Abstract
A xenogeneic DNA vaccination has been licensed for use in dogs with locally controlled stage II and III oral malignant melanoma (OMM). At present, there are limited outcome data for dogs with OMM treated with surgery and immunotherapy. The aim of this study is to retrospectively review the outcome and survival of 32 dogs affected by OMM that were treated with a combination of surgery and the xenogeneic DNA vaccination (with the addition of radiotherapy in some cases) and to determine the influence of surgical margins and delay in receiving vaccination. The overall median survival time (MST) was 335 days (95% CI: 301–540 days), and the overall median progression-free survival (PFS) was 160 days (mean 182 days, 95% CI: 132–232 days). Stage, completeness of surgical margins and delay in administration of the vaccine did not appear to statistically influence survival or PFS, although these results may reflect the low statistical power of the study due to small numbers. Further studies are required to assess whether the addition of any adjuvant treatment to surgery, including immunotherapy, is able to significantly prolong survival in cases of canine oral melanoma.
Keywords: canine, immunotherapy, oral melanoma, radiotherapy, xenogeneic DNA vaccination
Malignant melanoma is the most common oral neoplasm in dogs. This tumor can potentially arise from any oral mucosal site, although the gingival location is the one most frequently reported. A breed predisposition has been described in Cocker Spaniels, Scottish Terriers, Golden Retrievers, Chow Chows and Miniature Poodles [11, 14].
Local and distant metastases occur frequently and are reported in approximately 70–95% of dogs [6, 15, 26], with a predilection for the regional lymph nodes and ultimately development of pulmonary metastases, although other sites may be affected including the skin and central nervous system. A number of clinical prognostic factors have been identified, including location, tumor size and presence of metastases at the time of diagnosis [15].
Both surgery and radiation therapy have been described for treatment of oral malignant melanoma (OMM) [1, 4, 10, 16, 20, 22, 24, 26]. Mandibulectomy or maxillectomy can achieve complete excision in gingival tumors, although it may result in microscopically incomplete resection in cases of extensive and invasive neoplasms [26]. In patients that are treated surgically, the median survival time (MST) correlates with WHO stage [12] and, most importantly, with tumor size (Table 1). MST is reported to be 17–18 months for stage I, 5–6 months for stage II and 3 months for stage III disease [18].
Table 1. WHO staging system for canine oral melanoma.
| Stage I | Stage II | Stage III | Stage IV |
|---|---|---|---|
| ≤2 cm diameter | 2–4 cm diameter | >4 cm | Any size |
| Negative nodes | Negative nodes | +/−Metastatic lymph nodes | Distant metastasis |
Radiotherapy (RT) has been shown to result in complete response (CR) in 51–69% of cases and partial response (PR) in 25–30% of cases in one study [1], although anecdotal evidence suggests that different responses can be expected. Radiation response also appears to be dependent on stage [24] and protocols [1, 4]. Reported survival time (ST) with RT alone is between 5.3 and 11.9 months [10, 20, 22].
Given the aggressive metastatic behavior of OMM, it is not surprising that survival times after local treatment with surgery or radiation alone are disappointing, and many patients succumb to metastatic disease. Adjuvant treatment has been investigated throughout the years, with chemotherapy been associated with no survival advantage when used in addition to surgery or RT alone [20, 23], although a small number of individual dogs treated with surgery and carboplatin may have had some survival benefit [8]. In addition, another study showed improved survival (MST 440 days) when using carboplatin in association with surgery (with or without radiotherapy) [9]. A MST of 119 days was achieved using piroxicam in combination with cisplatin [5].
To address the lack of response to traditional adjunctive chemotherapy, immunotherapy has been used in patients affected by oral melanoma [19, 25, 27]. Human tyrosinase has been evaluated for the treatment of OMM. This specific glycoprotein is expressed in melanocytes, and it appears to be sufficiently different to canine tyrosinase to induce an immune response, which then targets tumor cells [2, 17]. A vaccine (Oncept™, Merial®, Duluth, GA, U.S.A.) carrying the human gene for tyrosinase has been licensed for veterinary use. In a phase I clinical trial of Oncept™, 9 vaccinated dogs experienced an overall MST of 389 days, and prolonged survival times have been reported in 2 patients with Stage II/III (501 and 496 days, respectively) with good loco-regional control [3]. Subsequent studies have shown encouraging results, with 14 stage III dogs achieving an individual survival time of 338 days, although all the 58 dogs receiving the human tyrosinase vaccine in this study did not reach MST [13]. A retrospective, controlled study evaluating immunotherapy with Oncept™ in 22 vaccinated versus 23 non-vaccinated patients [21] found that the MST of stage I, II and III patients in the vaccinated group was 485 days versus 585 days for the non-vaccinated group, with no statistical significance between groups.
