HR), mean (MAP) and pulsatile arterial pressure (PAP) from an unanesthetized rat, representative of the group, showing the cardiovascular changes during and after the exposure to AIH. The grey line indicates baseline MAP levels before AIH.
Figure 2. Cardiovascular parameters in conscious rats after AIH.
Average values of baseline mean arterial pressure (MAP; Panel A), heart rate (HR; Panel B); power of low (LF; panel C) and high frequency (HF; panel D) components of systolic arterial pressure; LF/HF ratio of pulse interval (Panel E) and spontaneous baroreflex gain (SBG; Panel F) before and after AIH exposure in unanesthetized rats (n=9); * - P <0.05 - different from respective baseline values.
Sympathetic and respiratory motor outputs in in situ rat preparations after AIH
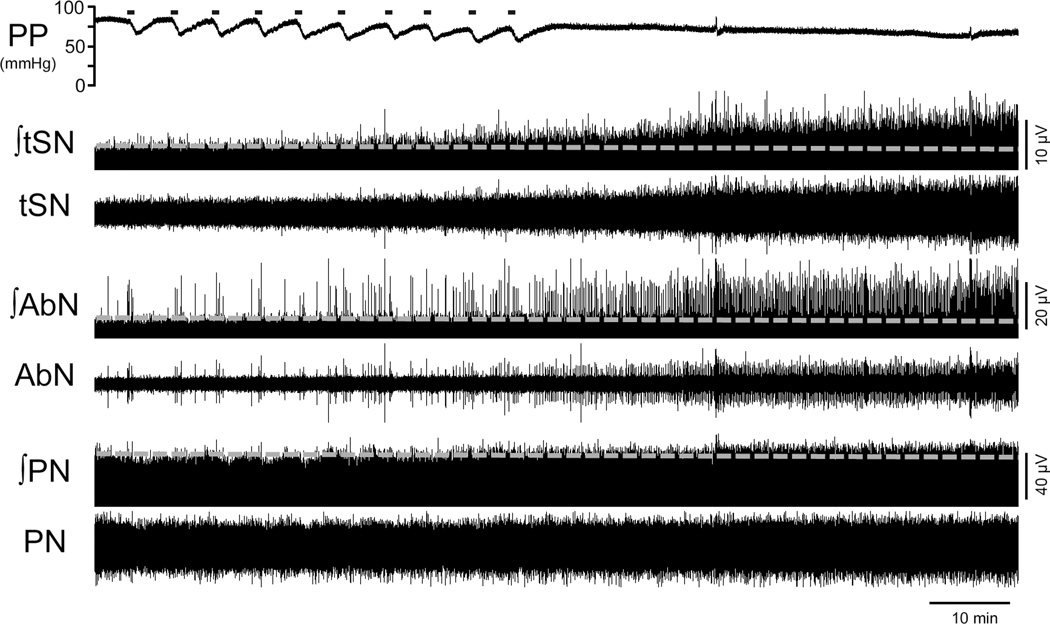
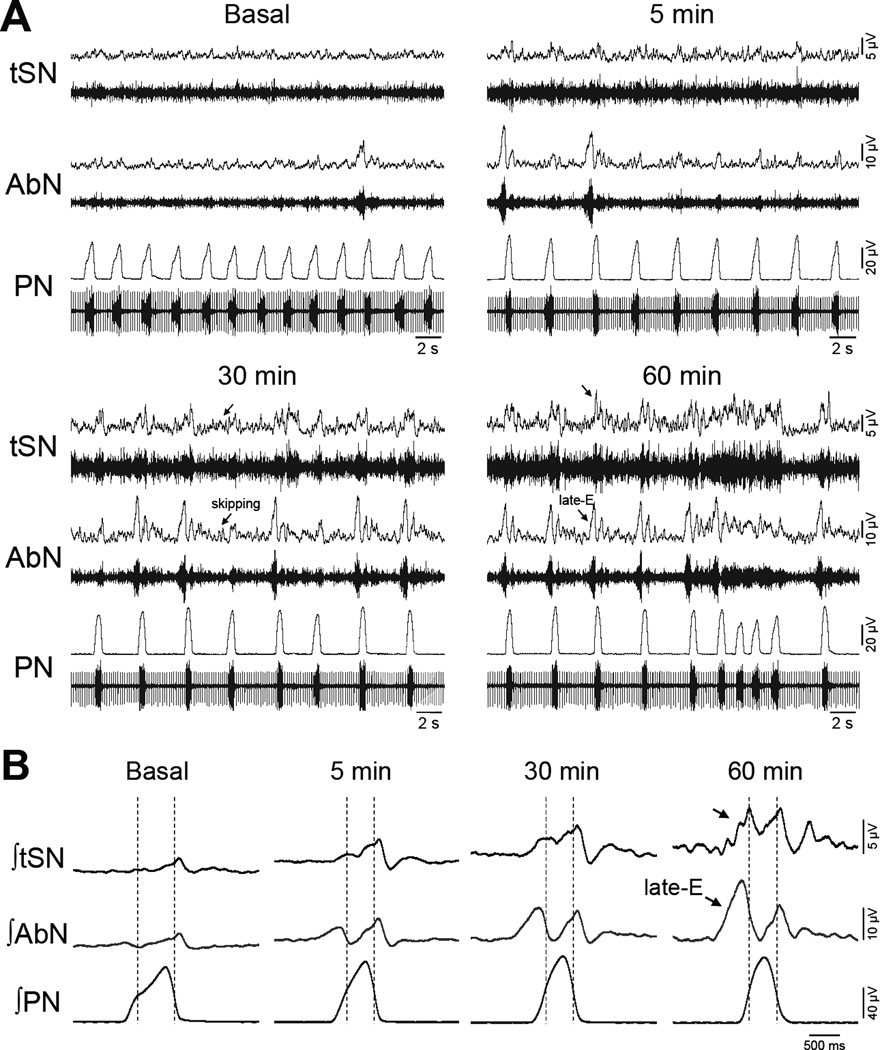
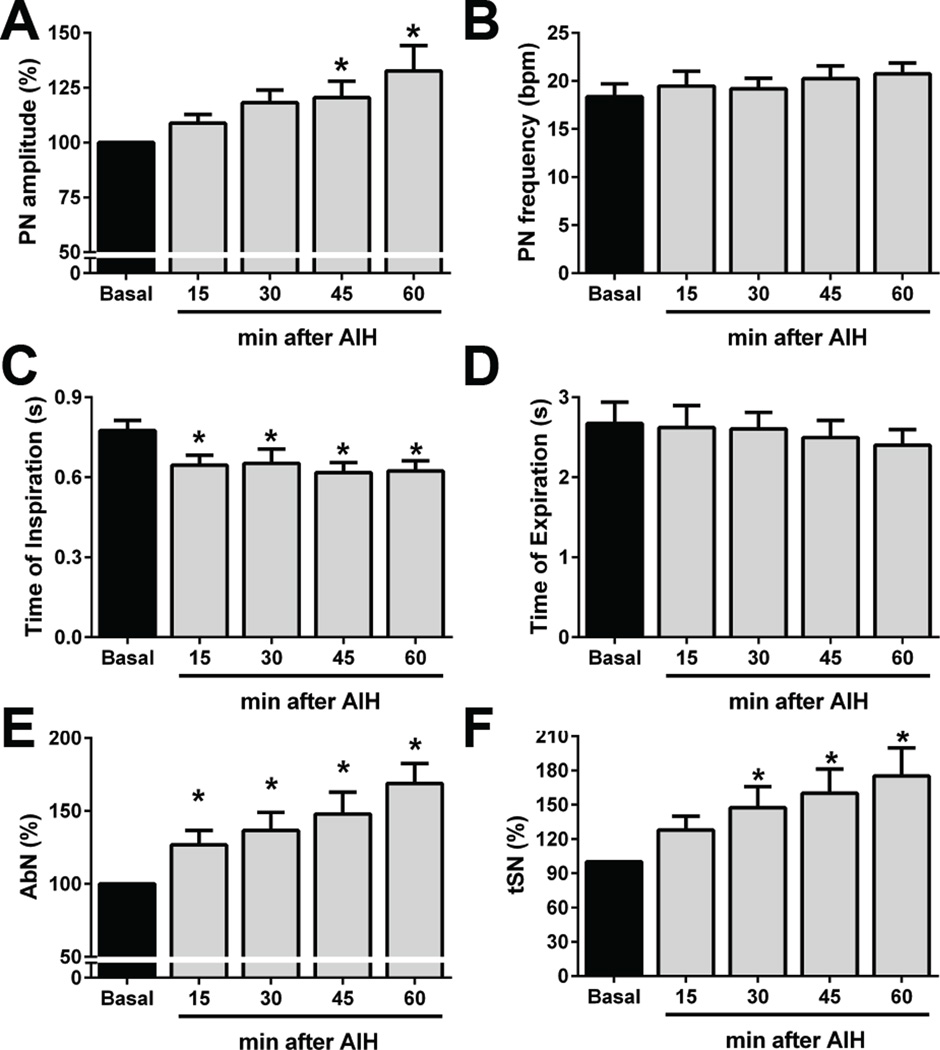
Figure 3 demonstrates the effects of AIH on PN, AbN and tSN activities of in situ preparations. At baseline conditions, PN bursts showed a ramping pattern of discharge, AbN presented a low-amplitude activity and tSN exhibited a marked inspiratory/post-inspiratory modulation (Figure 4), as previously described (Zoccal et al., 2008, Moraes et al., 2014). The hypoxic episodes in situ, at the level and duration used in the present study, evoked decreases in perfusion pressure and slight increases in tSN and AbN