Abstract
With the rapid development of nanotechnology, metallic (metal or metal oxide) nanoparticles (NPs) are widely used in many fields such as cosmetics, the food and building industries, and bio-medical instruments. Widespread applications of metallic NP-based products increase the health risk associated with human exposures. Studies revealed that the brain, a critical organ that consumes substantial amounts of oxygen, is a primary target of metallic NPs once they are absorbed into the body. Oxidative stress (OS), apoptosis, and the inflammatory response are believed to be the main mechanisms underlying the neurotoxicity of metallic NPs. Other studies have disclosed that antioxidant pretreatment or co-treatment can reverse the neurotoxicity of metallic NPs by decreasing the level of reactive oxygen species, up-regulating the activities of antioxidant enzymes, decreasing the proportion of apoptotic cells, and suppressing the inflammatory response. These findings suggest that the neurotoxicity of metallic NPs might involve a cascade of events following NP-induced OS. However, additional research is needed to determine whether NP-induced OS plays a central role in the neurotoxicity of metallic NPs, to develop a comprehensive understanding of the correlations among neurotoxic mechanisms and to improve the bio-safety of metallic NP-based products.
Keywords: Metallic nanoparticles, Brain, Neurotoxicity, Oxidative stress, Reactive oxygen species
Review
Introduction
Metallic nanoparticles (NPs), with particle sizes ranging from 1 to 100 nm, possess superior physicochemical characteristics. This makes them useful in cosmetics [1], as food additives [2], in the biomedical industry [3], for environmental applications [4], and in the construction industry [5]. The widespread application of metallic NPs in many fields increases the risk human exposures. After exposure, NPs may be absorbed into the body and redistributed into secondary target organs. Numerous in vivo studies have revealed that, after animals were exposed to metallic NPs through intravenous injection [6], oral administration [7], intranasal instillation [8], and intraperitoneal injection [9], these particles can be absorbed and detected in many organs including the brain, liver, lung, spleen, and kidneys. The brain, as the most important organ, is vulnerable to the toxic effects induced by accumulated metallic NPs. Feng et al. [10] concluded that oxidative stress (OS), apoptosis, autophagy, the inflammatory response, and disturbed signaling pathways might be the main mechanisms underlying the neurotoxicity of metallic NPs. However, the interrelationships among those mechanisms remain obscure.
In view of the core role of OS (Fig. 1), we have summarized relevant in vivo and in vitro studies about the relationship between metallic NP-induced OS status and neurotoxicity. We conclude from available data that OS is implicated in the neurotoxicity of NPs in most situations. In addition to OS, other mechanisms are involved in the neurotoxicity of metallic NPs. Furthermore, a few rescue studies have exposed neuronal cells or animals to metallic NPs together with antioxidants. Findings from these studies show that antioxidants can reverse the neurotoxicity of metallic NPs by decreasing ROS production, up-regulating the activities of antioxidant enzymes, suppressing inflammation, and reducing the proportion of apoptotic cells. These findings suggest that the neurotoxicity of metallic NPs might involve a cascade of events following NP-induced OS. However, available data from rescue studies are insufficient to draw the definite conclusion that OS are the central mechanism of NP-induced neurotoxicity. We expect that the potential central role of OS in the neurotoxicity induced by metallic NPs might explain the complicated correlations among their neurotoxic mechanisms. However, additional rescue research is needed to determine whether OS induced by metallic NPs plays a core role in neurotoxicity.
Fig. 1.

Roles of ROS in cellular responses [104]. CDK-2 cyclin-dependent kinase 2; COX-2 cyclooxygenase-2; GSH glutathione; HSP70 70 kDa heat shock protein; IGF insulin-like growth factor; IL interleukin; NAC N-acety- l -cysteine; NF-kB necrosis factor kappa B; NOS nitric oxide synthase; ROS reactive oxygen species
Applications of Metallic NPs and Their Bio-distribution in the Brain
With the rapid development of nanotechnology, metallic NPs or NP-based products, due to their outstanding physicochemical characteristics, are widely used in many fields such as cosmetics [11–13], the food industry [14–17], building materials [18, 19], biomedical applications [20–23], painting [24–26], and decontaminants [27–29]. However, widespread applications imply that humans might be unintentionally exposed to metallic NPs. After exposure, metallic NPs can be absorbed into the body and re-distributed into the main organs, possibly leading to tissue damage. Hence, metallic NP-based products become a potential threat to human health [30–32].
The brain is the most important organ, and injury to this organ is generally irreversible. Recent in vivo studies have shown that, after animals are exposed to metallic NPs such as titanium dioxide (TiO2), zinc oxide (ZnO), iron oxide, silica dioxide (SiO2), silver (Ag), or gold, these particles can enter into the body and be translocated into the brain. The limited excretion rate out of the brain leads to a gradual accumulation of metallic NPs in this organ. This, in turn, could damage neuronal cells and impair brain function, leading to permanent brain injury.
After exposure via various routes of administration, metallic NPs are translocated into the rat/mouse brain. Wu et al. [33] demonstrated that, when hairless mice were treated with TiO2 NPs through dermal exposure for 60 days, the Ti content in their brains was increased. Similarly, the Ti level increased in the rat brain when these animals were exposed to TiO2 NPs through intravenous injection [34, 35]. Female mice administered TiO2 NPs through intranasal instillation for 30 days exhibited an increased Ti concentration in the brain [36]. After male mice were injected intravenously with ZnO NPs, Zn ions were detected in their brain [37]. Repeated oral administration of ZnO NPs led to increased Zn ion content in the rat brain [38]. Gold NPs were detected in the brain after rats/mice were treated with gold NPs through intravenous injection [6, 39] or inhalation [40]. The Ag content in the brain increased after rats were exposed to Ag NPs through subcutaneous injection [41], intravenous injection [42, 43], oral gavage [44], other oral exposure [7, 45], or intranasal instillation [8]. The Ag level in the brain increased when mice were exposed to silver NPs through intraperitoneal injection [9], repeated oral administration [46], or intravenous injection [47]. Rabbits that were treated with Ag NPs intravenously demonstrated increased Ag content in the brain [48]. TiO2 and silica NPs even passed the placental barrier to accumulate in the fetal brain when pregnant mice were exposed to TiO2 NPs [49], which suggested a potential for neurodevelopmental toxicity.