A recent multicenter study [7] showed no difference in survival time when surgery was used alone or in combination, with reported survival of 352 days for the patients treated surgically, versus 335 days for those dogs receiving adjunctive treatment (including chemotherapy, radiotherapy and immunotherapy). However, it is hard to draw conclusions as only 14 dogs received the licensed vaccine in this study.
At present, there are limited outcome data for dogs with OMM treated with surgery and adjuvant treatments, and immunotherapy deserves further investigation to better assess its efficacy in the adjuvant setting. The purpose of this study is to retrospectively review the outcome and survival of dogs with OMM that were treated with surgery and the adjuvant xenogeneic DNA vaccination, with the addition of RT in some cases, and to assess whether completeness of surgical margins and time between surgery and vaccination could have influenced ST or progression-free survival (PFS).
The database of our institution was searched for cases of oral melanoma of dogs presenting during 2009–2012. Inclusion criteria were histologically confirmed diagnosis of OMM and complete staging at presentation with no evidence of distant metastases. Patients were classified according to the WHO staging system for OMM and were stages I to III. All the patients had staging performed prior to starting treatment (including CBC, serum biochemistry, three view thoracic radiographs and abdominal ultrasound Computed tomography [CT], cytology of the ipsilateral and contralateral mandibular lymph nodes). CT of the head was also performed in cases of invasive tumors where mandibulectomy or maxillectomy was planned.
All dogs received surgery (with the addition of RT in some cases) and pursued immunotherapy treatment. In most of the cases, surgery was performed at our institution by a qualified specialist surgeon, specifically by means of maxillectomy or mandibulectomy to excise gingival tumors presenting with gross disease, or after incomplete excision or excisional biopsy was performed by the primary veterinarian. Revision surgery was also performed in other locations, if there was evidence of incomplete margins on histopathology. The mandibular nodes were routinely removed for staging purposes or if cytologically diagnosed as metastatic.
Radiotherapy was used as an adjunct in those cases where a second surgery was performed at our institution and incomplete margins were subsequently found on histopathology, or to treat microscopic disease when the owners declined a second surgery at the time of referral. No dogs with macroscopic disease or local recurrence underwent radiotherapy.
The RT protocol consisted of 8–9 Gy/fraction once weekly for 4 weeks and included the regional lymph node in the field for Stage III patients in which the node had not been surgically removed. The patients were assessed for evidence of local recurrence before each radiotherapy treatment. If any local recurrence was found on clinical examination, further investigations to check for evidence of metastatic disease were performed at the same time and RT discontinued.
The vaccine was administered at biweekly intervals for a total of 4 cycles via a transdermal injection into the medial thigh following the label’s instructions. Follow-up consisted in monthly reassessment of the patients for the first 3 months and then every 3 months thereafter. Restaging (consisting of thoracic radiographs and cytology of the lymph nodes where indicated) was performed prior to each booster vaccination for those dogs that continued immunotherapy treatment. Boosters were administered every 6 months to patients that did not show any evidence of distant metastases after the first vaccination course.
Dogs that were lost to follow-up or were still alive at the time of writing were censored. Surgery was considered as the primary treatment when calculating ST and PFS: if dogs received a second surgery, survival was calculated from the time this was performed at our institution. ST was defined as the time between surgery and death from any causes. PFS was defined as the time between surgery and local recurrence, development of distant metastases or death from any causes. Data were entered into an Excel spreadsheet (Microsoft Corporation, 2007, Redmond, WA, U.S.A.) and analyzed using IBM SPSS Statistics 20 (New York, NY, U.S.A.).
The distribution of continuous data, including time to progression and ST, was evaluated using the Kolmogorov-Smirnov test for normality. Descriptive statistics were used for variables of breed, sex, age and body weight at diagnosis. ST and PFS were examined using Kaplan-Meier methodology and the grouped data compared using log rank analysis. A P value of <0.05 was considered to be statistically significant.
