Abstract
Calcinated egg shell (Egg-CaO), of which the main component is calcium oxide, was evaluated in the forms of powder and aqueous solutions for their efficacies as disinfectants against avian influenza virus (AIV), Newcastle disease virus (NDV), infectious bursal disease virus (IBDV), Salmonella Infantis and Escherichia coli. Egg-CaO powder inactivated these viruses within 3 min in the presence of 33% of fetal bovine serum (FBS). In Egg-CaO solutions, except AIV, all pathogens were inactivated within 1 hr, even in the presence of 5% of FBS. Without FBS, all pathogens, except AIV, were inactivated within 3 min, and AIV within 1 hr. In addition, persistence of virucidal activity against AIV and NDV of Egg-CaO powder was confirmed after exposure to sunlight for 2 weeks or resuspension with water for 7 times, simulating field harsh environments. Chick growth test was conducted to ensure the safety of the use of Egg-CaO powder in chicken cages and showed that it is safe to add Egg-CaO in litter or feed. In conclusion, Egg-CaO can be useful for the enhancement of biosecurity at farms.
Keywords: calcinated egg shell (Egg-CaO), HACCP at farm level, inactivation of avian pathogen, livestock biosecurity
For the food safety, the Ministry of Agriculture, Forestry and Fisheries (MAFF) in Japan announced the certification standard for “hazard analysis critical control point (HACCP) at livestock farm level: Farm HACCP” in August, 2009 [13, 17]. Accreditation of farms that are recognized as Farm HACCP adopted farms was started since 2011 by the certification organizations of Farm HACCP [7]. HACCP at farm level aims at the improvement of the security and the production of livestock products in the primary production sites, namely “livestock farms”. At the farm, biological hazards include Salmonella sp., Escherichia coli (E. coli), avian influenza virus (AIV), Newcastle disease virus (NDV), infectious bursal disease virus (IBDV), etc. Some of these pathogens are zoonotic, and some are infective towards domestic animals only; however, enhancement of biosecurity at farms is most important for HACCP at farm level.
Since 2003, highly pathogenic avian influenza (HPAI) caused by H5N1 subtype virus became endemic in Asian countries, mainly because some of the Asian countries are using vaccine against HPAI [5, 16]. HPAI is one of the most serious diseases in the poultry industry in the world [1]. The outbreaks by HPAIV subtypes of H5N8 and H5N2 in United States brought about depopulation of around 50 million birds [23]. Newcastle disease (ND) is also highly contagious and constitutes a big concern for poultry industry all over the world [2].
Enhancement of biosecurity at farms is very important to control infectious diseases. Slaked lime, of which the main component is calcium hydroxide (Ca (OH)2), is widely used as disinfectant at farms in Japan, but there is a problem about eyes; if humans get slaked lime into eyes, they get blind [14]. Disinfection effect of slaked lime is maintained for a long period if it is dry powder, but if it turns wet by rain, it becomes hardened [22]. In addition, it was reported that when slaked lime was combined with carbon dioxide in the air or rain water, it became calcium carbonate (CaCO3) and the pH got lowered, bringing about the disappearance of disinfection effect [15]. When spraying the disinfectant in a farm, it is necessary to respray diligently, because rainfall cannot be avoided. Namely, biosecurity strengthening material which is cheaper, without loss of effect even when it gets wet with water and has long-term effect even in the presence of organic matter has been demanded.
The Kewpie Group (Tokyo, Japan) handles around 250,000 tons, or 10%, of the chicken eggs produced domestically in Japan each year, from which 25,000 tons of egg shell per year occur as a by-product [8]. The main constituent of calcinated egg shell powder is calcium oxide (CaO), after which we here call the calcinated egg shell ‘Egg-CaO’. When calcium oxide is mixed with water, the aqueous solution becomes calcium hydroxide and its pH is more than 12. It has been reported that AIV (H7N9) retained its infectivity when exposed to pH 12 for 24 hr [24]. We also reported that scallop shell powder solution at pH 12.3 did not inactivate AIV (H7N1), however, at pH 13.0 it did [20]. Therefore, aqueous solution above pH12.5 seems to be able to inactivate the influenza viruses. To our knowledge, there is no report that examined the anti-viral activity of Egg-CaO.
