Abstract
In isolated hepatocytes, the environmental estrogen bisphenol A (BPA) is metabolized into a mono-glucuronide and a glucuronide/sulfate diconjugate. Little is known about the fate of the diconjugate in the liver. The present study focused on the metabolism and dispostion of BPA diconjugate in the liver using a perfusion method. In Sprague-Dawley rats, BPA (15,150 or 1,500 nmol) was applied into the liver. In male rats, the infused BPA was conjugated to both glucuronide and a diconjugate during passage through the liver. The diconjugate was observed at high-dose application of the substrate. In female rats, the chemical was conjugated almost exclusively to the glucuronide in all doses utilized in this study. In both the male and female rats, the resultant metabolites were preferentially excreted into the bile. These results suggest that BPA is conjugated primarily to mono-glucuronide in rat liver; and that in males, diconjugate production occurs under conditions of high-dose exposure to BPA.
Keywords: bisphenol A, diconjugation, glucuronide conjugation, liver perfusion, sulfate conjugation
Bisphenol A (BPA, 2,2-bis[4-hydroxyphenyl]propane), a compound widely employed in the manufacture of plastics used in food containers and tableware [10], has been shown to act as an endocrine disruptor with estrogenic properties [7]. Traces of the compound, leached from plastic products, can be taken up by humans through eating and drinking [2, 19]. Calafat et al. showed that measurable levels of BPA were detected in 95% of the urinary samples from people in the United States [3].
After oral exposure, BPA undergoes an elimination process that is mediated by drug metabolism enzymes and chemical transporters. Recent studies showed that animals orally administered BPA excreted BPA metabolites in their urine and feces [8, 13]. Previously, we found that BPA is highly glucuronidated by rat liver microsomes and that the glucuronidation is mediated by UGT2B1, an isoform of UDP-glucuronosyltransferase [20]. It is well established that conjugation plays an important role in xenobiotic detoxification by converting lipophilic compounds to hydrophilic molecules, which are more easily eliminated from the body [1, 9]. During elimination of the chemical to the urine, the conjugates flow into the general circulation, potentially exposing target organs, such as the uterus and testes. Our previous data suggest that BPA glucuronide in the general circulation was transferred to the fetus during passage through the placenta and that the glucuronide is deconjugated in the fetal tissue [11]. In light of these findings, it is important that the disposition of BPA metabolites as well as the chemical conjugation process be elucidated to unveil the mechanisms responsible for the adverse effects caused by BPA.
The perfusion method, an established experimental model in physiology, provides information on both the actual metabolism and the dynamic disposition of the chemical of interest in living organs. Our previous investigation of liver perfusion with BPA demonstrated that BPA is glucuronidated during passage through the liver and is then mainly excreted into the bile [5]. In contrast, the fates of other BPA metabolites remain unclear. In primary cultures of rat hepatocytes incubated with BPA, Pritchett et al. showed that BPA is biotransformed into two major metabolites, mono-glucuronide and glucuronide/sulfate [14]. The present study focused on the metabolism and disposition of BPA via diconjugate formation within the living liver.
MATERIALS AND METHODS
Chemicals: BPA was purchased from Kanto Chemical Co. (Tokyo, Japan); BPA glucuronide and BPA glucuronide/sulfate diconjugate were obtained from Frontier Science Co. (Ishikari, Japan); and all other chemicals for the liver perfusion study and high performance liquid chromatography (HPLC) analysis were purchased from Kanto Chemical Co.
Animals: Male (330–400 g) and female (240–280 g) Sprague-Dawley rats (9–11 weeks old) were used. Before use in experiments, all rats were housed under standard conditions and given food and water ad libitum. The animals were handled according to the Laboratory Animal Control Guidelines of Rakuno Gakuen University (ethics committee protocol approval number ES23A06, approved Jan 13, 2012).
Surgical procedure for perfusion: To study perfusion, the rats were anesthetized by intraperitoneal injection of pentobarbital sodium (1.3 ml/100 g body weight). Whole liver perfusion was prepared according to the method described previously [5]. Briefly, after anesthesia, the abdomen was surgically opened, and the portal vein and common bile duct were cannulated and the caudal vena cava was incised. Oxygenated Krebs-Ringer buffer, described below, was infused by roller pump (MP-32N; EYELA, Tokyo, Japan) through the liver via the portal vein at a constant rate of 30 ml/min. Once perfusion was begun, a polyethylene catheter was inserted into the vena cava. The thorax was then opened, and the cranial vena cava was ligated. The liver was not excised; all experiments were performed in situ. After insertion of the polyethylene catheter, each animal was euthanized by exsanguination under anesthesia.