TiO2 and Ag NPs are employed frequently to examine the bio-distribution of metallic NPs after systematic administration. In order to fully illustrate how metallic NPs are absorbed into the body, translocated into the brain, and excreted from the brain, more relevant research that employs different metallic NPs besides TiO2 and Ag is needed. In addition, the potential for neurodevelopmental toxicity of metallic NPs should be investigated.
The Role of OS Induced by Metallic NPs in Neurotoxicity
Brief Description of Oxidative Stress and its Relationship with Brain Disorders
OS can be defined as disturbed redox homeostasis caused by excessive reactive oxygen species (ROS) or/and reactive nitrogen species (RNS) production, or decreased activities of antioxidant enzymes in response to harmful stimuli. Excessive ROS and RNS production can, in turn, damage DNA (determined by measuring the level of 8-hydroxy-2′-deoxyguanosine), oxidize proteins (determined by measuring the level of carbonyls), and induce lipid peroxidation, all of which can lead to tissue damage. In the process of OS, the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSH-Px), are inhibited in most situations. Meanwhile, non-enzymatic antioxidants such as vitamin C, vitamin E, and glutathione (GSH), are also depleted [50].
Lipids are abundant in brain tissue, and oxygen consumption in the brain accounts for nearly a quarter of the whole body’s consumption. Hence, the brain is more sensitive to hypoxic injury than other tissues and is vulnerable to oxidative damage. The pathology of neurodegenerative diseases [51–53] such as Alzheimer’s disease, Parkinson’s disease, and psychiatric disorders [54–56] (e.g., anxiety, autism, major depression) are closely related to the OS status in the brain. Meanwhile, environmental stimuli, such as air pollution, can induce oxidative damage in the brain, potentially leading to neurodegenerative diseases [57, 58].
OS is involved in heavy metal-induced neurotoxicity [59–61]. Metallic NPs, as another type of “environmental stimuli,” also affect the OS status in the brain. Recent studies have revealed that OS is implicated in the neurotoxicity of metallic NPs [62, 63]. In addition to oxidative stress, the inflammatory response, apoptosis, autophagy, and cell signaling pathways are the main mechanisms underlying the neurotoxicity of metallic NPs [10]. However, the correlations among these mechanisms are complex. It is possible that one mechanism plays a dominant role in the neurotoxicity of metallic NPs. In view of the pivotal role of OS in brain disorders, we have summarized relevant in vivo and in vitro published articles dealing with the correlations between metallic NP-induced OS and neurotoxicity.
In Vivo Studies About the Involvement of OS in the Neurotoxicity of Metallic NPs
TiO2 NPs impair mitochondrial functions and lead to OS in the rat and mouse brain [64, 65]. Although TiO2 NPs could not be detected in the brain zones after mice were exposed through nasal instillation, the activities of SOD, CAT, GSH-Px, and acetylcholine esterase were inhibited in the brain, probably indirectly [66]. After mice were administered TiO2 NPs orally, OS biomarkers showed differentiated responses. Although the activities of SOD and GSH-Px in the cortex and hippocampus were inhibited, levels of malondialdehyde (MDA; an index of lipid peroxidation) and ROS production remained unaffected [67]. Ze et al. [68] treated mice with three doses of TiO2 NPs nasally for 90 days and found that the levels of superoxide (O2−), H2O2, MDA, protein carbonyls, and 8-hydroxy-2′-deoxyguanosine in the mouse brain were increased in all groups compared with control animals. Furthermore, microarray analyses showed that the expression of OS-related genes in the mouse brain was also changed.
Inhalation exposure of mice to TiO2 NPs increased the brain levels of H2O2 and MDA [69]. After Meena et al. [70] injected rats with TiO2 NPs intravenously, the Ti content in the brain increased, leading to excessive ROS production and MDA, accompanied by inhibited activities of SOD and GSH-Px. In addition to OS, the proportion of apoptotic cells increased and the expression of nuclear factor-kB (NF-kB), p38, nitric oxide, interferon-γ, and tumor necrosis factor-α in the brain were elevated. Based on those findings, they concluded that TiO2 NP-induced OS in the rat brain might lead to inflammation and apoptosis, which contributed to the neurotoxicity of TiO2 NPs. Hu et al. [71] also reported that, after exposure to TiO2 NPs, the Ti content in the mouse brain increased, inducing ROS production and inhibiting antioxidant activities in the hippocampal areas, and increasing the proportion of apoptotic cells. They concluded that apoptosis was initiated by NP-induced OS in the brain. After rats were administered TiO2 and Ag NPs through a single injection, there was a change in the expression of OS-related genes in the rat brain [72]. At the same time, the antioxidant capability as well as the renin-angiotensin system in the brain was disrupted.
Ag NPs up-regulated heme oxygenase-1 (HO-1) expression at both the gene and protein levels in the hippocampus, but not in the cortex, after mice were exposed through intranasal instillation [73]. Another study using microarray analyses [74] found that, after mice were treated with Ag particles (25 nm), the expression of 18 genes in the caudate nucleus, 14 in the frontal cortex, and 29 in the hippocampal area were altered. Ag NPs (23 and 29 nm) administered by intraperitoneal injection for 7 days inhibited the activities of SOD and GSH-Px and increased MDA production in the temporal cortex of rats. In addition, the short-term memory of rats was impaired, and they performed poorly in behavioral tests [75]. These findings suggested that the expression of OS-related biomarkers in response to NPs might be regionally specific in the brain.
OS, determined by increased ROS production and decreased expression of CuZn-SOD and Mn-SOD, was induced in the mouse brain after administration of TiO2, ZnO, or Al2O3 NPs [76]. In another study, ZnO NPs inhibited the activities of SOD and GSH-Px and increased the MDA content in the mouse brain after intraperitoneal injection [77]. These effects appeared to contribute to impaired learning and memory ability.