In view of the above, Egg-CaO is considered to be useful for enhancement of biosecurity at farms. In the present study, AIV, NDV and IBDV were targeted as the viruses that often cause serious infectious diseases in poultry. In addition, Salmonella Infantis (SI) and E. coli, which cause food poisoning, were also used to evaluate Egg-CaO as a material for enhancement of biosecurity at farms.
MATERIALS AND METHODS
Calcinated egg shell powder: Calcinated egg shell (Egg-CaO) powder heated at 900°C, the average diameter of the powder particle being 15 µm, was provided by Kewpie Egg Co., Ltd (Tokyo, Japan). The suspensions of 3% or 10% (w/v) of Egg-CaO were prepared in redistilled water (dW2) and then centrifuged at 12,000 ×g for 3 min, and the resulted supernatants were used as 3% or 10% Egg-CaO solutions, respectively. Both of the solutions showed pH at 12.7.
Viruses: A low pathogenic AIV (LPAIV), A/duck/Aomori/395/04 (H7N1) [6] and NDV strain Sato [18] were propagated in embryonated chicken eggs. After aliquot, these viruses were stored at −80°C until used. IBDV vaccine strain D78 (Intervet Co., Ltd., Tokyo, Japan) was purchased.
Cell cultures: Madin-Darby canine kidney (MDCK) cells were used for AIV and NDV. Chicken embryo fibroblasts (CEF) were prepared from 10-day-old embryonated eggs as described [18] and used for IBDV titration. The growth medium (GM) was prepared with Eagle’s minimum essential medium (MEM, Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 5% fetal bovine serum (FBS), L-glutamine 0.3 mg/ml, NaHCO3 1.4 mg/ml and antibiotic–fungicide cocktail (penicillin 100 IU/ml, Streptomycin 0.1 mg/ml and amphotericin B 0.5 µg/ml). Maintenance medium (MM) was prepared like the GM, without FBS.
Viruses were diluted in serial 10-fold dilution and inoculated on Madin-Darby canine kidney (MDCK) cells for AIV and NDV and on CEF for IBDV in 96-well tissue culture plates (4 wells per dilution, 200 µl final volume in each well). For AIV, 0.2 µg/ml trypsin (final concentration, trypsin from bovine pancreas 10,000 BAEE units/mg protein, Sigma, St. Louis, MO, U.S.A.) was added to each well. The plates were incubated at 37°C in the presence of 5% CO2. For AIV and NDV, at 3 day-post inoculation (dpi), virus-induced cytopathic effect (CPE) was observed, and hemagglutinin (HA) activity of the culture supernatant was checked with 0.5% chicken red blood cells. For IBDV, CPE was observed and titrated at 5 dpi. The 50% tissue culture infective dose (TCID50)/ml was determined by the method of Behrens and Kärber [12].
Bacteria: E. coli strain NBRC106373 was purchased from National Institute of Technology and Evaluation Biological Resource Center (NBRC) (Chiba, Japan), and Salmonella Infantis (SI) was kindly provided by Prof. Hiroshi Fujikawa (Laboratory of Public Health, Department of Veterinary Medicine, Tokyo University of Agriculture and Technology, Tokyo, Japan). Both bacteria were stocked in 10% skim milk at −80°C until used. When used, these bacteria were sub-cultured onto desoxycholate hydrogen sulfide lactose (DHL) agar and then incubated at 37°C overnight. Colonies were picked up and cultivated in Luria-Bertani (LB) medium (1% Bacto Tryptone, 0.5% Bacto Yeast Extract and 1%NaCl, pH 7.4) and titrated on DHL agar as described. To remove organic materials, LB medium was centrifuged at 1,750 ×g for 20 min, and the precipitate was re-suspended in PBS. This was centrifuged and re-suspended in PBS twice. This re-susupension was used as washed bacteria.
E. coli and SI were diluted in serial 10-fold dilution and inoculated on DHL agar. The dishes were incubated at 37°C overnight, and the number of colonies was enumerated. The titer was calculated as colony forming units (CFU)/ml.