Liver perfusion: Krebs-Ringer buffer (NaCl 115 mM, KCl 5.9 mM, MgCl2 1.2 mM, NaH2PO4 1.2 mM, Na2SO4 1.2 mM, CaCl2 2.5 mM, NaHCO3 25 mM and glucose 10 mM) was used in all experiments. The buffer solution was aerated using 95% O2 + 5% CO2, and the pH was adjusted to 7.4. BPA was added to the substrate buffer solution at a final concentration of 0.1 µM, 1 µM or 10 µM. The buffer solutions were maintained in separate water baths at 37°C. The liver perfusion was carried out in flow-through mode. Preliminary perfusion with Krebs-Ringer solution was performed for 10 min, followed by 5 min inflow of the substrate buffer solution, and then reperfusion of Krebs-Ringer solution for 55 min. Once perfusion of the substrate buffer had begun, the excreted bile and a small amount of the perfusate in the vein were collected independently at 5-min intervals for 1 hr.
High performance liquid chromatography (HPLC) analysis of reaction products: The perfusate samples were independently centrifuged for 3 min at 9,000 g, and the supernatant fraction was collected. Each bile sampling was dissolved in distilled water at a dilution of 1:200. The supernatant and the bile solutions were stored at −80°C until analysis. The samples were analyzed by a HPLC (Tosoh, Tokyo, Japan) system consisting of an LC-8020 pump, CO-8020 oven and UV-8020 UV detector. The samples were subjected to Unizon UK-C18 reversed phase column (4.6 mm i.d. × 150 mm; Imtakt, Tokyo, Japan) chromatography and eluted with a linear gradient of methanol/water at flow rate of 1 ml/min (solution A: methanol/water (24/76 v/v) and 10 mM ammonium acetate to solution B: methanol) over 15 min. The eluted samples were analyzed at 222 nm, and the results were recorded using LC-8020 integration software (Tosoh). The elution peaks of BPA, BPA glucuronide and BPA glucuronide/sulfate were noted, and the concentrations were compared with the standards.
Liquid chromatography-mass spectrometry conditions: Liquid chromatography-electrospray ionization-time of flight mass spectrometry (LC-ESI-TOF MS) analysis was conducted using a LCT Premier mass spectrometer (Waters, Milford, MA, U.S.A.). The mass spectrometer electrospray source capillary voltage was set to 2.2 kV, cone voltage to 20 V, source block temperature to 120°C and desolvation temperature to 350°C. The nitrogen nebulizing and desolvation gas flows were set to 50 l/hr and 650 l/hr, respectively. To identify the metabolites, [M−H]− ions were monitored.