Wu et al. [78] found that, after rats received SiO2 NPs through intranasal instillation, oxidative damage that induced an inflammatory response and disturbed neurotransmitters was observed in the striatum. They confirmed those findings in vitro by exposing rat pheochromocytoma cell line (PC12) to SiO2 NPs and showing that excessive ROS caused by the NPs was accompanied by increased apoptosis and a decreased number of cells in the G2/M phase of the cell cycle through the p53-mediated signaling pathway and reduced dopamine production. The same research group conducted another in vivo and in vitro study to examine the neurotoxicity of iron oxide NPs. They found that, after rats received iron oxide NPs through intranasal instillation, the expression of OS-related biomarkers in the brain showed region-specific changes. Although the GSH content in the striatum was increased, it remained unchanged in the hippocampus. H2O2 levels were elevated in the striatum and hippocampus, but SOD activity and MDA levels were unaffected in these areas. Findings obtained from PC12 cells exposed to iron oxide NPs were consistent with their previous research [79].
Parveen et al. [80] exposed rats to silica NPs through intranasal instillation and discovered that the silica content in the rat corpus striatum was elevated. This accumulation increased levels of H2O2, O2−, and protein carbonyls, inhibited activities of SOD, GSH-Px, and CAT, and decreased the GSH level in the rat corpus striatum. Meanwhile, the expression of genes and proteins related to apoptosis, such as bax, p53, bcl-2, and cytochrome c, was changed in the rat corpus striatum. Together, these findings indicated that silica NP-induced OS in the rat corpus striatum might lead to apoptosis, which contributed to the poor performance of animals in behavioral tests.
Although OS is clearly implicated in the neurotoxicity of metallic NPs, how these NPs regulate the OS status in the brain remains unclear. Ze et al. [81] reported that TiO2 NPs were detected in the mouse brain after intranasal instillation. This accumulation induced OS in the mouse brain that was characterized by excessive levels of H2O2, O2−, MDA, protein carbonyls, and 8-hydroxy-2′-deoxyguanosine. TiO2 NP-induced OS contributed to spongiocyte proliferation and hemorrhage in the mouse brain. Further experiments showed that the expression of p38, Jun N-terminal kinase, NF-kB, nuclear factor-2 (Nrf-2), and HO-1 was up-regulated. This suggested that oxidative impairments were probably mediated through the p38-Nrf-2 signaling pathway. Other studies revealed that OS can be mediated by Nrf-2 [82, 83]. More research is needed to investigate comprehensively how metallic NPs mediate OS in the brain.
In Vitro Studies About the Involvement of OS in the Neurotoxicity of Metallic NPs
Long et al. [84, 85] demonstrated that TiO2 NPs increased the levels of ROS, H2O2, and O2− in BV2 cells (an immortalized mouse microglial cell line). These NPs also increased ROS production in the primary astrocytes as well as induced mitochondrial dysfunction and altered mitochondrial morphology, leading to decreased cell viability [86]. Wu et al. [87] discovered that TiO2 NPs reduced the viability of PC12 cells, enhanced production of ROS and MDA, decreased GSH levels, and inhibited SOD activity. They concluded that NP-induced OS reduced the mitochondrial membrane potential, induced apoptosis, and inhibited the cell cycle. Kim et al. [88] showed that OS and DNA damage were involved in the toxic effects of silica NPs on human neuronal cells (SH-SY5Y). After Yang et al. [89] exposed SK-N-SH (human neuroblastoma cell line) and neuro2a (mouse neuroblastoma cell line) cells to silica NPs, they found that ROS production was enhanced and cell viability reduced in both cell lines. In another study, silica NPs increased the production of ROS, RNS, and IL-1β in rat primary microglial cells [90]. Similarly, mesoporous silica NPs increased the production of ROS and MDA and decreased the level of GSH-Px in PC12 cells [91].
Ag NPs increased ROS production and up-regulated the expression of OS-related genes, such as those encoding HO-1 and matrix metalloproteinases-3, in PC12 cells in a size- and dose-dependent way. Apoptosis was also observed [92]. Kim et al. [93] found that, after primary cerebral cortical neurons were exposed to Ag NPs, cell viability was reduced, ROS production was elevated, and the proportion of apoptotic cells was increased. This indicated that NP-induced OS led to cell apoptosis. Copper oxide NPs reduced the viability of primary rat brain astrocytes and enhanced ROS production [94]. Xu et al. [95] reported that copper NPs reduced the viability of PC12 cells, increased ROS production, decreased SOD activity, and enhanced the proportion of apoptotic cells. Wang et al. [96] demonstrated that PC12 cells incubated with manganese, Ag, or copper NPs exhibited alterations in the expression of dopaminergic system-associated genes. At the same time, copper NPs up-regulated the expression of thioredoxin reductase 1. Down-regulated GSH-Px expression was detected in the copper and Ag NPs groups. Manganese NPs did not change the expression of thioredoxin reductase 1 or GSH-Px. These findings indicated that the expression of OS-related biomarkers was differentiated when neuronal cells were exposed to different metallic NPs.
Iron oxide NPs increased ROS production in SH-SY5Y cells. This was accompanied by impaired mitochondrial function and an increased proportion of apoptotic cells [97]. However, ROS production was not enhanced by iron oxide NPs in oligodendroglial cell lines [98]. However, it is not possible to conclude that OS was not induced because other OS-related biomarkers were not assessed.
Gold NPs induced ROS in the C17.2 neural progenitor cell line in a dose-dependent manner [99]. Although gold NPs did not induce cytotoxicity in human astrocytes, they increased ROS production, up-regulated the activity of NF-kB, and reduced micronuclei formation [100]. After Sruthi et al. [101] treated C6 cells (a rat glial cell line) with ZnO NPs for 3 and 6 h, ROS production was enhanced. However, after a 24-h exposure, the ROS levels in ZnO NP-treated cells decreased to the control group level. Additional studies examining other OS-related biomarkers, such as SOD and GSH-Px, are needed to further assess the NP-induced OS status in this system. Recently, zirconium oxide NPs were reported to reduce cell viability, enhance the production of ROS and MDA, reduce GSH levels, and induce genotoxic effects in the PC12 and N2a cell lines [102].
Huerta-Garcia et al. [103] found that TiO2 NPs-induced changes in ROS production and the activities of antioxidant enzymes in C6 and U373 (human glial cell) cells were not always consistent. Thus, TiO2 NP-induced OS is complicated and may be associated with exposure time. Future research should focus on the correlation between exposure time and metallic NP-induced OS.