Signalment: a total of 32 dogs were included. There were 19 male (8 entire) and 13 female (1 entire) dogs. The most common breed identified was Labrador Retriever (6), followed by Cocker Spaniel (5), Golden Retriever (4), Dalmatian (2) and Shar-Pei (2), with one each of the following: Springer Spaniel, Poodle, Hungarian Viszla, Pekingese, Chow Chow, Border Terrier, Airedale Terrier and Staffordshire Bull Terrier. The remainder were all cross-breed dogs. The median age of the treated dogs was 10.4 years (range 5–14), and the median body weight was 24.8 kg (range 4.35–67) on presentation. Nine dogs were classified as stage I, 17 dogs as stage II and 6 dogs as stage III, 2 of which presented with nodal metastasis (ipsilateral mandibular node). The tumors were located at the level of the gingival mucosa (17), lip (8), tongue (5) and palate (2).
Histopathology: all the tumors had a histopathological diagnosis of OMM, either after surgical removal or excisional biopsy, performed by a board-certified pathologist. Immunohistochemistry (IHC) including melanocytic markers (S100, Melan-A) was performed in 2 cases. Excision was histologically classified as complete or incomplete. Margins were found to be complete in 24 (75%) and incomplete in 8 (25%) dogs. Margins were complete in 6 dogs for stage I, 12 for stage II and 6 for stage III. Incomplete margins were found in 3 dogs belonging to stage I (gingival location) and 5 dogs belonging to stage II (2 palatal, 2 lingual and 1 gingival location).
Treatment: surgery was used as the primary treatment of choice in all the 32 dogs in the study: they subsequently received the adjuvant xenogeneic DNA vaccination, and 7 patients (21%) were also treated with the addition of radiotherapy. Twenty-five dogs (78%) had their first surgery performed at our institution and were classified as follows: stage I, 7; stage II, 14; and stage III, 4. In 4 cases (stage II, 2 and stage III, 2), surgery was carried out at our institution for the second time (including lymph node removal), achieving incomplete margins in 3/4 cases. In one case, the tumor was removed following local recurrence and, despite complete margins on histopathology, radiation was used adjunctively; the remainder of cases also received RT to treat residual disease. Similarly, RT was used in 3 additional cases (stage I, 2 and stage II, 1) where the tumor was incompletely excised before referral and the owners declined a second surgery: in these dogs, the local lymph node was not removed and hence included in the radiation field.
With regards to the immunotherapy protocol, 19 dogs (59%) received a total of 4 administrations of the vaccine (stage I, 5; stage II, 9 and stage III, 5) and 6 dogs (18%, classified as stage I, 3 and stage II, 3) had one or more boosters every 6 months after completion of the initial 4 treatments course. Seven dogs (21%) did not reach the end of the induction protocol (stage I, 1; stage II, 5 and stage III, 1).
RT to treat microscopic disease was usually started 2 weeks after surgery. Four planned fractions of radiation were completed in 5/7 (71%) patients (stage I, 2; stage II, 2 and stage III, 1), and RT treatment was discontinued due to local disease progression or metastasis in the remainder of patients. The median time between the first radiotherapy treatment and the first delivered vaccination was 29 days (range 3–64 days). No adverse events were recorded when the vaccine was used in association with RT.
Time from surgery to first vaccination: in a total of 32 patients, 15 dogs received vaccination within 56 days after surgery was performed (range, 6–50 days), whereas 17 dogs received the vaccine beyond 56 days post surgery (range, 58–147 days). The median time between surgery and the first delivered vaccination was 58.5 days (range, 6–147 days). No metastases were detected prior to each vaccine administration.
Survival times: in a total of 32 patients, only 1 dog was lost to follow-up and therefore censored, and 2 patients were still alive at the time of writing. Hence, these 2 patients were censored for survival analysis, but included for the assessment of PFS. The median follow-up time was 910 days (range 455–1,460 days).
The overall median survival time (MST) was 335 days (95% CI: 301–540 days), with an MST of 373 days for stage I (range 163–913 days, mean 661 days and 95% CI: 329–993 days), 383 days for stage II (range 60–1,078, mean 495 days and 95% CI: 271–555 days) and 189 days for stage III (range 60–428, mean 236 days and 95% CI: 51–135 days, [Fig. 1]). There was no statistically significant difference in ST when comparing dogs belonging to different stages of the disease (9 stage I vs 17 stage II dogs: P=0.265; 9 stage I vs 6 stage III dogs: P=0.58 and 17 stage II vs 6 stage III dogs: P=0.51).
Fig. 1.