amplitude and PN burst frequency. After the AIH, the in situ preparations (n=9) showed: i) augmented PN burst amplitude (ΔPN: 21±8 and 33±12 %, respectively 45 and 60 min after AIH, P<0.05; Figure 5A), but no significant changes in PN burst frequency (18±1 vs 21±1 cpm, respectively basal and 60 min after AIH; Figure 5B); ii) reduced of time of inspiration (0.78±0.03 vs 0.65±0.04, 0.65±0.05, 0.62±0.04 and 0.62±0.04 s, respectively basal, 15, 30, 45 and 60 min after AIH; P<0.05, Figure 5C); iii) no changes in expiratory time (2.67±0.26 vs 2.4±0.19 s, respectively basal and 60 min after AIH, Figure 5D); iv) increased AbN activity (ΔAbN: 27±10, 37±12, 48±15, 69±14 %, respectively 15, 30, 45 and 60 min after AIH, P<0.05, Figure 5E) and v) augmented tSN activity (ΔtSN: 48±18, 60±21 and 75±25%, respectively 30, 45 and 60 min after AIH, P<0.05; Figure 5F). The analyses of respiratory and sympathetic pattern (Figure 4A) demonstrated that the increased AbN activity was dependent upon the appearance of bursts during the late part of expiratory phase, or late-expiration (late-E). Moreover, the progressive increase in the tSN after AIH was associated with the emergence of additional bursts during the late-E phase, coupled with the occurrence of late-E activity in the AbN (Figure 4B). Taken together, these data indicated that AIH in situ promotes LTF in the inspiratory, expiratory and sympathetic motor outputs.