Findings from the abovementioned studies suggested that OS was involved in the neurotoxicity of NPs in most situations. Although numerous studies have shown that OS can increase apoptosis (Fig. 2) [104], activate signaling pathways [105] (Fig. 3), affect cell cycling (Fig. 4) [106], and induce inflammation (Fig. 5) [107], it is still not possible to draw the definite conclusion that the cellular responses involved in the neurotoxicity of metallic NPs are mediated by NP-induced OS.
Fig. 2.

Schematic representation of apoptosis signals induced by ROS [104]. AIF apoptosis-inducing factor; Apaf-1 apoptotic protease activating factor 1; DISC death-inducing signaling complex; ROS reactive oxygen species; TRAIL tumor necrosis factor-alpha-related apoptosis-inducing ligand
Fig. 3.
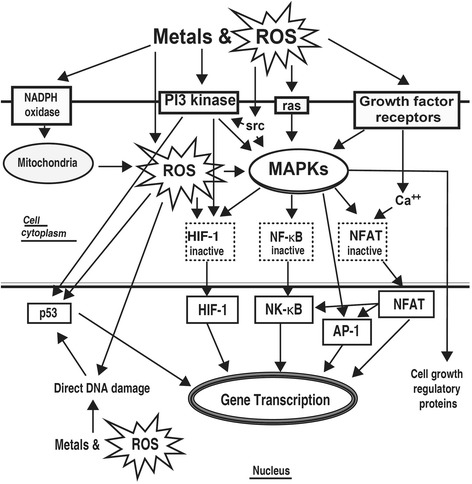
Signaling pathways activated by ROS [127]. ROS reactive oxygen species; NADPH reduced nicotine adenine dinucleotide phosphate; MAPK mitogen-activated protein kinase; HIF-1 hypoxia-inducible factor 1; NF-kB necrosis factor kappa B; NFAT nuclear factor of activated T cells; AP-1 activator protein-1
Fig. 4.

The effects of ROS on cell cycle regulation [106]. EGFR epidermal growth factor receptor; EGF epidermal growth factor; ROS reactive oxygen species
Fig. 5.

Inflammatory response mediated by OS [107]. DAMPs damage-associated molecular pattern molecules; TLR toll-like receptor
Rescue Studies About the Involvement of OS in the Neurotoxicity of Metallic NPs
Rescue studies examining the role of OS in the neurotoxicity of NPs might help determine whether the neurotoxicity of metallic NPs involves a cascade of events following NP-induced OS. N-acetyl-l-cysteine (NAC) exhibited both antioxidant and neuroprotective capabilities and decreased the production of ROS induced by ZnO NPs in rat primary astrocytes [108–110]. At the same time, the Jun N-terminal kinase signaling pathway was suppressed, mitochondrial impairment was relieved, and the proportion of apoptotic cells was decreased in cells pretreated with NAC 6 h before NP exposure. In another study [111], NAC reversed the elevated proportion of apoptotic cells induced by ZnO NPs in U87 human glial cells. These findings suggested that NP-mediated OS activated cell signaling pathways, mitochondrial injury, and apoptosis. ZnO NPs reduced cell viability, enhanced ROS production, increased the glutathione disulfide level, inhibited GSH-Px activity, and increased apoptosis in SH-SY5Y cells. Those toxic effects were reversed by pretreating cells with NAC or esculetin [112]. Esculetin possesses antioxidant properties [113–115].
Chlorophyllin is an effective ROS scavenger. It also possesses antioxidant properties [116–118]. DNA damage, determined by the comet assay, was detected in the mouse brain after mice were exposed to TiO2 NPs. This damage was prevented by co-treatment with chlorophyllin [119]. This indicated that NP-induced OS can lead to DNA damage. Niska et al. [120] found that, after HT22 cells were incubated with copper NPs, cell viability was reduced, the activities of GSH-Px and SOD were inhibited, ROS production was increased, and the proportion of apoptotic cells was elevated. However, pretreating cells with crocetin (an antioxidant with neuroprotective capabilities that can counteract OS [121–123]) 1 h before NP exposure prevented those changes. Pretreating PC12 cells with N-(mercaptopropionyl)-glycine (another type of ROS scavenger [124]) inhibited the apoptosis induced by TiO2 [125] and ZnO NPs [126]. The reduced cell viability caused by TiO2 NPs was also ameliorated [125]. These findings suggested that NP-induced OS can lead to cell apoptosis.
Brief Summary
The findings described in this review support the conclusion that OS is involved in the neurotoxicity of metallic NPs. However, the NP-induced OS status was mainly assessed by measuring ROS production and the activities of antioxidant enzymes. Including measurements of RNS production and levels of non-enzymatic antioxidants would provide an improved basis for assessing the NP-induced OS status comprehensively.
Other studies reviewed here implicate apoptosis, inflammation, and cell cycle arrest in the neurotoxicity of metallic NPs. Findings from a few rescue studies suggest that pretreatment or co-treatment with antioxidants can inhibit the inflammatory response, reduce the proportion of apoptotic cells, and reverse NP-induced neurotoxicity. These findings indicate that NP-induced OS might be a central mechanism underlying the neurotoxicity of metallic NPs. However, more rescue research studies are needed to understand the core role of OS in the neurotoxicity of metallic NPs.
Conclusions
With the widespread application of metallic NP-based products, the toxic effects induced by these particles have become a significant threat to brain health. Relevant studies have revealed that OS and other mechanisms, such as apoptosis and the inflammatory response, are involved in the neurotoxicity of metallic NPs. However, correlations among these mechanisms are unclear and do not fully support causality. In view of the purported central role of OS, a few recent rescue studies pretreating neuronal cells or co-treating animals, with antioxidants suggest that the neurotoxicity of metallic NPs might involve a cascade of events triggered by OS.
Based on the potentially pivotal role of OS in the neurotoxicity of metallic NPs, here are some suggestions for future research:
The bio-distribution of different metallic NPs should be investigated comprehensively
More rescue research is needed to ascertain the core role of OS
The mechanisms by which metallic NPs trigger OS and how NP-induced OS mediates other mechanisms of neurotoxicity should be studied in detail
The correlation among OS status, OS-related biomarkers, and biological effects induced by NPs should be explored
NP-induced OS status should be investigated in more detail by assessing multiple biomarkers such as the production of both ROS and RNS, activities of antioxidant enzymes, and the levels of non-enzymatic antioxidants
Because OS-related biomarkers are probably region-specific in the brain, it is inappropriate to measure biomarkers in whole brain tissue
Additional comparisons about the OS status in the brain induced by different metallic NPs are needed
Changing the physicochemical property of metallic NPs to inhibit NP-induced OS should be investigated as a feasible means for reducing their neurotoxicity
Overall, we expect that in-depth investigations of the central role of OS in metallic NP-induced neurotoxicity will help define how best to prevent this toxicity and can help us unravel the complicated correlations among neurotoxic mechanisms of metallic NPs.