Evaluation of the virucidal activity of Egg-CaO powder: Egg-CaO powder of 200 mg was mixed with 100 µl of virus, namely AIV, NDV or IBDV, in a micro-tube. To evaluate virucidal activity in the presence of organic materials, the virus was mixed with half volume of FBS, and then, 150 µl of the mixture was added to 300 mg Egg-CaO powder in a micro-tube. After 3 min incubation at room temperature, the viruses were recovered with 900 µl or 850 µl of MM, respectively, centrifuged at 17,400 ×g for 3 min and titrated in each sensitive cell, respectively.
Evaluation of the inactivation activity of Egg-CaO solutions against pathogens: Four hundreds micro-litter of 3% or 10% Egg-CaO solutions were mixed with 100 µl of viruses (AIV, NDV or IBDV) or bacteria (SI or E. coli) in a micro-tube, incubated for indicated time (3 min or 1 hr) at room temperature and then neutralized with 500 µl of 1 M HEPES (pH 7.2) for viruses or 1 M Tris-HCl (pH 7.2) for bacteria, which made pH of the tested solutions around 8.2. The neutralized samples were titrated in each sensitive cell or DHL agar plates. To evaluate inactivating activity of the solutions with organic materials, 25 µl of FBS was added to 100 µl of viruses or bacteria and then mixed with 375 µl of Egg-CaO solutions in a micro-tube, and then, the pH was neutralized with 500 µl of 1 M HEPES for viruses and 1 M Tris-HCl for bacteria. To confirm the effect of neutralization of 1 M HEPES or 1 M Tris-HCl, tested solutions and 1 M HEPES or 1 M Tris-HCl were mixed before adding viruses or bacteria (treatment of 0 sec). Each solution was tested in triplicates, and the titers were shown in mean ± standard error (SE).
Calculation of reduction factor (RF): Inactivation efficacy against pathogens was determined using reduction factor (RF), calculated by the following equation:
RF=log10 (amount of untreated intact pathogens/ml) −log10 (amount of treated pathogen with Egg-CaO/ml). Inactivation was considered to be satisfied when RF was ≥3 [21].
Evaluation of virucidal effect under harsh conditions: Egg-CaO powder was assessed for the persistence of its efficacy to inactivate AIV and NDV after being applied into the environment, as follows. Egg-CaO powder with the amount of 3 g was poured into 90 mm petri dishes, thus simulating field conditions around 0.5 kg/m2 [15]. The dishes were kept under sunlight from morning to evening for 8 weeks.
Another set of dishes containing Egg-CaO powder was also prepared and kept separately for measuring its persisting efficacy after being kept under wet and dry conditions. Egg-CaO powder in a dish was suspended with chlorine free tap water, 10 ml per dish. These dishes were kept under sunlight until dried up. Each dish was re-suspended with the same volume of water following the evaporation. This procedure was repeated 10 times.
The portions of Egg-CaO powder in dishes were collected for 8 weeks under sunlight, as well as for 10 times after being dried. Egg-CaO powder of 100 mg in micro-tubes was incubated with 50 µl of AIV or NDV. The viruses were recovered with 450 µl of MM, made serial 10 fold dilution and inoculated into MDCK cells.
Chick growth test of Egg-CaO powder safety for feed and litter additives: The possibility of proposing Egg-CaO powder as feed additive was evaluated using 18 chicks of 8-day-old, according to a protocol of MAFF as described [19]. Animal work was performed in strict accordance with Animal Care guidelines of Tokyo University of Agriculture and Technology (Tokyo, Japan) with permit numbers 26–45, 27–20. Day-old commercial layer male chicks, with no vaccination, hereafter designated as “conventional chicks”, were purchased from Kanto Co., Ltd. (Maebashi, Japan), kept 6 chicks per group in a rat cage (CLEA-0108–3, Clea Japan, Inc., Tokyo, Japan) inside the isolator (CL-5443, Clea Japan) until 8-day-old and then used for the experiments.
One cage was supplied with feed with 0.1% Egg-CaO powder and normal litter, and one was supplied with normal feed and litter with 10% Egg-CaO powder, whereas another cage was kept as a control, which was supplied with normal feed and normal litter. The body weight gain was observed for 6 days.