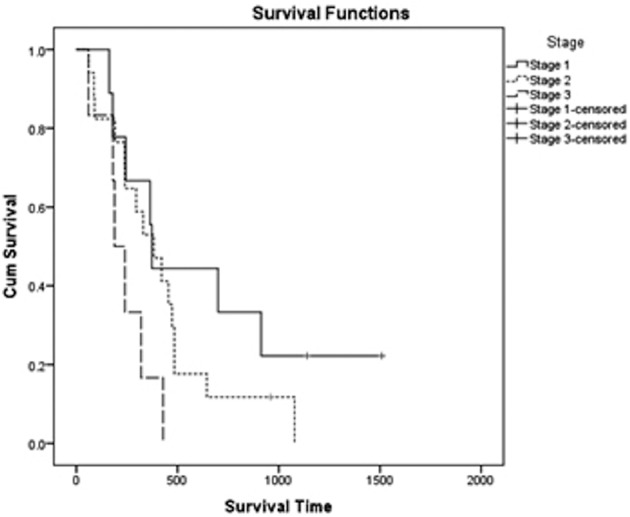
Kaplan Meier plot for survival time of dogs receiving surgery, vaccination and radiotherapy, stratified according to the WHO stage. Overall median survival time (MST) was 335 days. MST for Stage I patients (solid line) was 373 days; stage II patients (dotted line) achieved a MST of 383 days, and stage III patients (dashed line) had a MST of 189 days.
The overall median PFS was 160 days (mean 182 days and 95% CI: 132–232 days). The median PFS was 127 days for stage I patients (mean 174 days and 95% CI: 115–232 days), 120 days for stage II patients (mean 187 days and 95% CI: 119–265 days) and 180 days for stage III patients (mean 171 days and 95% CI: 131–212 days, [Fig. 2]). There was no significant difference in PFS when comparing dogs belonging to different stages of the disease (9 stage I vs 17 stage II dogs: P=0.95; 9 stage I vs 6 stage III dogs: P=0.51 and 17 stage II vs 6 stage III dogs: P=0.98).
Fig. 2.
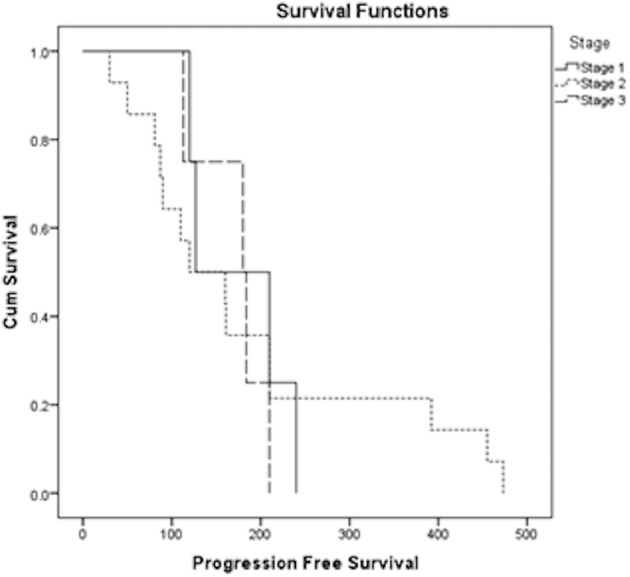
Kaplan Meier plot for progression-free survival (PFS) of dogs receiving surgery, vaccination and radiotherapy, stratified according to the WHO stage. Overall median PFS was 160 days. Median PFS was 127 days for Stage I patients (solid line), 120 days for stage II patients (dotted line) and 180 days for stage III patients (dashed line).
Survival time and PFS were compared for dogs in which complete excision was achieved and those with incomplete margins. The P value was not significantly different between the 2 groups with regards to ST (P=0.186) and PFS (P=0.589).
Delay in receiving vaccination was also assessed. ST in those 15 dogs that received the vaccine within 56 days of surgery was not significantly different from those 17 dogs that received vaccination >56 days after surgery was performed. There was no significant difference in ST (P=0.925) or PFS (P=0.764) between the two groups.
Survival time for those 7 dogs receiving RT ranged between 180–645 days and PFS between 81–645 days, with the majority of dogs (5/7) completing treatment protocol as planned.
In this study, 62% of dogs (20) died from OMM. Of these, 4 dogs were euthanazed, because of loco-regional metastasis (2, metastasis of the ipsilateral mandibular node, diagnosed on cytology) and 2 for suspected distant metastases to the lungs (detected on radiographs). Five dogs were euthanazed, because of local recurrence associated with metastasis to the ipsilateral mandibular lymph node diagnosed on cytology. Eleven dogs were euthanazed, because of local recurrence and deterioration of their quality of life.