Acknowledgements
This work was supported by the Science and Technology Foundation of the Health and Family Planning Commission of Guizhou province, China (gzwjkj2015-1-026), the National Natural Science Foundation of China (81550011), and the Natural Science Foundation of Guangdong Province of China (2015A030313299).
Footnotes
Competing Interests
The authors declare that they have no competing interests.
Authors’ Contributions
BS collected and reviewed the data and drafted the manuscript. All authors helped in drafting the first version of the manuscript and in revisions. All authors read and approved the final manuscript.
Contributor Information
Bin Song, Email: 17055224@qq.com.
YanLi Zhang, Email: 541292702@qq.com.
Jia Liu, Email: liujia1988dr@163.com.
XiaoLi Feng, Email: 867770038@qq.com.
Ting Zhou, Email: zhouting187@126.com.
LongQuan Shao, Phone: +86-20-15989283921, Email: shaolongquan@smu.edu.cn.
References
- 1.Wiechers JW, Musee N. Engineered inorganic nanoparticles and cosmetics: facts, issues, knowledge gaps and challenges. J Biomed Nanotechnol. 2010;6(5):408–431. doi: 10.1166/jbn.2010.1143. [DOI] [PubMed] [Google Scholar]
- 2.Sanguansri P, Augustin MA. Nanoscale materials development—a food industry perspective. Trends Food Sci Technol. 2006;17(10):547–556. doi: 10.1016/j.tifs.2006.04.010. [DOI] [Google Scholar]
- 3.Singh R, Nalwa HS. Medical applications of nanoparticles in biological imaging, cell labeling, antimicrobial agents, and anticancer nanodrugs. J Biomed Nanotechnol. 2011;7(4):489–503. doi: 10.1166/jbn.2011.1324. [DOI] [PubMed] [Google Scholar]
- 4.Li QL, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D, et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. 2008;42(18):4591–4602. doi: 10.1016/j.watres.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Lee J, Mahendra S, Alvarez PJJ. Nanomaterials in the construction industry: a review of their applications and environmental health and safety considerations. ACS Nano. 2010;4(7):3580–3590. doi: 10.1021/nn100866w. [DOI] [PubMed] [Google Scholar]
- 6.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips A, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29(12):1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 7.van der Zande M, Vandebriel RJ, Van Doren E, Kramer E, Rivera ZH, Serrano-Rojero CS, et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano. 2012;6(8):7427–7442. doi: 10.1021/nn302649p. [DOI] [PubMed] [Google Scholar]
- 8.Wen RX, Yang XX, Hu LG, Sun C, Zhou QF, Jiang GB. Brain-targeted distribution and high retention of silver by chronic intranasal instillation of silver nanoparticles and ions in Sprague-Dawley rats. J Appl Toxicol. 2016;36(3):445–453. doi: 10.1002/jat.3260. [DOI] [PubMed] [Google Scholar]
- 9.Daniel S, Tharmaraj V, Sironmani TA, Pitchumani K. Toxicity and immunological activity of silver nanoparticles. Appl Clay Sci. 2010;48(4):547–551. doi: 10.1016/j.clay.2010.03.001. [DOI] [Google Scholar]
- 10.Feng XL, Chen AJ, Zhang YL, Wang JF, Shao LQ, Wei LM. Central nervous system toxicity of metallic nanoparticles. Int J Nanomed. 2015;10:4321–4340. doi: 10.2147/IJN.S78308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu PJ, Huang SC, Chen YP, Chiueh LC, Shih DYC. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J Food Drug Anal. 2015;23(3):587–594. doi: 10.1016/j.jfda.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melo A, Amadeu MS, Lancellotti M, de Hollanda LM, Machado D. The role of nanomaterials in cosmetics: national and international legislative aspects. Quim Nova. 2015;38(4):599–603. [Google Scholar]
- 13.Kapuscinska A, Igielska-Kalwat J, Goscianska J, Nowak I. Use of metal nanoparticles in cosmetics. Przemysl Chemiczny. 2015;94(4):566–570. [Google Scholar]
- 14.Hannon JC, Kerry J, Cruz-Romero M, Morris M, Cummins E. Advances and challenges for the use of engineered nanoparticles in food contact marterials. Trends Food Sci Technol. 2015;43(1):43–62. doi: 10.1016/j.tifs.2015.01.008. [DOI] [Google Scholar]
- 15.Bumbudsanpharoke N, Choi J, Ko S. Applications of nanomaterials in food packaging. J Nanosci Nanotechnol. 2015;15(9):6357–6372. doi: 10.1166/jnn.2015.10847. [DOI] [PubMed] [Google Scholar]
- 16.Martirosyan A, Schneider YJ. Engineered nanomaterials in food: implications for food safety and consumer health. Int J Environ Res Public Health. 2014;11(6):5720–5750. doi: 10.3390/ijerph110605720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yada RY, Buck N, Canady R, DeMerlis C, Duncan T, Janer G, et al. Engineered nanoscale food ingredients: evaluation of current knowledge on material characteristics relevant to uptake from the gastrointestinal tract. Compr Rev Food Sci Food Saf. 2014;13(4):730–744. doi: 10.1111/1541-4337.12076. [DOI] [PubMed] [Google Scholar]
- 18.Shandilya N, Le Bihan O, Bressot C, Morgeneyer M. Emission of titanium dioxide nanoparticles from building materials to the environment by wear and weather. Environ Sci Technol. 2015;49(4):2163–2170. doi: 10.1021/es504710p. [DOI] [PubMed] [Google Scholar]
- 19.Sang LX, Zhao YX, Burda C. TiO2 nanoparticles as functional building blocks. Chem Rev. 2014;114(19):9283–9318. doi: 10.1021/cr400629p. [DOI] [PubMed] [Google Scholar]
- 20.Wu SL, Weng ZY, Liu XM, Yeung KWK, Chu PK. Functionalized TiO2 based nanomaterials for biomedical applications. Adv Funct Mater. 2014;24(35):5464–5481. doi: 10.1002/adfm.201400706. [DOI] [Google Scholar]
- 21.Sasidharan A, Monteiro-Riviere NA. Biomedical applications of gold nanomaterials: opportunities and challenges. Wiley Interdisciplinary Reviews-Nanomedicine Nanobiotechnology. 2015;7(6):779–796. doi: 10.1002/wnan.1341. [DOI] [PubMed] [Google Scholar]