Figure 3. AIH promotes expiratory and sympathetic long-term facilitations (LTF) in the in situ preparations.
Recordings of perfusion pressure (PP) and of the thoracic sympathetic (tSN), abdominal (AbN) and phrenic nerve (PN) activities [raw and integrated (∫)] of an in situ rat preparation, representative from the group, illustrating baseline activities before, during and after exposure to AIH. The grey lines represent the level of baseline activities before AIH.
Figure 4. Effects of AIH on the sympathetic and respiratory pattern of the in situ preparations.
Panel A: Raw and integrated (∫) activities recorded from the thoracic sympathetic (tSN), abdominal (AbN) and phrenic nerve (PN) activities, of an in situ preparation representative of the group, illustrating the effects of AIH on the inspiratory, expiratory and sympathetic motor pattern before and after AIH exposure. Panel B: Phrenic-triggered averages of AbN and tSN, obtained from the recordings shown in Panel A, illustrating the changes on sympathetic-respiratory coupling after the exposure to AIH. The dotted lines delineate the inspiratory and expiratory phases. Note that the emergence of additional bursts in the tSN activity during the expiratory phase entrained with the emergence of late-E bursts in the AbN activity (arrows).
Figure 5. Effects of AIH on the sympathetic and respiratory activities of the in situ preparations.
Average changes in phrenic (PN) burst amplitude (Panel A) and frequency (Panel B); inspiratory (Panel C) and expiratory times (Panel D) and in the mean abdominal (AbN; Panel E) and thoracic sympathetic nerve activities (tSN; Panel F) of in situ preparations submitted to AIH (n=9); * - P <0.05 - different from respective baseline values.
Effects of RTN/pFRG inhibition on arterial pressure and respiratory motor activity after AIH
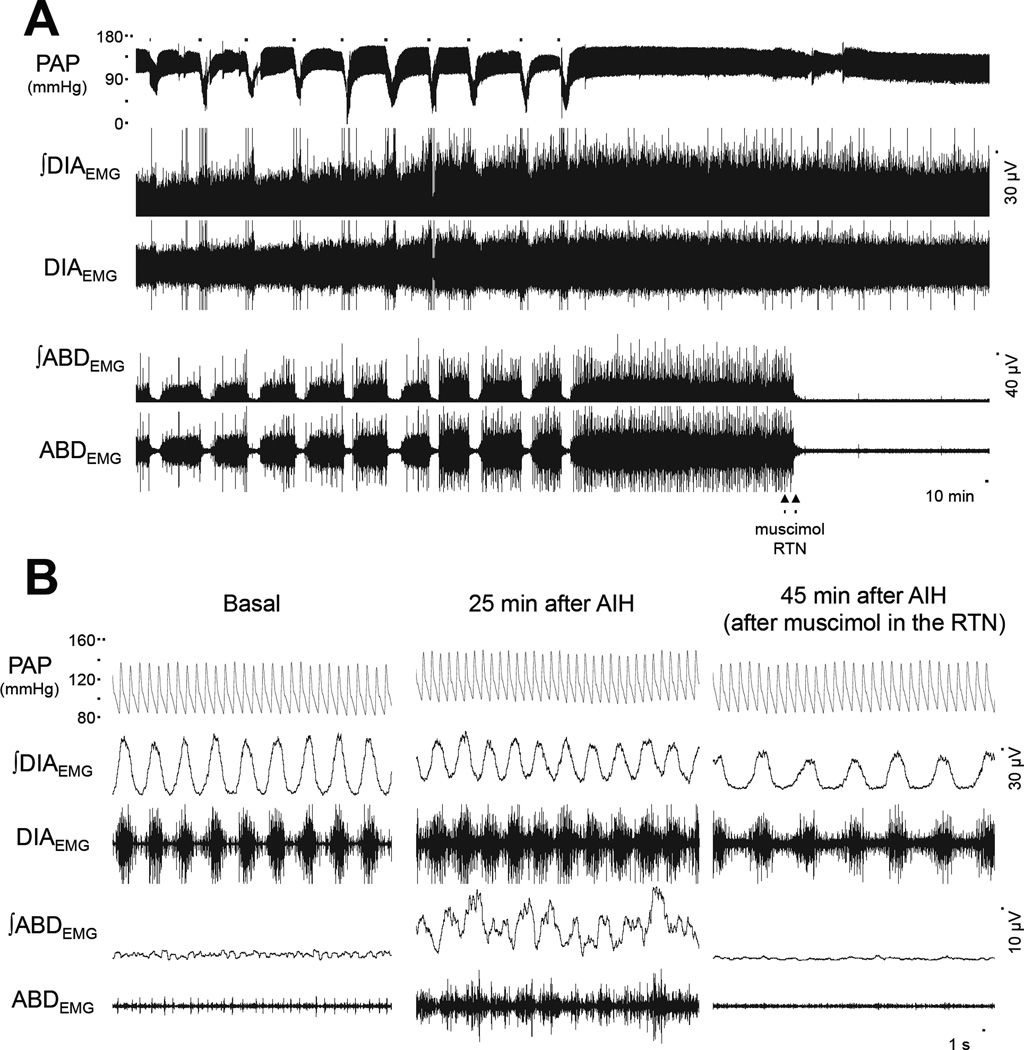
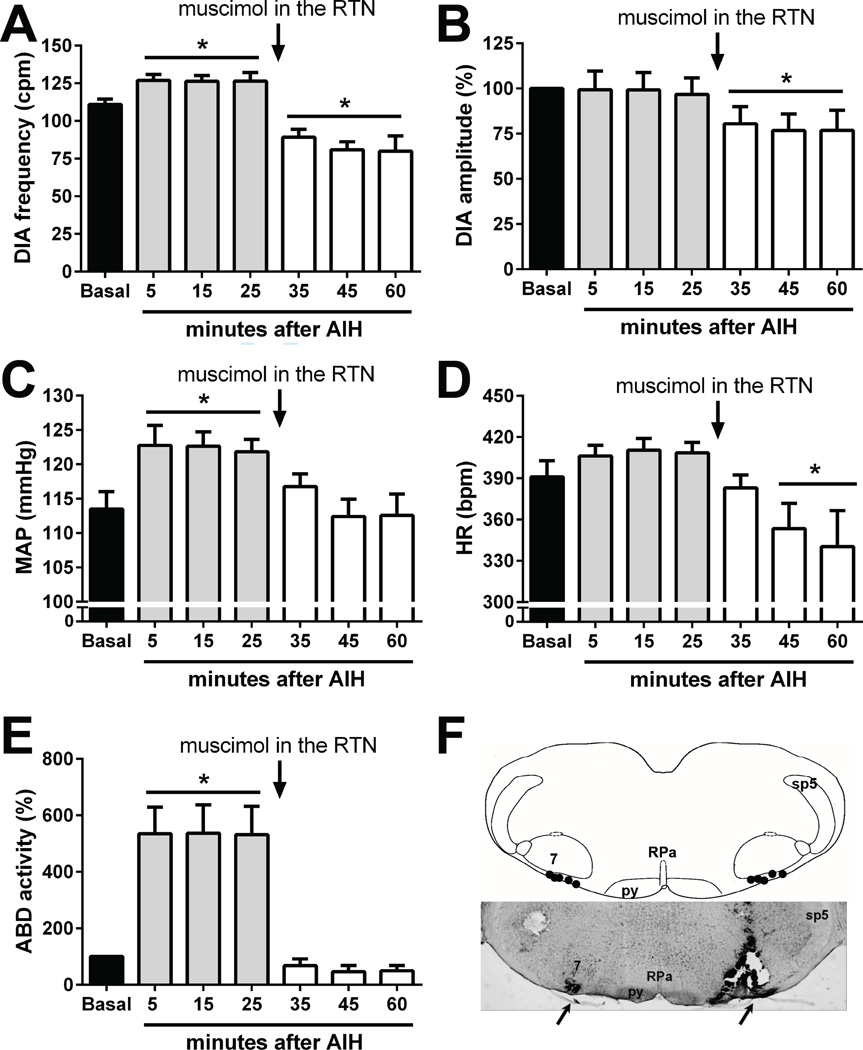