SPSS software (IBM corporation, Tokyo, Japan) was applied for statistical analysis. The obtained body weight was analyzed by independent Student’s t-test and analysis of variance (ANOVA) with using Duncan’s multiple range test, where applicable. The difference between parameters was regarded as significant when the P-value was less than 0.05.
RESULTS
Evaluation of the virucidal activity of Egg-CaO powder: As shown in Table 1, Egg-CaO powder inactivated all viruses within 3 min incubation under the detectable level (2.5 log10 TCID50/ml), and this ability was not affected by the presence of organic materials, namely 33% of FBS. When the RF was calculated, it was revealed that Egg-CaO powder reduced viral titer by more than 3 log, which is effective, within 3 min.
Table 1. The virucidal efficacy of Egg-CaO powder.
| Virus | AIV | NDV | IBDV | ||||
|---|---|---|---|---|---|---|---|
| FBS at 33% | + | - | + | - | + | - | |
| Incubation period | Positive control | 7.50a) | 7.88 | 7.75 | 8.42 | 7.13 | 7.13 |
| 3min | <2.50 | <2.50 | <2.50 | <2.50 | <2.50 | <2.50 | |
| RFb) | >5.00 | >5.38 | >5.25 | >5.92 | >4.63 | >4.63 | |
a) Figures are shown as log10TCID50/ml. b) Reduction factor (RF)=log10 (amount of untreated intact virus/ml) −log10 (amount of treated virus with Egg-CaO/ml).
Evaluation of the inactivation activity of Egg-CaO solutions against pathogens: When pH of the solutions was neutralized with 1 M Tris-HCl for bacteria or 1 M HEPES for viruses before adding the pathogens (0 sec), no pathogens titer reduction was observed in comparison with the positive controls (Tables 2 and 3, for 10% solution data not shown), which means 1 M Tris-HCl or 1 M HEPES prevented pathogen inactivation by the tested solutions.
Table 2. The bactericidal effect of 3% Egg-CaO solution.
| Bacteria | SI | E. coli | |||
|---|---|---|---|---|---|
| FBS | + | - | + | - | |
| Positive control | 8.68 ± 0.29a) | 9.03 ± 0.39 | 8.38 ± 0.04 | 9.06 ± 0.33 | |
| Incubation period | 0 sec | 8.17 ± 0.14 | 8.28 ± 0.11 | 8.19 ± 0.04 | 8.73 ± 0.31 |
| 3 min | 2.10 ± 0.10 | <2.00 ± 0.00 | <2.00 ± 0.00 | <2.00 ± 0.00 | |
| RFb) | 6.07 | >6.58 ± 0.19 | >7.03 ± 0.39 | >6.38 ± 0.04 | |
a) Figures are shown as log10TCID50/ml (mean ± SE). b) Reduction factor (RF)=log10 (amount of untreated intact virus/ml) −log10 (amount of treated virus with Egg-CaO/ml).
Table 3. The virucidal effect of 3% suspension.
| Virus | AIV | NDV | IBDV | |||
|---|---|---|---|---|---|---|
| FBS | - | + | - | + | - | |
| Positive control | 8.33 ± 0.22 | 7.42 ± 0.44a) | 7.00 | 6.88 ± 0.16 | 6.87 ± 0.20 | |
| Incubation period | 0 sec | 7.91 ± 0.08 | 6.17 ± 0.44 | 7.00 | 7.37 ± 0.24 | 7.36 ± 0.24 |
| 3 min | NTb) | 3.75 ± 0.75 | <2.50 | 1.90 ± 0.10 | 1.90 ± 0.10 | |
| 1 hr | 3.83 ± 0.79 | NT | NT | NT | NT | |
| RFc) | 4.50 ± 0.66 | 3.67 ± 0.94 | >4.50 | 4.98 ± 0.06 | 4.97 ± 0.10 | |
a) Figures are shown as log10TCID50/ml (mean ± SE). b) Not tested. c) Reduction factor (RF)=log10 (amount of untreated intact virus/ml) −log10 (amount of treated virus with Egg-CaO/ml).