Statistical analysis: Area under the curve (AUC) was used for comparison of bilious and venous excretion of BPA and its conjugates. Comparisons were made by either a student’s t test or analysis of variance, and a P value of 0.05 was taken to be significant. All values are presented as the mean ± S.E.
RESULTS
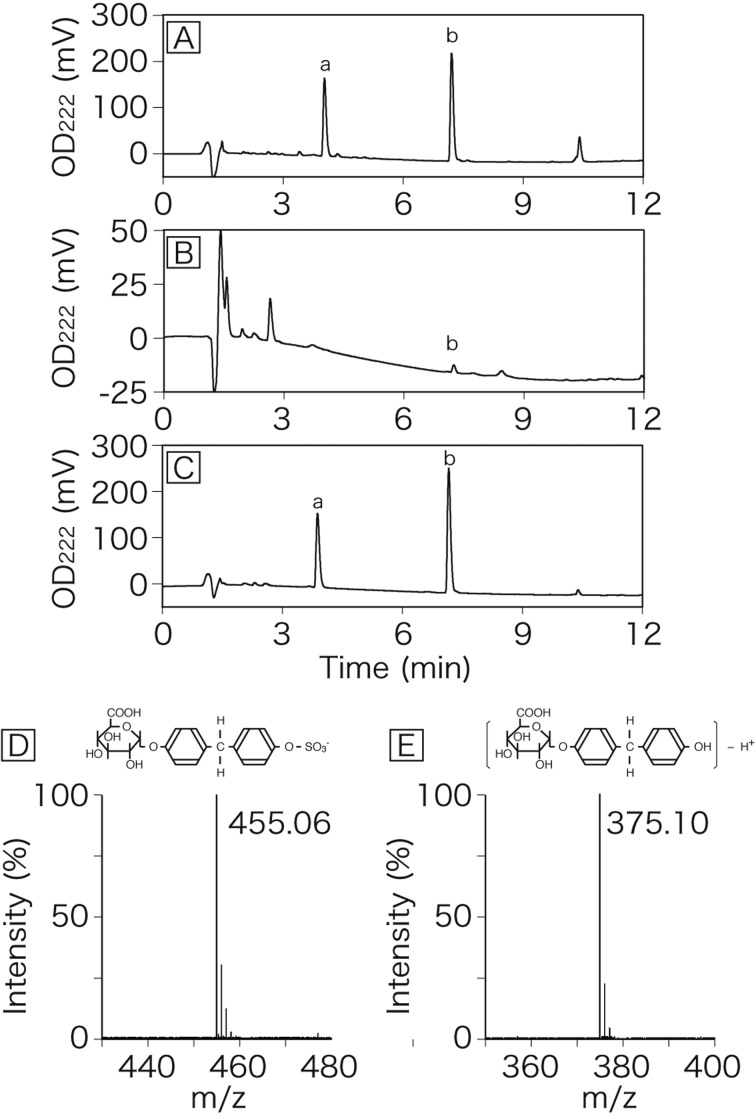
HPLC analysis of reaction products: In the HPLC samples obtained from rat liver perfused with 10 µM BPA in Krebs-Ringer solution, two major peaks were eluted at 3.9 min and 7.2 min (Fig. 1). In LC-MS analysis, m/z of peak a (RT=3.9 min) and peak b (RT=7.2 min) were 455.06 and 375.10, respectively. The first peak (peak a), detected mainly in the bile, was identified as BPA glucuronide/sulfate diconjugate (Fig. 1D). The second peak (peak b), found in both the bile and venous perfusate, was identified as BPA mono-glucuronide (Fig. 1E). The limits of detection for the bisphenol A glucuronide/sulfate diconjugate and the mono-glucuronide were 0.1 µM and 0.2 µM, respectively. The standard curves for the diconjugate and the mono-glucuronide resulted in a high linearity in a range of 1–500 µM.
Fig. 1.
HPLC chromatograms of bile and venous perfusate derived from rat liver perfused with BPA. The liver was perfused for 5 min with Krebs-Ringer buffer containing BPA and then perfused with normal Krebs-Ringer buffer. Bile (A) and venous perfusate (B) were collected at 15 min after application of BPA to the liver. Peaks of BPA glucuronide/sulfate diconjugate (a) and BPA mono-glucuronide (b) are indicated. Panel C is a HPLC chromatogram of a standard solution of BPA conjugates. Panels D and E are a mass spectrum of peak a and peak b, respectively.
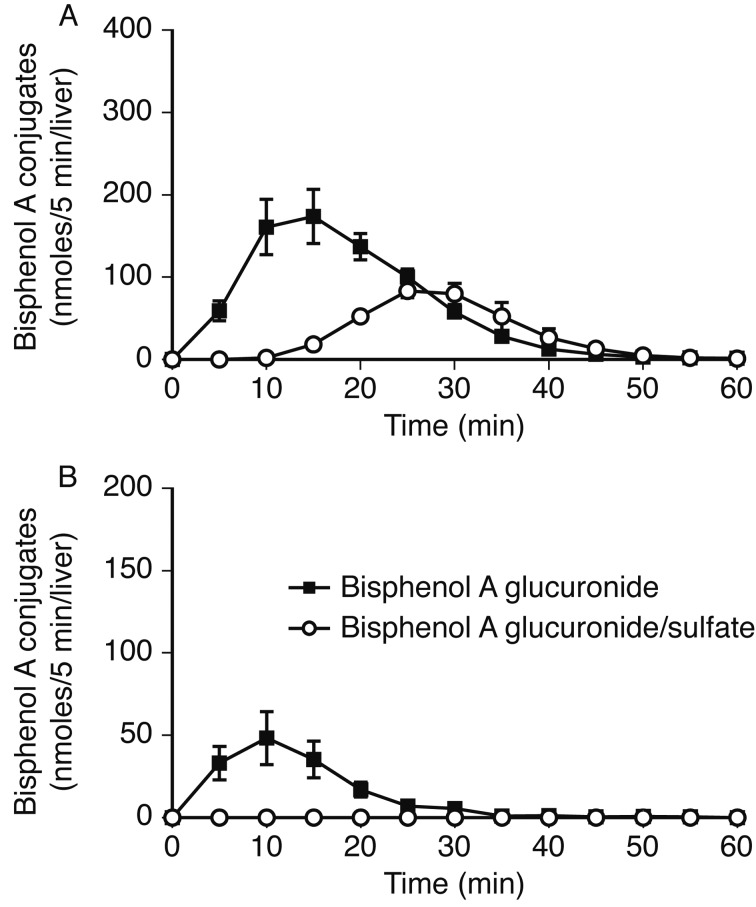
BPA conjugation and excretion in the perfused liver of male rats: On perfusion of the liver with 10 µM BPA in Krebs-Ringer solution, 99.6% of the substrate was transferred to the liver. BPA mono-glucuronide and glucuronide/sulfate diconjugate were subsequently excreted from the liver. The greatest glucuornide excretion was observed 10–15 min after substrate application (Fig. 2A). The diconjugate excretion peak was observed 10 min after that of the glucuronide. Venous excretion of diconjugate was almost nil in the present experiments (Fig. 2B).