Four dogs (12%) died of unrelated causes. Causes of death (suspected brain metastasis, spinal mass, seizures, pathological fracture due to a bone mass and stroke) were controversial in 5 dogs (15%). The owners elected for euthanasia, but necropsy was declined in all cases. It was difficult to establish with certainty whether these patients died because of distant metastasis due to OMM, but they were considered dead of disease. There was no statistically significant difference in PFS (P=0.813), but a significant difference in ST was found for those dogs that died of melanoma-related causes versus those that died of unrelated causes (P=0.009), with dogs dying of melanoma living significantly less.
The purpose of this study was to retrospectively review the outcomes and survival in a referral population of dogs with OMM that received immunotherapy and in some cases RT as an adjunct to surgery. Limitations include, but are not limited to, the lack of a control group, stage heterogeneity and the lack of histopathology review for the assessment of prognostic factors. In addition, small numbers may have resulted in a type II error, hence influencing the final results.
One more source of bias is represented by the lack of adequate local control in 25% of cases. However, the majority of dogs included in this study (78%) were initially treated at our institution and even in the case referred at the time of local recurrence, complete excision was achieved and the patient staged negative for the presence of metastatic disease. Hence, immunotherapy seemed to be the most sensible approach after complete tumor excision, although certainly the timing of referral may have influenced the outcome in this case, potentially allowing metastatic disease to develop more rapidly. Additionally, in those cases where a second surgery was declined or incomplete margins found following revision surgery, microscopic disease was addressed by using RT and, in no cases, this treatment modality was used to treat macroscopic disease. In a preliminary study about the vaccine [3], the authors’ recommendations were to obtain a complete excision in order to achieve the best chances of a long-term survival. However, a later paper supporting the use of the vaccine [13] included 28/51 (54%) dogs with incomplete margins confirmed on histopathology, and did not find any statistically significant difference in ST between dogs with completely and incompletely excised tumors, suggesting that the vaccine could be considered in dogs with evidence of gross local disease control, as deemed able to possibly activate the host’s immune response [13]. Also, a more recent paper [21] included 16/22 dogs (nearly 73%) with incomplete or equivocal margins in the vaccinated group, although this was not correlated to ST or PFS.
Possibly, one of the interesting findings in our study is indeed the different outcomes seen in our population, compared to the paper from Grosenbaugh et al. [13]; perhaps, this represents variation on pathologist reporting of margins, aggressiveness of surgical approach between the U.S.A. and U.K., or simply more aggressive tumors in this particular population compared to that study. In addition, incomplete margins on histopathology are likely to have biased treatment choices and recommendations by different clinicians (e.g. multiple surgeries, use of RT). In our population, the completeness of surgical margins did not seem to influence the outcome: we could not find any significant difference between the two groups, and similarly no significant difference in ST or PFS, in agreement with the findings of Grosenbaugh et al. [13], although this could simply represent a type II error due to small numbers. Additionally, this finding may also reflect the fact that a significant proportion of dogs die or are euthanased because of metastatic rather than recurrent disease and completeness of margins may not influence survival in such cases. Interestingly, in the current study, within the 8 dogs that had incomplete margins found on histopathology only 4 experienced local recurrence, whereas 3 developed metastatic disease and 1 died of causes unrelated to OMM.
Overall, 7 of 32 (21%) dogs in this study did not complete the initial course of 4 vaccinations, due to local recurrence or onset of metastatic disease. Most of these dogs belonged to stages II and III, possibly reflecting a shorter PFS or a higher metastatic potential. In addition, clinical findings in these cases may have resulted in owners’ perception of poor quality of life and therefore decision to perform euthanasia. This result is in agreement with the Ottnod et al. study, where immunotherapy treatment was not completed in 5/22 vaccinated dogs [21], whereas a previous study reports that 4/58 dogs did not complete treatment course [13], further reinforcing the need for loco-regional disease control before immunotherapy is started.