The bactericidal effects of 3% Egg-CaO solution against SI and E. coli are shown in Table 2. The titers of tested SI in log10CFU/ml were 8.17 ± 0.14 and 8.28 ± 0.11 at 0 sec incubation with or without 5% FBS, respectively. The SI titers were reduced down to 2.10 ± 0.10 and less than 2.00 at 3 min, with or without FBS, respectively, when treated with Egg-CaO solution. The RF was calculated more than 6. E. coli was also inactivated within 3 min under the detectable level. With 10% solution, the results were similar, and all bacteria were inactivated to undetectable level within 3 min incubation (data not shown).
The virucidal effects of 3% solution against AIV,NDV and IBDV are summarized in Table 3. In a preliminary experiment, AIV was not inactivated within 3 min (data not shown), whereas after 1 hr incubation, AIV was inactivated without FBS (Table 3). However, with FBS, AIV was not inactivated after 1 hr incubation in 3% nor 10% Egg-CaO solutions (data not shown). NDV and IBDV were inactivated within 3 min with or without FBS.
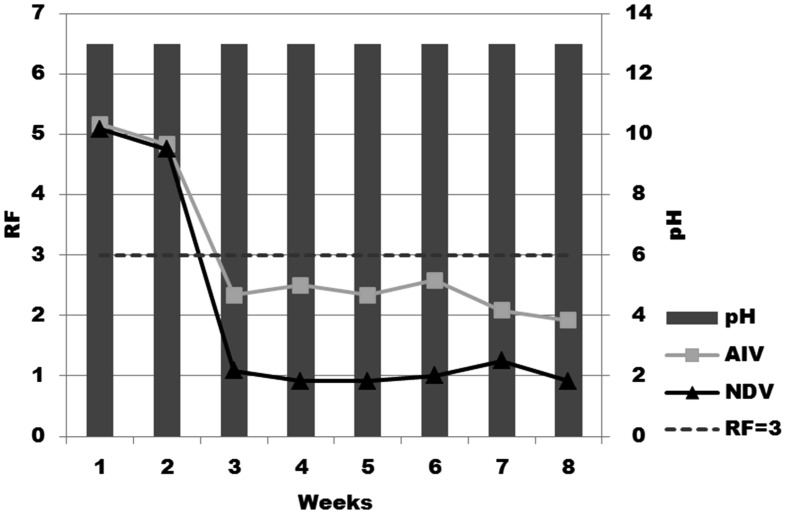
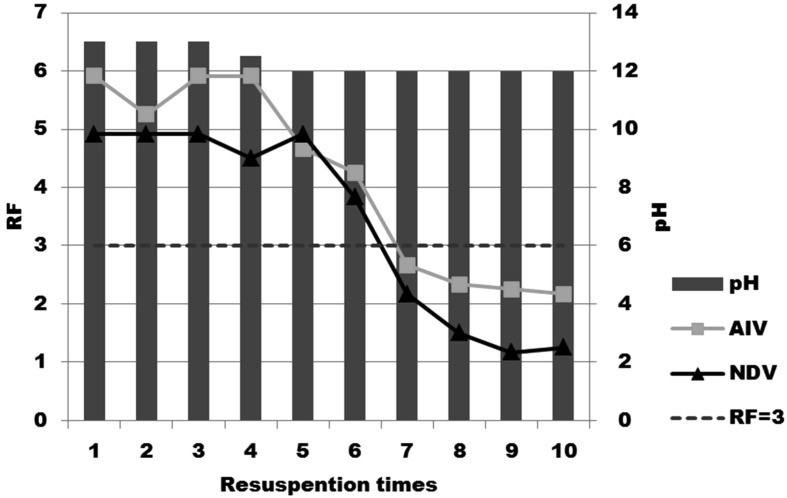
Evaluation of virucidal effect under harsh conditions: As shown in Fig. 1, Egg-CaO powder kept under sunlight in a dry condition retained its efficacy (RF>3) for 2 weeks when the samples were evaluated using AIV and NDV for 3 min incubation. Egg-CaO powder kept under wet and dry conditions, with a 3-min incubation period, demonstrated high efficacy (RF>3) up to six times resuspention when the samples were evaluated using AIV and NDV for 3 min (Fig. 2).