Fig. 2.
BPA conjugates excreted into the bile (A) and vein (B) after liver perfusion of male Sprague-Dawley rats with BPA. The liver was perfused for 5 min with 10 µM BPA in Krebs-Ringer solution and for 55 min without substrate. Bile and venous perfusate were collected and analyzed by HPLC. Parameters are shown as the mean ± S.E.
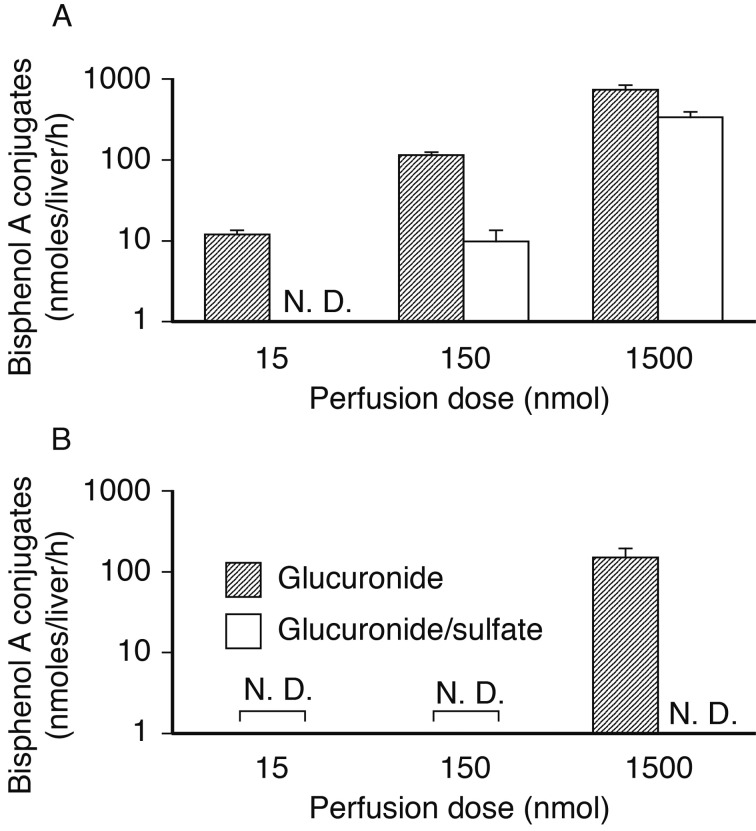
On application of low-doses of BPA to the perfused liver, the excretion of the conjugates diminished in a dose-dependent manner (Fig. 3). In the bile, the excretion of diconjugate was not detected following 15 µmol application (0.1 µM perfusion; Fig. 3A). The venous excretion of glucuronide/sulfate was below quantifiable levels in all trials in this study (Fig. 3B).
Fig. 3.
BPA metabolites after liver perfusion in male rats. Columns show total excretion of BPA glucuronide (hatched line) and diconjugate (empty columns) into the bile (A) and venous system (B) during 1-hr perfusion. Parameters are shown as the mean ± S.E.