Administration of the vaccine in dogs with OMM has been shown to produce a detectable humoral response 3 to 9 months after completion of a 4-dose, biweekly protocol [17]. In subsequent studies [13], it has been hypothesized that vaccination at an earlier stage would be more likely to trigger an immune response able of controlling disease progression, although the authors acknowledged that a delay in receiving vaccination was inevitable in their population. Nearly half of the dogs in our study were not able to receive early treatment with the vaccine either due to the manufacturer’s availability or to a delay in referral, and in these cases, time between surgery and vaccination may have negatively influenced the outcome of these patients. Delay in receiving vaccination was assessed by choosing a time to vaccination (<56 days and>56 days) that would have allowed similar numbers of dogs in the two groups. Delay in vaccine administration did not appear to influence ST or PFS, with no significant difference seen between dogs receiving earlier vaccination and those in which treatment was delayed. However, this result may simply reflect a type II error given the heterogeneity between groups (different WHO stage, number of treatments offered and type of treatment used) and the low statistical power of the study, other than being related to a proportion of well-differentiated tumors that may have resulted in longer ST or PFS in some cases. This treatment delay was unfortunately inevitable in our cases, given that dogs were often seen in a referral setting weeks to months after surgery was performed by the primary veterinarian (as referral is usually sought depending on owner’s decision/finances and once a histopathological diagnosis becomes available). Regardless, immunotherapy should be started as early as possible in order to activate the host’s immune response.
The RT group included only a small number of patients. The selection criteria for patients receiving RT were dependent on completeness of excision and the owner’s choice to decline any further surgery. Only 5 patients completed the full course of RT, and the decision of performing treatment was affected by other factors. This group could not be statistically assessed in comparison to those patients that received surgery and adjuvant vaccination only. It remains unclear whether treating residual disease with the addition of RT may have affected the outcome in these cases, and the role of this treatment modality as an adjunct in this setting warrants further investigation.
The overall MST of the present study was similar to the ones reported previously [7,8,9] for dogs receiving surgery and adjuvant treatment. Other authors evaluating immunotherapy [13, 20] when local control was already achieved by means of surgery, did find a variable survival time (338 and 485 days, respectively) in the vaccinated dogs. Survival for dogs with stage II (383 days) and stage III (189 days) tumors was lower than previously reported in early studies [3, 13]. This may reflect the different population of dogs and different individual immune responses; alternatively, it may be due to a type II error and poor loco-regional disease control.
There was no significant difference in PFS for patients that received immunotherapy after achieving loco-regional disease control, regardless of stage, nor in those receiving different treatment modalities. This result could be the direct consequence of small numbers and/or poor statistical power and a type II error. Overall median PFS in the present study (160 days) was similar to disease-free interval and PFS in the vaccinated dogs (171 and 199 days, respectively) previously reported [21]. However, our results may reflect an overestimation of the true time to progression (as PFS is sometimes dependent on recognition of progressive disease and/or recurrence by the owners) or simply lack of adequate tumor control.
A significant difference in survival was found for those dogs that died of melanoma-related causes versus those that died of unrelated causes (which survived longer), but the ST in our patients did not differ between WHO stages of the disease. These findings may reflect again a type II error, due to small numbers and degree of heterogeneity in treatment (confounding factors including one definitive versus multiple surgeries; differences in surgical “dose” due to the anatomical location; treatment with or without adjunctive RT) and does not mean that stage II-III OMM do not require to be treated aggressively.
In conclusion, our population of dogs with OMM treated with surgery and adjunct treatment with the xenogeneic DNA vaccination and RT achieved a MST of 335 days, with a variable outcome for each WHO stage of the disease and no statistical difference seen between groups. Disease stage did not appear to influence survival or PFS, as well as completeness of surgical margins and delay in administration of the vaccine. RT can be considered to treat residual disease when available [4, 24], although no recommendations can be made based on the number of dogs treated within our population.
Our study suffers all the limitations of a retrospective study, including a low statistical power due to small numbers and group heterogeneity, which may have influenced the final results by simply lacking the power to identify associations. In light of this, further prospective, controlled studies are required to assess whether the addition of any adjuvant treatment to surgery, including immunotherapy, is able to significantly prolong survival in cases of canine oral melanomas.