In anesthetized spontaneously-breathing animals with vagus nerve intact, hypoxia produced hypotension, augmented DIA burst amplitude and frequency and a brief increase in ABD amplitude followed by inhibition (Figure 6), as previous described (Lemes and Zoccal, 2014). In agreement with previous observations (Janssen and Fregosi, 2000), the anesthetized vagus-intact animals exposed to AIH (n=5) presented a inspiratory motor facilitation, mostly dependent on an increase in the DIA frequency than in amplitude (Figure 7 A and B). Moreover, AIH also produced a long-lasting elevation of baseline arterial pressure (Figure 7C), but not in HR (Figure 7D), accompanied by a progressive and sustained increase in the ABD activity (Figure 7E). All the cardiorespiratory changes elicited by AIH persisted for, at least, 30 min after the 10th exposure to hypoxia (Figure 6). At this time, bilateral microinjections of muscimol (agonist of GABAA receptors) were performed in the RTN/pFRG, which caused: i) reduction in the DIA burst frequency and amplitude (Figures 7 A and B); ii) normalization of baseline MAP levels (Figure 7C); iii) fall in the HR (Figure 7D); and iv) elimination of the AIH-induced ABD overactivity (Figure 7E). The sites of microinjections in the RTN/pFRG are illustrated in Figure 7F and all the data aforementioned are described in table 1.
Figure 6. Bilateral microinjections of muscimol in the RTN/pFRG eliminate the expiratory long-term facilitation elicited by AIH in anesthetized vagus-intact rats.
Panel A: Raw and integrated (∫) electromyographic recordings of the diaphragm (DIAEMG) and abdominal muscles (ABDEMG), and recordings of the pulsatile arterial pressure (PAP), from an anesthetized rat representative of the group, illustrating the effects of AIH exposure and the pharmacological inhibition of the RTN/pFRG with muscimol (1 mM) on the arterial pressure and respiratory motor outputs. Panel B: expanded recordings from Panel A, at basal, after AIH (25 min) and after muscimol microinjections in the RTN/pFRG (45 min). Note that the pharmacological inhibition of the RTN/pFRG normalized the levels of arterial pressure and eliminated the active expiration induced by AIH.