- 22.Haider A, Kang IK. Preparation of silver nanoparticles and their industrial and biomedical applications: a comprehensive review. Advances in materials science and engineering. 2015. [Google Scholar]
- 23.Wu W, Wu ZH, Yu T, Jiang CZ, Kim WS. Recent progress on magnetic iron oxide nanoparticles: synthesis, surface functional strategies and biomedical applications. Sci Technol Adv Mater. 2015;16(2). [DOI] [PMC free article] [PubMed]
- 24.Zuin S, Massari A, Ferrari A, Golanski L. Formulation effects on the release of silica dioxide nanoparticles from paint debris to water. Sci Total Environ. 2014;476:298–307. doi: 10.1016/j.scitotenv.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Hincapie I, Caballero-Guzman A, Hiltbrunner D, Nowack B. Use of engineered nanomaterials in the construction industry with specific emphasis on paints and their flows in construction and demolition waste in Switzerland. Waste Manag. 2015;43:398–406. doi: 10.1016/j.wasman.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 26.El-Feky OM, Hassan EA, Fadel SM, Hassan ML. Use of ZnO nanoparticles for protecting oil paintings on paper support against dirt, fungal attack, and UV aging. J Cult Herit. 2014;15(2):165–172. doi: 10.1016/j.culher.2013.01.012. [DOI] [Google Scholar]
- 27.Prasad GK, Ramacharyulu P, Singh B. Nanomaterials based decontaminants against chemical warfare agents. J Sci Ind Res. 2011;70(2):91–104. [Google Scholar]
- 28.Viswanathan S, Manisankar P. Nanomaterials for electrochemical sensing and decontamination of pesticides. J Nanosci Nanotechnol. 2015;15(9):6914–6923. doi: 10.1166/jnn.2015.10724. [DOI] [PubMed] [Google Scholar]
- 29.Sundarrajan S, Chandrasekaran AR, Ramakrishna S. An update on nanomaterials-based textiles for protection and decontamination. J Am Ceram Soc. 2010;93(12):3955–3975. doi: 10.1111/j.1551-2916.2010.04117.x. [DOI] [Google Scholar]
- 30.McCall MJ. Environmental, health and safety issues nanoparticles in the real world. Nat Nanotechnol. 2011;6(10):613–614. doi: 10.1038/nnano.2011.169. [DOI] [PubMed] [Google Scholar]
- 31.Conti JA, Killpack K, Gerritzen G, Huang L, Mircheva M, Delmas M, et al. Health and safety practices in the nanomaterials workplace: results from an international survey. Environ Sci Technol. 2008;42(9):3155–3162. doi: 10.1021/es702158q. [DOI] [PubMed] [Google Scholar]
- 32.Papp T, Schiffmann D, Weiss D, Castranova V, Vallyathan V, Rahman Q. Human health implications of nanomaterial exposure. Nanotoxicology. 2008;2(1):9–27. doi: 10.1080/17435390701847935. [DOI] [Google Scholar]
- 33.Wu JH, Liu W, Xue CB, Zhou SC, Lan FL, Bi L, et al. Toxicity and penetration of TiO2 nanoparticles in hairless mice and porcine skin after subchronic dermal exposure. Toxicol Lett. 2009;191(1):1–8. doi: 10.1016/j.toxlet.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Geraets L, Oomen AG, Krystek P, Jacobsen NR, Wallin H, Laurentie M, et al. Tissue distribution and elimination after oral and intravenous administration of different titanium dioxide nanoparticles in rats. Part Fibre Toxicol. 2014;11:1. doi: 10.1186/1743-8977-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Disdier C, Devoy J, Cosnefroy A, Chalansonnet M, Herlin-Boime N, Brun E, et al. Tissue biodistribution of intravenously administrated titanium dioxide nanoparticles revealed blood-brain barrier clearance and brain inflammation in rat. Part Fibre Toxicol. 2015;12:27. doi: 10.1186/s12989-015-0102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Li Y, Li W, Chen C, Li B, Zhao Y. Biological effect of intranasally instilled titanium dioxide nanoparticles on female mice. Nano. 2008;3(4):279–285. doi: 10.1142/S1793292008001325. [DOI] [Google Scholar]
- 37.Yeh TK, Chen JK, Lin CH, Yang MH, Yang CS, Chou FI, et al. Kinetics and tissue distribution of neutron-activated zinc oxide nanoparticles and zinc nitrate in mice: effects of size and particulate nature. Nanotechnology. 2012;23(8):085102. doi: 10.1088/0957-4484/23/8/085102. [DOI] [PubMed] [Google Scholar]
- 38.Cho WS, Kang BC, Lee JK, Jeong J, Che JH, Seok SH. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part Fibre Toxicol. 2013;10:9. doi: 10.1186/1743-8977-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonavane G, Tomoda K, Makino K. Biodistribution of colloidal gold nanoparticles after intravenous administration: effect of particle size. Colloid Surf B-Biointerfaces. 2008;66(2):274–280. doi: 10.1016/j.colsurfb.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Balasubramanian SK, Poh KW, Ong CN, Kreyling WG, Ong WY, Yu LE. The effect of primary particle size on biodistribution of inhaled gold nano-agglomerates. Biomaterials. 2013;34(22):5439–5452. doi: 10.1016/j.biomaterials.2013.03.080. [DOI] [PubMed] [Google Scholar]
- 41.Tang JL, Xiong L, Wang S, Wang JY, Liu L, Li JG, et al. Distribution, translocation and accumulation of silver nanoparticles in rats. J Nanosci Nanotechnol. 2009;9(8):4924–4932. doi: 10.1166/jnn.2009.1269. [DOI] [PubMed] [Google Scholar]
- 42.Lankveld DPK, Oomen AG, Krystek P, Neigh A, Troost-de Jong A, Noorlander CW, et al. The kinetics of the tissue distribution of silver nanoparticles of different sizes. Biomaterials. 2010;31(32):8350–8361. doi: 10.1016/j.biomaterials.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 43.Dziendzikowska K, Gromadzka-Ostrowska J, Lankoff A, Oczkowski M, Krawczynska A, Chwastowska J, et al. Time-dependent biodistribution and excretion of silver nanoparticles in male Wistar rats. J Appl Toxicol. 2012;32(11):920–928. doi: 10.1002/jat.2758. [DOI] [PubMed] [Google Scholar]