Fig.1.
Virucidal efficacies of Egg-CaO powder after being kept under sunlight. Virucidal efficacies against AIV and NDV were examined by mixing harvested powder with viruses and incubation for 3 min.
Fig.2.
Virucidal efficacies of Egg-CaO powder after kept under wet and dry conditions. Virucidal efficacies against AIV and NDV were examined by mixing harvested powder with viruses and incubated for 3 min.
Effect of Egg-CaO powder in feed or litter on growth performance of chicks: According to a protocol of MAFF as described [19], in the safety test, initial body weights (BW) were compared among Egg-CaO powder treatment groups and control group. There were no significant differences in all groups at starting age. The final body weights after 6 days were evaluated and showed no significant difference among groups. The growth rates were displayed in terms of average daily gain (ADG), which is the important value that could demonstrate whether the effect on growth performance of Egg-CaO powder took place or not. The results reveal no difference when compared with a control of each tested starting age (Table 4).
Table 4. Growth performance of chicks kept with Egg-CaO powder.
| Initial BW (g) | Final BW (g) | ADG (g)a) | |
|---|---|---|---|
| Control | 79.40 ± 5.84b) | 112.23 ± 4.19 | 5.47 ± 1.26 |
| Treatment (10% litter) | 78.03 ± 3.93 | 109.58 ± 6.28 | 5.26 ± 0.45 |
| Treatment (0.5% feed) | 74.72 ± 1.82 | 111.52 ± 4.53 | 6.14 ± 0.68 |
a) ADG=average daily gain. b) Figures are shown as mean ±SD of 6 chicks in the same column with non-statistically significant difference at the same starting age (P>0.05).
DISCUSSION
Egg-CaO powder could inactivate AIV, NDV and IBDV even in the presence of organic matter, 33% FBS, within 3 min. The observed virucidal activity of Egg-CaO powder is appropriate for using this substance under field condition, taking into account that there are many organic matters in the poultry farms, including feces, taking into litter, food etc. Hydrated lime (slaked lime: Ca (OH)2) and quicklime (CaO) were also shown to have efficacy to inactivate bacteria in litter [3, 11].
AIV is relatively easy to inactivate due to the lipid envelope that increases its sensitivity to dehydration, detergents and surfactants [9]. However, it is reported that AIV (H7N2) lost 100% of its infectivity when exposed to pH 2 for 5 min, but exposure to pH 12 for 15 min had no effect on infectivity [10]. Thammakarn et al. also reported that pH 12.3 could not inactivate AIV, however, at pH 13.0 could [20]. As shown in Table 3, AIV was not inactivated by 3% solution of Egg-CaO in the absence of FBS within 3 min treatment, but after 1 hr treatment, it was. In the presence of 5% FBS, even after 1 hr incubation, AIV was not inactivated (data not shown).
As shown in Tables 2 and 3, 3% Egg-CaO solution inactivated all pathogens, except AIV, within 3 min even in the presence of 5% FBS (RF>3). The main determinant of inactivation seems to be high pH around 13, because when pH of the solution was neutralized with 1M HEPES or 1M Tris-HCl before adding pathogens, no inactivation was demonstrated (Tables 2 and 3, 0 sec). These data demonstrated that AIV is very resistant to high pH, as shown previously [10, 20].
In harsh conditions, it is reported that slaked lime maintained its virucidal efficacy (RF>3) under sunlight for 3 weeks and that it showed high efficacy (RF>3) under wet and dry conditions for 3 times resuspension when the samples were evaluated using LPAIV H7N1 for 3 min [21]. In addition, it is suggested that after rain, slaked lime became hardened and it was difficult to take samples from the surface of the lime [22]. Considering these points, Egg-CaO had longer efficacy when kept under wet and dry conditions up to 6 times than slaked lime.