Figure 7. Effects of AIH and the bilateral microinjection of muscimol in the RTN/pFRG on the cardiorespiratory parameters in anesthetized vagus-intact rat.
Average changes in the DIA burst frequency (Panel A) and amplitude (panel B), mean arterial pressure (MAP, panel C), heart rate (HR, panel D) and mean abdominal muscle activity (ABD, panel E) induced by AIH before (5–25 min) and after (35–60 min) bilateral microinjections of muscimol in the RTN/pFRG of anesthetized animals with vagus nerve intact. The microinjections were performed thirty minutes after the AIH (arrows). * - P <0.05 - different from respective baseline values. Panel F: schematic drawing of the ventral surface of the medulla, combined with a photomicrography of coronal section of the brainstem of a rat, representative of the group, illustrating sites of injection in the RTN/pFRG. Abbreviations: RPa, raphe pallidus nucleus, 7, facial nucleus, sp5, spinal trigeminal nucleus, py, pyramidal tract.
Table 1.
Average values of baseline mean arterial pressure (MAP), heart rate (HR), diaphragm burst amplitude (DIAamp) and frequency (DIAf) and abdominal mean activity (ABD) before (basal) and after exposure to AIH in anesthetized vagus-intact rats (n=5) that received bilateral microinjections of muscimol in RTN/pFRG.
| After AIH | After AIH + RTN/pFRG inhibition |
||||||
|---|---|---|---|---|---|---|---|
| Basal | 5 min | 15 min | 25 min | 35 min | 45 min | 60 min | |
| MAP, mmHg | 113±3 | 123±3* | 122±2* | 122±2* | 116±1 | 113±3 | 113±3 |
| HR, bpm | 391±12 | 406±8 | 410±8 | 409±7 | 383±9 | 353±18* | 340±26* |
| DIAamp, % | 100 | 99±10 | 99±9 | 97±9 | 80±9* | 77±9* | 77±11* |
| DIAf, cpm | 111±4 | 127±4* | 126±3* | 126±6 * | 89±5* | 80±10 * | 80±10* |
| ABD, % | 100 | 535±94* | 536±100* | 532±100* | 67±23 | 46±22 | 50±18 |
- P <0.05 - different from respective baseline values.
DISCUSSION
In the present study, we describe a novel component of the respiratory LTF elicited by AIH. From measurements of the abdominal activity, we verified that anesthetized rats and in situ rat preparations exposed to AIH presented a sustained increase in the abdominal expiratory motor activity referred as expiratory LTF. Remarkably, the progression of the expiratory LTF was accompanied by the development of high levels of sympathetic activity and arterial pressure. Bilateral microinjections of GABAA receptor agonist (muscimol) in the RTN/pFRG – a region critically involved with the generation of active expiration (Abdala et al., 2009, Pagliardini et al., 2011, Moraes et al., 2012) – eliminated the expiratory LTF and normalized the high levels of arterial pressure induced by AIH, indicating that ventromedullary expiratory neurons are necessary for the emergence of both expiratory and sympathetic LTF. Our findings indicate that AIH exposure generates active expiration at rest, which is determinant, at least in part, for the development of sustained high levels of sympathetic activity observed in this condition.
Although distinct paradigms of AIH are described in the literature (e.g., level of oxygen, number and length of cycles), there is a consensus that AIH evokes LTF of inspiratory motor activity, including phrenic (Baker and Mitchell, 2000, Dick et al., 2007), hypoglossal (Fuller et al., 2001) and intercostal nerves (Fregosi and Mitchell, 1994). These sustained elevations of the upper airway and inspiratory pump muscle activities are suggested to underpin the compensatory increase in minute ventilation (Olson et al., 2001, Turner and Mitchell, 1997). In agreement with previous studies, we verified that the in situ preparations submitted to AIH presented a long-lasting increase in the phrenic burst amplitude. On the other hand, anesthetized spontaneous-breathing rats with intact vagus nerves did not show consistent increments in the diaphragm burst amplitude, but exhibited a significant increase in the respiratory frequency after AIH. The differences in the pattern of the AIH-induced inspiratory LTF observed in our study may be consequent to the activation of pulmonary feedback afferents, which were absent in the in situ preparations and may have suppressed the increase in the diaphragm burst amplitude in anesthetized animals. Along with inspiratory LTF, both in situ preparations and anesthetized animals also presented a progressive increase in the expiratory motor activity that persisted for at least 60 min after AIH. We previously demonstrated that the recruitment of abdominal muscles during hypoxia and hypercapnia is accompanied by increases in the expiratory flow and tidal volume (Lemes and Zoccal, 2014). Based on that, we theorize that the persistent increase in abdominal motor activity represents a new component of the compensatory ventilatory response to AIH, possibly to recruit expiratory reserve volume and then increase tidal volume.