- 44.Loeschner K, Hadrup N, Qvortrup K, Larsen A, Gao XY, Vogel U, et al. Distribution of silver in rats following 28 days of repeated oral exposure to silver nanoparticles or silver acetate. Part Fibre Toxicol. 2011;8:18. doi: 10.1186/1743-8977-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espinosa-Cristobal LF, Martinez-Castanon GA, Loyola-Rodriguez JP, Patino-Marin N, Reyes-Macias JF, Vargas-Morales JM, et al. Toxicity, distribution, and accumulation of silver nanoparticles in Wistar rats. J Nanopart Res. 2013;15(6):1–12. doi: 10.1007/s11051-013-1702-6. [DOI] [Google Scholar]
- 46.Park EJ, Bae E, Yi J, Kim Y, Choi K, Lee SH, et al. Repeated-dose toxicity and inflammatory responses in mice by oral administration of silver nanoparticles. Environ Toxicol Pharmacol. 2010;30(2):162–168. doi: 10.1016/j.etap.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 47.Chen R, Zhao L, Bai R, Liu Y, Han LP, Xu ZF, et al. Silver nanoparticles induced oxidative and endoplasmic reticulum stresses in mouse tissues: implications for the development of acute toxicity after intravenous administration. Toxicol Res. 2016;5(2):602–608. doi: 10.1039/C5TX00464K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee Y, Kim P, Yoon J, Lee B, Choi K, Kil KH, et al. Serum kinetics, distribution and excretion of silver in rabbits following 28 days after a single intravenous injection of silver nanoparticles. Nanotoxicology. 2013;7(6):1120–1130. doi: 10.3109/17435390.2012.710660. [DOI] [PubMed] [Google Scholar]
- 49.Yamashita K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, Nozaki M, et al. Silica and titanium dioxide nanoparticles cause pregnancy complications in mice. Nat Nanotechnol. 2011;6(5):321–328. doi: 10.1038/nnano.2011.41. [DOI] [PubMed] [Google Scholar]
- 50.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8(9-10):1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 51.Emerit J, Edeas A, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomedicine & Pharmacotherapy. 2004;58(1):39–46. doi: 10.1016/j.biopha.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 52.Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 2006;97(6):1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- 53.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 54.Mhillaj E, Morgese MG, Trabace L. Early life and oxidative stress in psychiatric disorders: what can we learn from animal models? Curr Pharm Des. 2015;21(11):1396–1403. doi: 10.2174/1381612821666150105122422. [DOI] [PubMed] [Google Scholar]
- 55.Smaga I, Niedzielska E, Gawlik M, Moniczewski A, Krzek J, Przegalinski E, et al. Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacol Rep. 2015;67(3):569–580. doi: 10.1016/j.pharep.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 56.Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 57.Migliore L, Coppede F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res Genet Toxicol Environ Mutagen. 2009;674(1-2):73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 58.Farina M, Avila DS, da Rocha JBT, Aschner M. Metals, oxidative stress and neurodegeneration: a focus on iron, manganese and mercury. Neurochem Int. 2013;62(5):575–594. doi: 10.1016/j.neuint.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jomova K, Jenisova Z, Feszterova M, Baros S, Liska J, Hudecova D, et al. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31(2):95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 60.Flora SJS, Mittal M, Mehta A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res. 2008;128(4):501–523. [PubMed] [Google Scholar]
- 61.Farina M, Aschner M, Rocha JBT. Oxidative stress in MeHg-induced neurotoxicity. Toxicol Appl Pharmacol. 2011;256(3):405–417. doi: 10.1016/j.taap.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Win-Shwe TT, Fujimaki H. Nanoparticles and neurotoxicity. Int J Mol Sci. 2011;12(9):6267–6280. doi: 10.3390/ijms12096267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karmakar A, Zhang QL, Zhang YB. Neurotoxicity of nanoscale materials. J Food Drug Anal. 2014;22(1):147–160. doi: 10.1016/j.jfda.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nalika N, Parvez S. Mitochondrial dysfunction in titanium dioxide nanoparticle-induced neurotoxicity. Toxicol Mech Methods. 2015;25(5):355–363. doi: 10.3109/15376516.2015.1020183. [DOI] [PubMed] [Google Scholar]
- 65.Wang JX, Chen CY, Liu Y, Jiao F, Li W, Lao F, et al. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol Lett. 2008;183(1-3):72–80. doi: 10.1016/j.toxlet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Jeon YM, Park SK, Lee MY. Toxicoproteomic identification of TiO2 nanoparticle-induced protein expression changes in mouse brain. Anim Cells Syst. 2011;15(2):107–114. doi: 10.1080/19768354.2011.555144. [DOI] [Google Scholar]
- 67.Zhang R, Niu YJ, Li YW, Zhao CF, Song B, Li Y, et al. Acute toxicity study of the interaction between titanium dioxide nanoparticles and lead acetate in mice. Environ Toxicol Pharmacol. 2010;30(1):52–60. doi: 10.1016/j.etap.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 68.Ze Y, Hu R, Wang X, Sang X, Ze X, Li B, et al. Neurotoxicity and gene-expressed profile in brain-injured mice caused by exposure to titanium dioxide nanoparticles. J Biomed Mater Res Part A. 2014;102(2):470–478. doi: 10.1002/jbm.a.34705. [DOI] [PubMed] [Google Scholar]
- 69.Yin JL, Kang C, Li YF, Li QN, Zhang XY, Li WX. Aerosol inhalation exposure study of respiratory toxicity induced by 20 nm anatase titanium dioxide nanoparticles. Toxicol Res. 2014;3(5):367–374. doi: 10.1039/C4TX00040D. [DOI] [Google Scholar]