The safety tests of Egg-CaO powder toward chicks were performed according to the MAFF protocol [19] and started when chicks became 8-day-old. There were no significant differences in ADG among the treatment groups and the control group. This means Egg-CaO powder can be used safely as feed additive for chicks at 0.5% or added in litter at 10%, without harm, even if used during the critical age around 1 week old. Bennett et al. reported that lime in excess of 5% (w/v) in litter to day-of-hatch poults caused mild but apparent ocular and respiratory irritation, but not lime of 5% or less [4]. They kept poults up to 49-day-old.
The present study suggests that Egg-CaO has good efficacy for inactivating predominant viral and bacterial poultry pathogens, even under the field conditions, requiring only brief contact with pathogens. It is necessary to develop the calcination method for the low price to change the egg shell, which is industrial waste, into a valuable ‘Egg-CaO’.
Acknowledgments
The authors would like to thank by Kewpie Egg Co., Ltd. (Tokyo, Japan) for Egg-CaO powder providing in this experiment. This study was supported in part by Kieikai Research Foundation of 2014 and 2015.
REFERENCES
- 1.Alexander D. J., Brown I. H.2009. History of highly pathogenic avian influenza. Rev. - Off. Int. Epizoot. 28: 19–38. [DOI] [PubMed] [Google Scholar]
- 2.Alexander D. J., Senne D. A.2008. Newcastle disease. pp. 75–100. In: Diseases of Poultry, 12th ed. (Saif, Y. M., Fadly, A. M., Glisson, J. R., McDougald, L. R., Nolan, L. K. and Swayne, D. E. eds.), Blackwell, Oxford. [Google Scholar]
- 3.Bennett D. D., Higgins S. E., Moore R. W., Beltran R., Caldwell D. J., Byrd J. A., II, Hargis B. M.2003. Effects of lime on Salmonella enteritidis survival in vitro. J. Appl. Poult. Res. 12: 65–68. doi: 10.1093/japr/12.1.65 [DOI] [Google Scholar]
- 4.Bennett D. S., Higgins S. E., Moore R., Byrd J. A., Beltran R., Corsiglia C., Caldwell D., Hargis B. M.2005. Effect of addition of hydrated lime to litter on recovery of selected bacteria and poult performance. J. Appl. Poult. Res. 14: 721–727. doi: 10.1093/japr/14.4.721 [DOI] [Google Scholar]
- 5.Cha R. M., Smith D., Shepherd E., Davis C. T., Donis R., Nguyen T., Nguyen H. D., Do H. T., Inui K., Suarez D. L., Swayne D. E., Pantin-Jackwood M.2013. Suboptimal protection against H5N1 highly pathogenic avian influenza viruses from Vietnam in ducks vaccinated with commercial poultry vaccines. Vaccine 31: 4953–4960. doi: 10.1016/j.vaccine.2013.08.046 [DOI] [PubMed] [Google Scholar]
- 6.Jahangir A., Ruenphet S., Shoham D., Okamura M., Nakamaura M., Takehara K.2010. Haemagglutinin and neuraminidase characterization of low pathogenic H5 and H7 avian influenza viruses isolated from Northern pintails (Anas acuta) in Japan, with special reference to genomic and biogeographical aspects. Virus Genes 40: 94–105. doi: 10.1007/s11262-009-0423-5 [DOI] [PubMed] [Google Scholar]
- 7.Japan livestock industry association. Farm HACCP certification council. http://jlia.lin.gr.jp/haccp/ (accessed Dec 13, 2015).
- 8.Kewpie Corporation “Egg Products Business”, http://www.kewpie.co.jp/english/our_business/index03.html (accessed Dec 13, 2015).