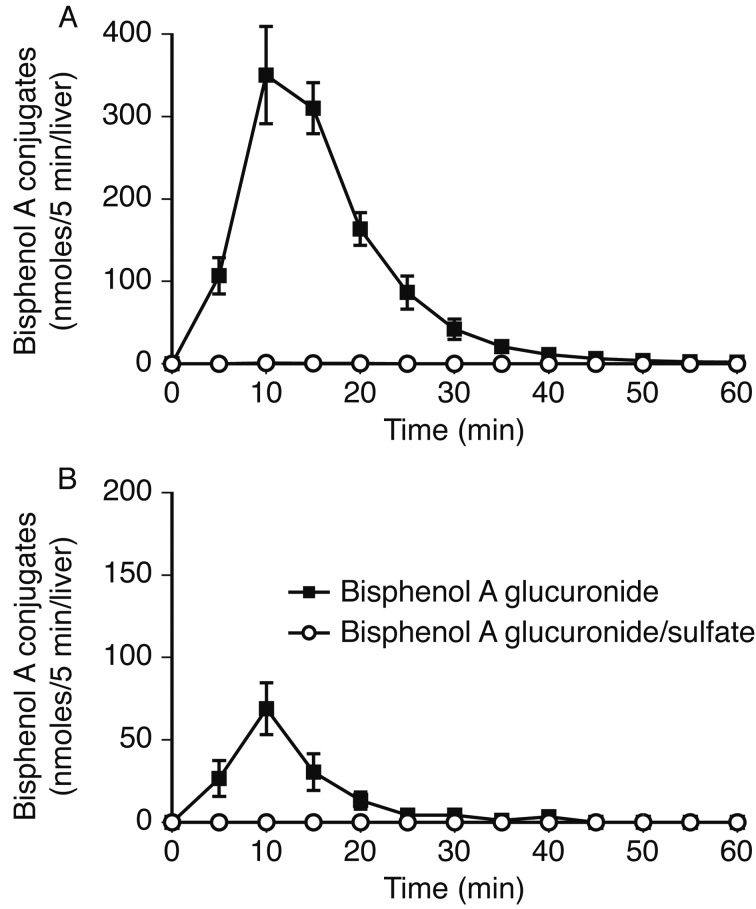
BPA conjugation and excretion in the liver of female rats: In female rats, liver perfusion of 10 µM BPA in Krebs-Ringer solution resulted in up to 100% of the infused substrate being absorbed into the liver tissue, which was subsequently glucuronidated and excreted into the bile and the venous system. The greatest glucuornide excretion was observed 10 min after application of the substrate (Fig. 4A). Bilious glucuronide excretion was estimated to be much higher than that of venous excretion. Negligible amounts of diconjugate were excreted from the liver of female rats (Fig. 4B).
Fig. 4.
BPA conjugates excreted into the bile (A) and venous system (B) after perfusion of the liver of female rats with BPA. The liver was perfused for 5 min with 10 µM BPA in Krebs-Ringer solution and for 55 min without substrate. Parameters are shown as the mean ± S.E.
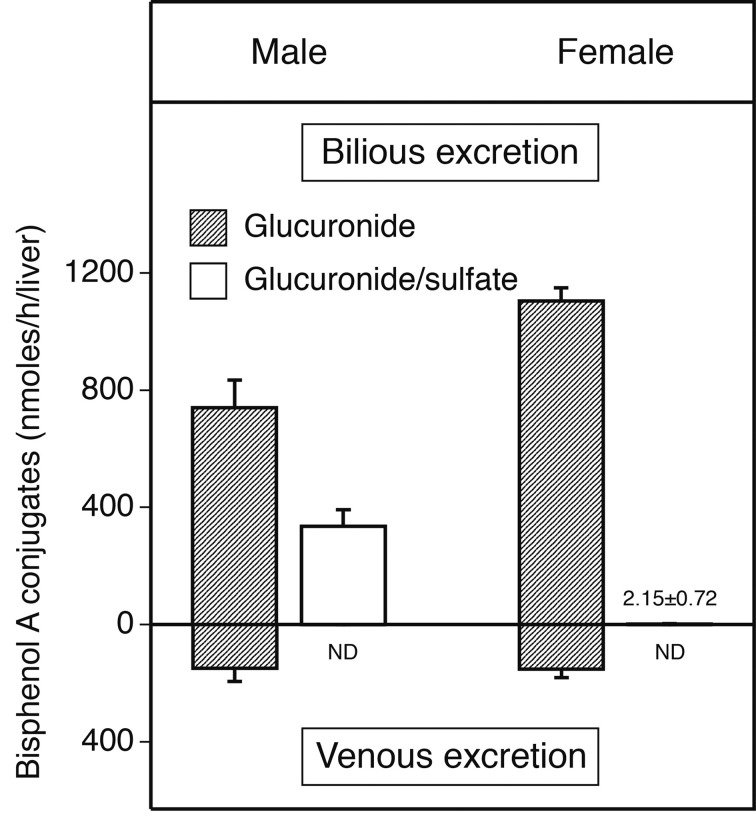
Bilious and venous excretions of the resulting conjugates during 1-hr perfusion in male and female rats are illustrated in Fig. 5. In male rats, the amounts of bilious and venous excretions of mono-glucuronide were 49.4% and 9.9% of the infused BPA, respectively. In female rats, the amounts of bilious and venous glucuronide were 73.7% and 10.1%, respectively. The excretion via glucuronide/sulfate diconjugate production in male rats was calculated as 22.3% of the infused BPA. Total recovery of the infused substrate was 81.9% in male rats and 83.9% in female rats.