We found that the expression of the expiratory LTF after AIH was dependent on the integrity of cells located ventral to the caudal pole of the facial nucleus, in the RTN/pFRG. Bilateral microinjections of muscimol in the RTN/pFRG eliminated completely the expiratory motor hyperactivity elicited by AIH. The RTN/pFRG neurones are well known for their critical role in the CO2 detection and regulation of breathing (Mulkey et al., 2004, Li and Nattie, 2002). Recent studies have also suggested that this region is critical for the generation of active expiratory pattern (Huckstepp et al., 2015). The RTN/pFRG contains conditional late-expiratory neurones that are silent at rest but fire rhythmically in conditions of metabolic challenges (e.g., hypercapnia, hypoxia) or reduced inhibitory drive (Abdala et al., 2009, Pagliardini et al., 2011, Moraes et al., 2012), providing excitatory inputs to the bulbospinal expiratory neurons and producing late-E bursts in the abdominal activity (Molkov et al., 2010, Huckstepp et al., 2015). Activation of the late-expiratory neurons is also associated with modifications in the PN burst pattern (changing from ramping to square-like pattern) and with reduction in the inspiratory time (Abdala et al., 2009) – effects that were observed in the in situ preparations after AIH. Thereby, we hypothesize that AIH exposure promoted a long-lasting activation of the RTN/pFRG late-expiratory neurones, generating active expiration at rest. This might involve plastic changes in the late-E neuronal excitability elicited by serotonin- and reactive oxygen species-dependent plasticity mechanisms (MacFarlane et al., 2011, Bocchiaro and Feldman, 2004, Baker-Herman and Mitchell, 2002) or might be dependent on enhanced excitatory synaptic inputs, such as from central chemoreceptors (Molkov et al., 2011, Huckstepp et al., 2015) and nucleus of the solitary tract (Yamamoto et al., 2015). All these possibilities still require further experiments to be elucidated.
Previous studies demonstrated that bilateral microinjections of muscimol in the RTN/pFRG of anesthetized rats produced respiratory arrest due to the removal of CO2 tonic drive to breathe (Takakura et al., 2006). In our study, using the same experimental approach, we verified that pharmacological inhibition of the RTN/pFRG reduced, but not eliminated, the diaphragmatic activity of anesthetized rats exposed to AIH. We speculate that the differences between previous and our study could be related to the presence of plastic changes in the pre-motor and motor inspiratory neurones introduced by AIH (Mitchell and Johnson, 2003), which were able to maintain the breathing rhythm even in the absence of excitatory drive from chemosensitive neurones of the RTN/pFRG. These findings, therefore, emphasize the notion that AIH amplifies the activity of neurones responsible for the control of inspiratory activity.
In agreement with previous observations obtained in anesthetized rats (Xing and Pilowsky, 2010, Dick et al., 2007) we verified that AIH also promoted long-lasting changes in the sympathetic outflow. In unanesthetized rats, we demonstrated that AIH increased baseline arterial pressure and enlarged sympathetic modulation of blood vessels and of the heart, corroborating the concept that AIH heightens the sympathetic activity to the cardiovascular system. Parallel with the elevated levels of arterial pressure, we found that rats submitted to AIH exhibited higher spontaneous baroreflex gain. Although this analysis reflected the baroreflex operation in a narrow range of arterial pressure variation, these findings suggest that the cardiac baroreflex control is facilitated after AIH. These observations indicate that the sympathetic-mediated high blood pressure after AIH is not related to changes in baroreflex function, which, in fact, may be offsetting the augmented sympathetic activity and buffering the changes in the arterial pressure and heart rate. Studies by Dick et al. (2007) suggest that the sympathetic LTF may results, in part, from changes in the central respiratory activity, since the higher levels of sympathetic activity after AIH was synchronized with the respiratory cycle. Xing and Pilowsky (2010) reported that AIH-induced sympathetic LTF occurred in the absence of phrenic LTF, contrasting the idea that central respiratory-sympathetic coupling mechanisms may contribute to the sympathetic LTF after AIH. In our experiments, unanesthetized rats exhibited augmented respiratory-related variability of systolic arterial pressure after AIH, suggesting a higher impact of the respiratory activity on the vascular resistance control. Moreover, the progressive increase in sympathetic activity after AIH in situ was entrained with the development of expiratory LTF. Indeed, the analysis of sympathetic pattern revealed the presence of novel bursts of activity during the late-expiratory phase after AIH, coupled with the emergence of late-E bursts in the abdominal activity. Our findings, therefore, indicate that the mechanisms of coupling between expiratory and sympathetic neurons are strengthened after AIH and contribute to the development of sympathetic LTF.
The rostral ventrolateral medulla (RVLM) is a fundamental site for integration of respiratory and sympathetic activities (Zoccal et al., 2009b). In addition to its major role as a main source of glutamatergic inputs to pre-ganglionic neurones of the spinal cord (Ross et al., 1984), the RVLM pre-sympathetic neurones receive excitatory and inhibitory inputs from brainstem respiratory neurones, generating respiratory rhythmical oscillations in the sympathetic nerve discharge (Moraes et al., 2013, Haselton and Guyenet, 1989, Gilbey et al., 1986). Using mathematical models we previously suggested that the RTN/pFRG late-E neurones would be an important source of phasic excitatory drive to RVLM neurones during hypercapnia and might be necessary for the maintenance of hypertension and sympathetic overactivity in rats subjected to CIH (Molkov et al., 2011, Zoccal, 2015). In the present study, we showed that bilateral microinjections of muscimol in the RTN/pFRG were able to normalize the high levels of arterial pressure and abrogate the expiratory hyperactivity. We cannot exclude that the effects produced by muscimol microinjections in the RTN/pFRG were related to the spread of the drug to adjacent areas, such as the RVLM and the Bötzinger complex. However, considering that the responses described in our study were achieved immediately after the second microinjection in the RTN/pFRG (see figure 6), and previous studies showing that bilateral microinjections of muscimol in the RVLM and Bötzinger complex caused a large fall in arterial pressure and eliminated the respiratory activity (Schreihofer et al., 2005, Moraes et al., 2012), we are confident that the effects described in the present study were related to the inhibition of the RTN/pFRG cells. Therefore, we are providing functional evidence that the RTN/pFRG neurones are required for the emergence of coupled expiratory and sympathetic LTF, supporting the notion that the respiratory-sympathetic coupling mechanisms play a meaningful role for the development of sympathetic overactivity after AIH. The cellular sources involved in the coupling between RTN/pFRG and RVLM neurones require future studies.