- 9.Lombardi M. E., Ladman B. S., Alphin R. L., Benson E. R.2008. Inactivation of avian influenza virus using common detergents and chemicals. Avian Dis. 52: 118–123. doi: 10.1637/8055-070907-Reg [DOI] [PubMed] [Google Scholar]
- 10.Lu H., Castro A. E., Pennick K., Liu J., Yang Q., Dunn P., Weinstock D., Henzler D.2003. Survival of avian influenza virus H7N2 in SPF chickens and their environments. Avian Dis. 47Suppl: 1015–1021. doi: 10.1637/0005-2086-47.s3.1015 [DOI] [PubMed] [Google Scholar]
- 11.Maguire R. O., Hesterberg D., Gernat A., Anderson K., Wineland M., Grimes J.2006. Liming poultry manures to decrease soluble phosphorus and suppress the bacteria population. J. Environ. Qual. 35: 849–857. doi: 10.2134/jeq2005.0339 [DOI] [PubMed] [Google Scholar]
- 12.Matumoto M.1949. A note on some points of calculation method of LD50 by Reed and Muench. Jpn. J. Exp. Med. 20: 175–179. [PubMed] [Google Scholar]
- 13.Ministry of Agriculture Forestry and Fisheries. “The improvement of the breeding hygiene management standard in the stage of production of the domestic animal”, http://www.maff.go.jp/j/syouan/douei/katiku_yobo/k_haccp/index.html (accessed Jan 4, 2016).
- 14.Ministry of Education Culture, Sports, Science and Technology. “Handling of the lime to use for the lines of the athletic ground”, http://www.gankaikai.or.jp/info/pdf/20080101_monbu.pdf (accessed Jan 20, 2016).
- 15.Okubo Y., Tojyo H.2009. Evaluation of slaked lime for “Trapping” disinfection oeffect on bacteria and avian influenza virus. J. Jpn. Soci. Poult. Dis. 45: 84–90. [Google Scholar]
- 16.Sims L. D.2007. Lessons learned from Asian H5N1 outbreak control. Avian Dis. 51Suppl: 174–181. doi: 10.1637/7637-042806R.1 [DOI] [PubMed] [Google Scholar]
- 17.Takehara K.2013. Thoughts on health control management of meat and chicken from farm to table: hazard analysis, critical control points and basic assistance. JVM 66: 409–418. [Google Scholar]
- 18.Takehara K., Shinomiya T., Kobayashi H., Azuma Y., Yamagami T., Yoshimura M.1987. Characterization of Newcastle disease viruses isolated from field cases in Japan. Avian Dis. 31: 125–129. doi: 10.2307/1590784 [DOI] [PubMed] [Google Scholar]
- 19.Takehara K., Chinen O., Jahangir A., Miyoshi Y., Ueno Y., Ueda S., Takada Y., Ruenphet S., Mutoh K., Okamura M., Nakamura M.2009. Ceramic powder made from chicken feces: anti-viral effects against avian influenza viruses. Avian Dis. 53: 34–38. doi: 10.1637/8382-062008-Reg.1 [DOI] [PubMed] [Google Scholar]
- 20.Thammakarn C., Satoh K., Suguro A., Hakim H., Ruenphet S., Takehara K.2014. Inactivation of avian influenza virus, newcastle disease virus and goose parvovirus using solution of nano-sized scallop shell powder. J. Vet. Med. Sci. 76: 1277–1280. doi: 10.1292/jvms.14-0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thammakarn C., Tsujimura M., Satoh K., Hasegawa T., Tamura M., Kawamura A., Ishida Y., Suguro A., Hakim H., Ruenphet S., Takehara K.2015. Efficacy of scallop shell powders and slaked lime for inactivating avian influenza virus under harsh conditions. Arch. Virol. 160: 2577–2581. doi: 10.1007/s00705-015-2517-9 [DOI] [PubMed] [Google Scholar]
- 22.Tsujimura M., Thammakarn C., Yamada Y., Satoh K., Hasegawa T., Ruenphet S., Takehara K.2012. Antiviral activity of scallop-shell powder against avian influenza virus and goose parvovirus. Trans. Mater. Res. Soc. Jpn. 37: 567–570. doi: 10.14723/tmrsj.37.567 [DOI] [Google Scholar]
- 23.United States Department of Agriculture. Fall 2015 HPAI preparedness and response plan. Animal and Plant Health Inspection Service. September 18, 2015. (accessed Dec 13, 2015).
- 24.Zou S., Guo J., Gao R., Dong L., Zhou J., Zhang Y., Dong J., Bo H., Qin K., Shu Y.2013. Inactivation of the novel avian influenza A (H7N9) virus under physical conditions or chemical agents treatment. Virol. J. Sep 15; 10: 289. doi: . [DOI] [PMC free article] [PubMed]