Fig. 5.
BPA conjugates excreted into the bile (upper graph) and venous system (lower graph) during 1-hr perfusion. The livers of male (left graph) and female (right graph) rats were perfused for 5 min with 10 µM BPA in Krebs-Ringer solution and for 55 min without substrate. Results are shown as the mean ± S.E.
DISCUSSION
In Sprague-Dawley rats perfused with BPA, the infused compound was highly conjugated during passage through the liver, and the resultant metabolites were excreted from the liver. The main conjugate of the compound was mono-glucuronide. In male rats, approx. one-quarter of the infused BPA was eliminated as a glucuronide/sulfate diconjugate, whereas the diconjugate was virtually absent in female rats. These findings concur with a recent report, in which BPA, added to the medium of isolated hepatocytes, was metabolized into both mono-glucuronide and diconjugate; moreover, the diconjugate was almost nil in female rats [14]. BPA sulfo-conjugation is mediated by the SULT1 family, a phenol sulfotransferase isoform [17]. One member of the SULT1 family, SULT1A1, has been shown to have high-conjugation activity toward BPA [12]. The expression level of the SULT1 family is estimated to be higher in male than female rats [6]. In contrast to the sulfotransferases, the UGT2 isoenzyme, which mediates BPA glucuronidation, exhibits gender differences in mRNA expression; higher levels were shown in females compared to males [16]. These results suggest that sex differences in the conjugation pathways of BPA are responsible for the expression level of the conjugation enzymes.
In the present study, the excretion of BPA diconjugate followed the excretion of the mono-glucuronide. This phenomenon can be explained by the two-step conjugation of BPA, in which a carboxy radical of the substrate is glucuronidated and an addition radical is subsequently sulfated. UDP-glucuronosyltransferases are located on the endoplasmic reticulum [1], while sulfotransferases are found in the cytosol [12]. It is presumable that in hepatocytes, lipophilic BPA crosses the plasma membrane and reaches the endoplasmic reticulum, where it is glucuronidated and diffuses into the cytosol. After conjugation, the resulting hydrosoluble metabolites are likely transported from inside the cell to outside via chemical transporters. We found that in rat liver, the bilious excretion of BPA mono-glucuronide is mediated by Mrp2 (Abcc2), a member of the ATP-binding cassette (ABC) family [4]. Pharmacokinetic studies involving 4-methylumbelliferone and ethinyl estradiol demonstrate that Bcrp (Abcg2) mediates the transport of glucuronide and sulfate [21, 22]. While identification of the transporter exporting BPA diconjugate requires further investigation, our previous study and recent studies suggest that the ABC family mediates the export of diconjugate.
On application of low-dose BPA to the perfused liver in the present study, BPA diconjugate levels were negligible; however, the reaction increased in a dose-dependent manner. This indicates that BPA elimination via diconjugation occurs upon high exposure to the chemical. In animal studies of the health effects of BPA, long-term/low concentration exposure or short-term/high concentration exposure is typically employed. Our present results give rise to the view that, depending on the exposure level, different metabolic pathways are involved in the elimination of BPA. From bile, the excreted conjugates flow into the intestinal tract, where the metabolites are reabsorbed into the body. Our previous data confirmed that BPA glucuronide is reabsorbed in the intestine [15]. An in vivo study by Kurebayashi et al. showed that oral BPA exposure flows into the enterohepatic circulation before excretion to the urine and feces [8]. In light of these findings, we suggest that the BPA conjugates pass through internal organs, such as the heart, lung, kidney and brain, before being eliminated from the body. Of concern is that the conjugates also pass through the placenta. Our previous data showed that BPA glucuronide is absorbed from the maternal blood stream at the placenta [11]. Recently, chemical transporters involved in the movement of hormone-sulfates have been identified in the placenta [18, 23]. It is highly likely that BPA diconjugate is actively transferred into the fetus at the placenta. Pritchett et al. demonstrated the production of a BPA glucuronide/sulfate diconjugate in female mice using isolated hepatocytes [14]. Given that greater diconjugate production in hepatocytes has been found in females [14], it is important that further work be done to determine the fate of the diconjugate in the pathway before excretion.