REFERENCES
- 1.Bateman K. E., Catton P. A., Pennock P. W., Kruth S. A.1994. 0-7-21 radiation therapy for the treatment of canine oral melanoma. J. Vet. Intern. Med. 8: 267–272. doi: 10.1111/j.1939-1676.1994.tb03231.x [DOI] [PubMed] [Google Scholar]
- 2.Bergman P. J., Camps-Palau M. A., McKnight J. A., Leibman N. F., Craft D. M., Leung C., Liao J., Riviere I., Sadelain M., Hohenhaus A. E., Gregor P., Houghton A. N., Perales M. A., Wolchok J. D.2006. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine 24: 4582–4585. doi: 10.1016/j.vaccine.2005.08.027 [DOI] [PubMed] [Google Scholar]
- 3.Bergman P. J., McKnight J., Novosad A., Charney S., Farrelly J., Craft D., Wulderk M., Jeffers Y., Sadelain M., Hohenhaus A. E., Segal N., Gregor P., Engelhorn M., Riviere I., Houghton A. N., Wolchok J. D.2003. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin. Cancer Res. 9: 1284–1290. [PubMed] [Google Scholar]
- 4.Blackwood L., Dobson J. M.1996. Radiotherapy of oral malignant melanomas in dogs. J. Am. Vet. Med. Assoc. 209: 98–102. [PubMed] [Google Scholar]
- 5.Boria P. A., Murry D. J., Bennett P. F., Glickman N. W., Snyder P. W., Merkel B. L., Schlittler D. L., Mutsaers A. J., Thomas R. M., Knapp D. W.2004. Evaluation of cisplatin combined with piroxicam for the treatment of oral malignant melanoma and oral squamous cell carcinoma in dogs. J. Am. Vet. Med. Assoc. 224: 388–394. doi: 10.2460/javma.2004.224.388 [DOI] [PubMed] [Google Scholar]
- 6.Bostock D. E.1979. Prognosis after surgical excision of canine melanomas. Vet. Pathol. 16: 32–40. [DOI] [PubMed] [Google Scholar]
- 7.Boston S. E., Lu X., Culp W. T., Montinaro V., Romanelli G., Dudley R. M., Liptak J. M., Mestrinho L. A., Buracco P.2014. Efficacy of systemic adjuvant therapies administered to dogs after excision of oral malignant melanomas: 151 cases (2001-2012). J. Am. Vet. Med. Assoc. 245: 401–407. doi: 10.2460/javma.245.4.401 [DOI] [PubMed] [Google Scholar]
- 8.Brockley L. K., Cooper M. A., Bennett P. F.2013. Malignant melanoma in 63 dogs (2001-2011): the effect of carboplatin chemotherapy on survival. N. Z. Vet. J. 61: 25–31. doi: 10.1080/00480169.2012.699433 [DOI] [PubMed] [Google Scholar]
- 9.Dank G., Rassnick K. M., Sokolovsky Y., Garrett L. D., Post G. S., Kitchell B. E., Sellon R. K., Kleiter M., Northrup N., Segev G.2014. Use of adjuvant carboplatin for treatment of dogs with oral malignant melanoma following surgical excision. Vet. Comp. Oncol. 12: 78–84. doi: 10.1111/j.1476-5829.2012.00338.x [DOI] [PubMed] [Google Scholar]
- 10.Freeman K. P., Hahn K. A., Harris F. D., King G. K.2003. Treatment of dogs with oral melanoma by hypofractionated radiation therapy and platinum-based chemotherapy (1987-1997). J. Vet. Intern. Med. 17: 96–101. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt M. H.1985. Benign and malignant melanocytic neoplasms of domestic animals. Am. J. Dermatopathol. 7Suppl: 203–212. doi: 10.1097/00000372-198501001-00039 [DOI] [PubMed] [Google Scholar]
- 12.Goldschmidt M. H., Dunstan R. W., Stannard A. A., Von Tschamer C., Walder E. J., Yager J. A.1998. Histological classification of epithelial and melanocytic tumors of the skin of domestic animals. World Health Organization International Histological Classification of Tumors in Domestic Animals.III. [Google Scholar]
- 13.Grosenbaugh D. A., Leard A. T., Bergman P. J., Klein M. K., Meleo K., Susaneck S., Hess P. R., Jankowski M. K., Jones P. D., Leibman N. F., Johnson M. H., Kurzman I. D., Wolchok J. D.2011. Safety and efficacy of a xenogeneic DNA vaccine encoding for human tyrosinase as adjunctive treatment for oral malignant melanoma in dogs following surgical excision of the primary tumor. Am. J. Vet. Res. 72: 1631–1638. doi: 10.2460/ajvr.72.12.1631 [DOI] [PubMed] [Google Scholar]