In conclusion, our findings show that the respiratory LTF after AIH also involves a progressive and persistent increase in the expiratory motor activity, named as expiratory LTF. This phenomenon seems to be dependent on neuronal hyperactivity in the RTN/pFRG, which may provide additional excitatory drive to the bulbospinal expiratory neurones and generate the active expiratory pattern. Moreover, RTN/pFRG is also critical for the maintenance of long-lasting increase in arterial pressure after AIH, supporting the hypothesis that the development of sympathetic LTF is, at least in part, dependent on respiratory drive, particularly on changes in the expiratory pattern. Altogether, these data highlight that the development of cardiorespiratory compensatory adaptations to intermittent hypoxia involves complex and integrated central mechanisms that still require additional experiments to be fully elucidated. The identification of the cellular and molecular targets of intermittent hypoxia has also potential implications for the understanding of autonomic and respiratory disorders associated with pathological states with chemoreflex dysfunctions, as observed in patients with obstructive sleep apnoea, heart failure and essential hypertension.
PHYSIOLOGICAL RELEVANCE.
Acute intermittent hypoxia (AIH) elicits a compensatory and sustained increase in ventilation, which has been associated with a long-term facilitation (LTF) of the inspiratory motor activity. AIH also promotes a persistent increase in sympathetic activity that has been suggested to occur independently of ventilatory LTF. In the present study, we demonstrated that the ventilatory LTF also includes a sustained and progressive increase in expiratory motor activity, referred to as expiratory LTF. We also found that AIH-induced expiratory and sympathetic LTF are coupled events and depends on the integrity of neurones located in the ventrolateral medulla. Altogether, our data support the notion that expiratory and sympathetic neuronal coupling is critical for the development sympathetic overactivity in conditions associated with hyperactivity of peripheral chemoreflex, such as observed in patients with obstructive sleep apnoea, heart failure and neurogenic hypertension.
ACKNOWLEDGEMENTS
This research was funded by São Paulo Research Foundation (FAPESP, grant to DBZ – 2013/17251-6; grant to EC - 2009/54888-7; PhD fellowship to EVL – 2014/06976-2) and the National Council for Scientific and Technological Development (CNPq, grant 478640/2013-7).
Footnotes
CONFLICT OF INTEREST
There is no conflict of interest.
AUTHOR CONTRIBUTION
EVL, EC and DBZ designed the research. EVL, SA, CBO, CF, MB and DZ performed the experiments. EVL and DBZ analysed the data and drafted the manuscript. All authors revised and approved the final version of the manuscript.
REFERENCES
- Abdala AP, Rybak IA, Smith JC, Paton JF. Abdominal expiratory activity in the rat brainstem-spinal cord in situ: patterns, origins and implications for respiratory rhythm generation. J Physiol. 2009;587:3539–3559. doi: 10.1113/jphysiol.2008.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol (1985) 2001;1985;91:2751–2757. doi: 10.1152/jappl.2001.91.6.2751. [DOI] [PubMed] [Google Scholar]
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol. 2000;529(Pt 1):215–219. doi: 10.1111/j.1469-7793.2000.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi L, Porta C, Gabutti A, Spicuzza L, Sleight P. Modulatory effects of respiration. Auton Neurosci. 2001;90:47–56. doi: 10.1016/S1566-0702(01)00267-3. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol (1985) 1992;1992;73:2083–2088. doi: 10.1152/jappl.1992.73.5.2083. [DOI] [PubMed] [Google Scholar]
- Cerutti C, Gustin MP, Paultre CZ, Lo M, Julien C, Vincent M, Sassard J. Autonomic nervous system and cardiovascular variability in rats: a spectral analysis approach. Am J Physiol Heart Circ Physiol. 1991;261:H1292–H1299. doi: 10.1152/ajpheart.1991.261.4.H1292. [DOI] [PubMed] [Google Scholar]
- Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir Physiol Neurobiol. 2008;160:65–75. doi: 10.1016/j.resp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Morrison S, Coles SK, Prabhakar N. Entrainment pattern between sympathetic and phrenic nerve activities in the Sprague-Dawley rat: hypoxia-evoked sympathetic activity during expiration. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1121–R1128. doi: 10.1152/ajpregu.00485.2003. [DOI] [PubMed] [Google Scholar]
- Dick TE, Hsieh YH, Wang N, Prabhakar N. Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol. 2007;92:87–97. doi: 10.1113/expphysiol.2006.035758. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol. 1994;477(Pt 3):469–479. doi: 10.1113/jphysiol.1994.sp020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Baker TL, Behan M, Mitchell GS. Expression of hypoglossal long-term facilitation differs between substrains of Sprague-Dawley rat. Physiol Genomics. 2001;4:175–181. doi: 10.1152/physiolgenomics.2001.4.3.175. [DOI] [PubMed] [Google Scholar]