In conclusion, the present study assessed BPA conjugation with mono-glucuronide and glucuronide/sulfate using a liver perfusion method. The results suggest that in the rat liver, BPA is conjugated primarily to mono-glucuronide and that diconjugate production occurs when the animal is exposed to high concentrations of the substrate. The potential public health hazards of BPA remain unknown. To elucidate the adverse effects of BPA systemically, further work focusing on the changes in BPA metabolism according to the dose is required.
Acknowledgments
This study was supported in part by the Health Science Research Grant H23-Kagaku-004 from the Ministry of Health, Labour and Welfare, Japan; and a Feasibility Study among Fundamental Studies under the framework of EXTEND 2010 (Extended Tasks on Endocrine Disruption 2010) funded by the Ministry of the Environment, Japan.
REFERENCES
- 1.Bock K. W.2003. Vertebrate UDP-glucuronosyltransferases: functional and evolutionary aspects. Biochem. Pharmacol. 66: 691–696. doi: 10.1016/S0006-2952(03)00296-X [DOI] [PubMed] [Google Scholar]
- 2.Brotons J. A., Olea-Serrano M. F., Villalobos M., Pedraza V., Olea N.1995. Xenoestrogens released from lacquer coatings in food cans. Environ. Health Perspect. 103: 608–612. doi: 10.1289/ehp.95103608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calafat A. M., Kuklenyik Z., Reidy J. A., Caudill S. P., Ekong J., Needham L. L.2005. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 113: 391–395. doi: 10.1289/ehp.7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inoue H., Tsuruta A., Kudo S., Ishii T., Fukushima Y., Iwano H., Yokota H., Kato S.2005. Bisphenol A glucuronidation and excretion in liver of pregnant and nonpregnant female rats. Drug Metab. Dispos. 33: 55–59. doi: 10.1124/dmd.104.001537 [DOI] [PubMed] [Google Scholar]
- 5.Inoue H., Yokota H., Makino T., Yuasa A., Kato S.2001. Bisphenol A glucuronide, a major metabolite in rat bile after liver perfusion. Drug Metab. Dispos. 29: 1084–1087. [PubMed] [Google Scholar]
- 6.Klaassen C. D., Liu L., Dunn R. T.1998. Regulation of sulfotransferase mRNA expression in male and female rats of various ages. Chem. Biol. Interact. 109: 299–313. doi: 10.1016/S0009-2797(97)00141-5 [DOI] [PubMed] [Google Scholar]
- 7.Krishnan A. V., Stathis P., Permuth S. F., Tokes L., Feldman D.1993. Bisphenol-A: an estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 132: 2279–2286. [DOI] [PubMed] [Google Scholar]
- 8.Kurebayashi H., Nagatsuka S., Nemoto H., Noguchi H., Ohno Y.2005. Disposition of low doses of 14C-bisphenol A in male, female, pregnant, fetal, and neonatal rats. Arch. Toxicol. 79: 243–252. doi: 10.1007/s00204-004-0628-2 [DOI] [PubMed] [Google Scholar]
- 9.Mackenzie P. I., Owens I. S., Burchell B., Bock K. W., Bairoch A., Belanger A., Fournel-Gigleux S., Green M., Hum D. W., Iyanagi T., Lancet D., Louisot P., Magdalou J., Chowdhury J. R., Ritter J. K., Schachter H., Tephly T. R., Tipton K. F., Nebert D. W.1997. The UDP glycosyltransferase gene superfamily: recommended nomenclature update based on evolutionary divergence. Pharmacogenetics 7: 255–269. doi: 10.1097/00008571-199708000-00001 [DOI] [PubMed] [Google Scholar]
- 10.National Toxicology Program. 1982. Carcinogenesis Bioassay of Bisphenol A (CAS No. 80-05-7) in F344 Rats and B6C3F1 Mice (Feed Study). Technical Report Series, 215. [PubMed]