- 14.Hahn K. A. D. N. D., Richardson R. C., Hahn E. A.1994. Canine oral malignant melanoma: prognostic utility of an alternative staging system. J. Small Anim. Pract. 35: 251–256. doi: 10.1111/j.1748-5827.1994.tb03273.x [DOI] [Google Scholar]
- 15.Harvey H. J., MacEwen E. G., Braun D., Patnaik A. K., Withrow S. J., Jongeward S.1981. Prognostic criteria for dogs with oral melanoma. J. Am. Vet. Med. Assoc. 178: 580–582. [PubMed] [Google Scholar]
- 16.Kosovsky J. K., Matthiesen D. T., Marretta S. M., Patnaik A. K.1991. Results of partial mandibulectomy for the treatment of oral tumors in 142 dogs. Vet. Surg. 20: 397–401. doi: 10.1111/j.1532-950X.1991.tb00346.x [DOI] [PubMed] [Google Scholar]
- 17.Liao J. C., Gregor P., Wolchok J. D., Orlandi F., Craft D., Leung C., Houghton A. N., Bergman P. J.2006. Vaccination with human tyrosinase DNA induces antibody responses in dogs with advanced melanoma. Cancer Immun. 6: 8. [PMC free article] [PubMed] [Google Scholar]
- 18.MacEwen E. G., Patnaik A. K., Harvey H. J., Hayes A. A., Matus R.1986. Canine oral melanoma: comparison of surgery versus surgery plus Corynebacterium parvum. Cancer Invest. 4: 397–402. doi: 10.3109/07357908609017520 [DOI] [PubMed] [Google Scholar]
- 19.Mayayo S. L., Prestigio S., Maniscalco L., La Rosa G., Aricò A., De Maria R., Cavallo F., Ferrone S., Buracco P., Iussich S.2011. Chondroitin sulfate proteoglycan-4: a biomarker and a potential immunotherapeutic target for canine malignant melanoma. Vet. J. 190: e26–e30. doi: 10.1016/j.tvjl.2011.02.020 [DOI] [PubMed] [Google Scholar]
- 20.Murphy S., Hayes A. M., Blackwood L., Maglennon G., Pattinson H., Sparkes A. H.2005. Oral malignant melanoma - the effect of coarse fractionation radiotherapy alone or with adjuvant carboplatin therapy. Vet. Comp. Oncol. 3: 222–229. doi: 10.1111/j.1476-5810.2005.00082.x [DOI] [PubMed] [Google Scholar]
- 21.Ottnod J. M., Smedley R. C., Walshaw R., Hauptman J. G., Kiupel M., Obradovich J. E.2013. A retrospective analysis of the efficacy of Oncept vaccine for the adjunct treatment of canine oral malignant melanoma. Vet. Comp. Oncol. 11: 219–229. doi: 10.1111/vco.12057 [DOI] [PubMed] [Google Scholar]
- 22.Proulx D. R., Ruslander D. M., Dodge R. K., Hauck M. L., Williams L. E., Horn B., Price G. S., Thrall D. E.2003. A retrospective analysis of 140 dogs with oral melanoma treated with external beam radiation. Vet. Radiol. Ultrasound 44: 352–359. doi: 10.1111/j.1740-8261.2003.tb00468.x [DOI] [PubMed] [Google Scholar]
- 23.Rassnick K. M., Ruslander D. M., Cotter S. M., Al-Sarraf R., Bruyette D. S., Gamblin R. M., Meleo K. A., Moore A. S.2001. Use of carboplatin for treatment of dogs with malignant melanoma: 27 cases (1989-2000). J. Am. Vet. Med. Assoc. 218: 1444–1448. doi: 10.2460/javma.2001.218.1444 [DOI] [PubMed] [Google Scholar]
- 24.Théon A. P., Rodriguez C., Madewell B. R.1997. Analysis of prognostic factors and patterns of failure in dogs with malignant oral tumors treated with megavoltage irradiation. J. Am. Vet. Med. Assoc. 210: 778–784. [PubMed] [Google Scholar]
- 25.von Euler H., Sadeghi A., Carlsson B., Rivera P., Loskog A., Segall T., Korsgren O., Tötterman T. H.2008. Efficient adenovector CD40 ligand immunotherapy of canine malignant melanoma. J. Immunother. 31: 377–384. doi: 10.1097/CJI.0b013e31816a812d [DOI] [PubMed] [Google Scholar]
- 26.Wallace J., Matthiesen D. T., Patnaik A. K.1992. Hemimaxillectomy for the treatment of oral tumors in 69 dogs. Vet. Surg. 21: 337–341. doi: 10.1111/j.1532-950X.1992.tb01707.x [DOI] [PubMed] [Google Scholar]
- 27.Westberg S., Sadeghi A., Svensson E., Segall T., Dimopoulou M., Korsgren O., Hemminki A., Loskog A. S., Tötterman T. H., von Euler H.2013. Treatment efficacy and immune stimulation by AdCD40L gene therapy of spontaneous canine malignant melanoma. J. Immunother. 36: 350–358. doi: 10.1097/CJI.0b013e31829d8a1b [DOI] [PubMed] [Google Scholar]