- Gilbey MP, Numao Y, Spyer KM. Discharge patterns of cervical sympathetic preganglionic neurones related to central respiratory drive in the rat. J Physiol. 1986;378:253–265. doi: 10.1113/jphysiol.1986.sp016218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol. 1989;256:R739–R750. doi: 10.1152/ajpregu.1989.256.3.R739. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol. 1993;265:R811–R819. doi: 10.1152/ajpregu.1993.265.4.R811. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, Cardoza KP, Henderson LE, Feldman JL. Role of parafacial nuclei in control of breathing in adult rats. J Neurosci. 2015;35:1052–1067. doi: 10.1523/JNEUROSCI.2953-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen PL, Fregosi RF. No evidence for long-term facilitation after episodic hypoxia in spontaneously breathing, anesthetized rats. J Appl Physiol (1985) 2000;89:1345–1351. doi: 10.1152/jappl.2000.89.4.1345. [DOI] [PubMed] [Google Scholar]
- Lemes EV, Zoccal DB. Vagal afferent control of abdominal expiratory activity in response to hypoxia and hypercapnia in rats. Respir Physiol Neurobiol. 2014;203:90–97. doi: 10.1016/j.resp.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Leuenberger UA, Brubaker D, Quraishi S, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci. 2005;121:87–93. doi: 10.1016/j.autneu.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Vinit S, Mitchell GS. Serotonin 2A and 2B receptor-induced phrenic motor facilitation: differential requirement for spinal NADPH oxidase activity. Neuroscience. 2011;178:45–55. doi: 10.1016/j.neuroscience.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–492. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- McGuire M, Zhang Y, White DP, Ling L. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R334–R341. doi: 10.1152/ajpregu.00463.2003. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol (1985) 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Molkov YI, Abdala AP, Bacak BJ, Smith JC, Paton JF, Rybak IA. Late-expiratory activity: emergence and interactions with the respiratory CpG. J Neurophysiol. 2010;104:2713–2729. doi: 10.1152/jn.00334.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkov YI, Zoccal DB, Moraes DJ, Paton JF, Machado BH, Rybak IA. Intermittent hypoxia-induced sensitization of central chemoreceptors contributes to sympathetic nerve activity during late expiration in rats. J Neurophysiol. 2011;105:3080–3091. doi: 10.1152/jn.00070.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, Bonagamba LG, Costa KM, Costa-Silva JH, Zoccal DB, Machado BH. Short-term sustained hypoxia induces changes in the coupling of sympathetic and respiratory activities in rats. J Physiol. 2014;592:2013–2033. doi: 10.1113/jphysiol.2013.262212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, da Silva MP, Bonagamba LG, Mecawi AS, Zoccal DB, Antunes-Rodrigues J, Varanda WA, Machado BH. Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats. J Neurosci. 2013;33:19223–19237. doi: 10.1523/JNEUROSCI.3041-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes DJ, Dias MB, Cavalcanti-Kwiatkoski R, Machado BH, Zoccal DB. Contribution of retrotrapezoid/parafacial respiratory region to the expiratory-sympathetic coupling in response to peripheral chemoreflex in rats. J Neurophysiol. 2012;108:882–890. doi: 10.1152/jn.00193.2012. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol (1985) 2001;91:709–716. doi: 10.1152/jappl.2001.91.2.709. [DOI] [PubMed] [Google Scholar]
- Pagliardini S, Janczewski WA, Tan W, Dickson CT, Deisseroth K, Feldman JL. Active expiration induced by excitation of ventral medulla in adult anesthetized rats. J Neurosci. 2011;31:2895–2905. doi: 10.1523/JNEUROSCI.5338-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Rafacho A, Goncalves-Neto LM, Ferreira FB, Protzek AO, Boschero AC, Nunes EA, Zoccal DB. Glucose homoeostasis in rats exposed to acute intermittent hypoxia. Acta Physiol (Oxf) 2013;209:77–89. doi: 10.1111/apha.12118. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol. 1984;228:168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Ito S, Sved AF. Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1746–R1755. doi: 10.1152/ajpregu.00307.2005. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol. 2006;572:503–523. doi: 10.1113/jphysiol.2005.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol. 1997;499(Pt 2):543–550. doi: 10.1113/jphysiol.1997.sp021947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Pilowsky PM. Acute intermittent hypoxia in rat in vivo elicits a robust increase in tonic sympathetic nerve activity that is independent of respiratory drive. J Physiol. 2010;588:3075–3088. doi: 10.1113/jphysiol.2010.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Lalley P, Mifflin S. Acute intermittent optogenetic stimulation of nucleus tractus solitarius neurons induces sympathetic long-term facilitation. Am J Physiol Regul Integr Comp Physiol. 2015;308:R266–R275. doi: 10.1152/ajpregu.00381.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB. Peripheral chemoreceptors and cardiorespiratory coupling: a link to sympatho-excitation. Exp Physiol. 2015;100:143–148. doi: 10.1113/expphysiol.2014.079558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Bonagamba LG, Paton JF, Machado BH. Sympathetic-mediated hypertension of awake juvenile rats submitted to chronic intermittent hypoxia is not linked to baroreflex dysfunction. Exp Physiol. 2009a;94:972–983. doi: 10.1113/expphysiol.2009.048306. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Paton JF, Machado BH. Do changes in the coupling between respiratory and sympathetic activities contribute to neurogenic hypertension? Clin Exp Pharmacol Physiol. 2009b;36:1188–1196. doi: 10.1111/j.1440-1681.2009.05202.x. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]