- 11.Nishikawa M., Iwano H., Yanagisawa R., Koike N., Inoue H., Yokota H.2010. Placental transfer of conjugated bisphenol A and subsequent reactivation in the rat fetus. Environ. Health Perspect. 118: 1196–1203. doi: 10.1289/ehp.0901575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiyama T., Ogura K., Nakano H., Kaku T., Takahashi E., Ohkubo Y., Sekine K., Hiratsuka A., Kadota S., Watabe T.2002. Sulfation of environmental estrogens by cytosolic human sulfotransferases. Drug Metab. Pharmacokinet. 17: 221–228. doi: 10.2133/dmpk.17.221 [DOI] [PubMed] [Google Scholar]
- 13.Pottenger L. H., Domoradzki J. Y., Markham D. A., Hansen S. C., Cagen S. Z., Waechter J. M., Jr2000. The relative bioavailability and metabolism of bisphenol A in rats is dependent upon the route of administration. Toxicol. Sci. 54: 3–18. doi: 10.1093/toxsci/54.1.3 [DOI] [PubMed] [Google Scholar]
- 14.Pritchett J. J., Kuester R. K., Sipes I. G.2002. Metabolism of bisphenol a in primary cultured hepatocytes from mice, rats, and humans. Drug Metab. Dispos. 30: 1180–1185. doi: 10.1124/dmd.30.11.1180 [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto H., Yokota H., Kibe R., Sayama Y., Yuasa A.2002. Excretion of bisphenol A-glucuronide into the small intestine and deconjugation in the cecum of the rat. Biochim. Biophys. Acta 1573: 171–176. doi: 10.1016/S0304-4165(02)00418-X [DOI] [PubMed] [Google Scholar]
- 16.Shelby M. K., Cherrington N. J., Vansell N. R., Klaassen C. D.2003. Tissue mRNA expression of the rat UDP-glucuronosyltransferase gene family. Drug Metab. Dispos. 31: 326–333. doi: 10.1124/dmd.31.3.326 [DOI] [PubMed] [Google Scholar]
- 17.Shimizu M., Ohta K., Matsumoto Y., Fukuoka M., Ohno Y., Ozawa S.2002. Sulfation of bisphenol A abolished its estrogenicity based on proliferation and gene expression in human breast cancer MCF-7 cells. Toxicol. In Vitro 16: 549–556. doi: 10.1016/S0887-2333(02)00055-3 [DOI] [PubMed] [Google Scholar]
- 18.Ugele B., St-Pierre M. V., Pihusch M., Bahn A., Hantschmann P.2003. Characterization and identification of steroid sulfate transporters of human placenta. Am. J. Physiol. Endocrinol. Metab. 284: E390–398. doi: 10.1152/ajpendo.00257.2002 [DOI] [PubMed] [Google Scholar]
- 19.vom Saal F. S., Hughes C.2005. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 113: 926–933. doi: 10.1289/ehp.7713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokota H., Iwano H., Endo M., Kobayashi T., Inoue H., Ikushiro S., Yuasa A.1999. Glucuronidation of the environmental oestrogen bisphenol A by an isoform of UDP-glucuronosyltransferase, UGT2B1, in the rat liver. Biochem. J. 340: 405–409. doi: 10.1042/bj3400405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamek-Gliszczynski M. J., Day J. S., Hillgren K. M., Phillips D. L.2011. Efflux transport is an important determinant of ethinylestradiol glucuronide and ethinylestradiol sulfate pharmacokinetics. Drug Metab. Dispos. 39: 1794–1800. doi: 10.1124/dmd.111.040162 [DOI] [PubMed] [Google Scholar]
- 22.Zamek-Gliszczynski M. J., Hoffmaster K. A., Humphreys J. E., Tian X., Nezasa K., Brouwer K. L.2006. Differential involvement of Mrp2 (Abcc2) and Bcrp (Abcg2) in biliary excretion of 4-methylumbelliferyl glucuronide and sulfate in the rat. J. Pharmacol. Exp. Ther. 319: 459–467. doi: 10.1124/jpet.106.101840 [DOI] [PubMed] [Google Scholar]
- 23.Zhou F., Tanaka K., Soares M. J., You G.2003. Characterization of an organic anion transport system in a placental cell line. Am. J. Physiol. Endocrinol. Metab. 285: E1103–1109. doi: 10.1152/ajpendo.00182.2003 [DOI] [PubMed] [Google Scholar]