- 70.Meena R, Kumar S, Paulraj R. Titanium oxide (TiO2) nanoparticles in induction of apoptosis and inflammatory response in brain. J Nanopart Res. 2015;17(1):14. doi: 10.1007/s11051-015-2868-x. [DOI] [Google Scholar]
- 71.Hu R, Zheng L, Zhang T, Gao G, Cui Y, Cheng Z, et al. Molecular mechanism of hippocampal apoptosis of mice following exposure to titanium dioxide nanoparticles. J Hazard Mater. 2011;191(1-3):32–40. doi: 10.1016/j.jhazmat.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 72.Krawczynska A, Dziendzikowska K, Gromadzka-Ostrowska J, Lankoff A, Herman AP, Oczkowski M, et al. Silver and titanium dioxide nanoparticles alter oxidative/inflammatory response and renin-angiotensin system in brain. Food Chem Toxicol. 2015;85:96–105. doi: 10.1016/j.fct.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 73.Davenport LL, Hsieh H, Eppert BL, Carreira VS, Krishan M, Ingle T, et al. Systemic and behavioral effects of intranasal administration of silver nanoparticles. Neurotoxicol Teratol. 2015;51:68–76. doi: 10.1016/j.ntt.2015.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rahman MF, Wang J, Patterson TA, Saini UT, Robinson BL, Newport GD, et al. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol Lett. 2009;187(1):15–21. doi: 10.1016/j.toxlet.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 75.Hritcu L, Stefan M, Ursu L, Neagu A, Mihasan M, Tartau L, et al. Exposure to silver nanoparticles induces oxidative stress and memory deficits in laboratory rats. Cen Euro J Biol. 2011;6(4):497–509. [Google Scholar]
- 76.Shrivastava R, Raza S, Yadav A, Kushwaha P, Flora SJS. Effects of sub-acute exposure to TiO2, ZnO and Al2O3 nanoparticles on oxidative stress and histological changes in mouse liver and brain. Drug Chem Toxicol. 2014;37(3):336–347. doi: 10.3109/01480545.2013.866134. [DOI] [PubMed] [Google Scholar]
- 77.Tian L, Lin BC, Wu L, Li K, Liu HL, Yan J, et al. Neurotoxicity induced by zinc oxide nanoparticles: age-related differences and interaction. Sci Rep. 2015;5:12. doi: 10.1038/srep16117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu J, Wang C, Sun J, Xue Y. Neurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathways. ACS Nano. 2011;5(6):4476–4489. doi: 10.1021/nn103530b. [DOI] [PubMed] [Google Scholar]
- 79.Wu J, Ding TT, Sun J. Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus. Neurotoxicology. 2013;34:243–253. doi: 10.1016/j.neuro.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 80.Parveen A, Rizvi SHM, Mahdi F, Tripathi S, Ahmad I, Shukla RK, et al. Silica nanoparticles mediated neuronal cell death in corpus striatum of rat brain: implication of mitochondrial, endoplasmic reticulum and oxidative stress. J Nanopart Res. 2014;16(11):15. doi: 10.1007/s11051-014-2664-z. [DOI] [Google Scholar]
- 81.Ze Y, Zheng L, Zhao X, Gui S, Sang X, Su J, et al. Molecular mechanism of titanium dioxide nanoparticles-induced oxidative injury in the brain of mice. Chemosphere. 2013;92(9):1183–1189. doi: 10.1016/j.chemosphere.2013.01.094. [DOI] [PubMed] [Google Scholar]
- 82.Kumar KJS, Chu FH, Hsieh HW, Liao JW, Li WH, Lin JCC, et al. Antroquinonol from ethanolic extract of mycelium of Antrodia cinnamomea protects hepatic cells from ethanol-induced oxidative stress through Nrf-2 activation. J Ethnopharmacol. 2011;136(1):168–177. doi: 10.1016/j.jep.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 83.Jian Z, Li K, Liu L, Zhang Y, Zhou Z, Li CY, et al. Heme oxygenase-1 protects human melanocytes from h2o2-induced oxidative stress via the Nrf(2)-ARE pathway. J Investig Dermatol. 2011;131(7):1420–1427. doi: 10.1038/jid.2011.56. [DOI] [PubMed] [Google Scholar]
- 84.Long TC, Saleh N, Tilton RD, Lowry GV, Veronesi B. Titanium dioxide (P25) produces reactive oxygen species in immortalized brain microglia (BV2): implications for nanoparticle neurotoxicity. Environ Sci Technol. 2006;40(14):4346–4352. doi: 10.1021/es060589n. [DOI] [PubMed] [Google Scholar]
- 85.Long TC, Tajuba J, Sama P, Saleh N, Swartz C, Parker J, et al. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect. 2007;115(11):1631–1637. doi: 10.1289/ehp.10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wilson CL, Natarajan V, Hayward SL, Khalimonchuk O, Kidambi S. Mitochondrial dysfunction and loss of glutamate uptake in primary astrocytes exposed to titanium dioxide nanoparticles. Nanoscale. 2015;7(44):18477–18488. doi: 10.1039/C5NR03646A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu J, Sun JA, Xue Y. Involvement of JNK and P53 activation in G2/M cell cycle arrest and apoptosis induced by titanium dioxide nanoparticles in neuron cells. Toxicol Lett. 2010;199(3):269–276. doi: 10.1016/j.toxlet.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 88.Kim YJ, Yu M, Park HO, Yang SI. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by silica nanomaterials in human neuronal cell line. Mol Cell Toxicol. 2010;6(4):337–344. [Google Scholar]
- 89.Yang XF, He CE, Li J, Chen HB, Ma Q, Sui XJ, et al. Uptake of silica nanoparticles: neurotoxicity and Alzheimer-like pathology in human SK-N-SH and mouse neuro2a neuroblastoma cells. Toxicol Lett. 2014;229(1):240–249. doi: 10.1016/j.toxlet.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Choi J, Zheng QD, Katz HE, Guilarte TR. Silica-based nanoparticle uptake and cellular response by primary microglia. Environ Health Perspect. 2010;118(5):589–595. doi: 10.1289/ehp.0901534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou M, Xie LL, Fang CJ, Yang H, Wang YJ, Zhen XY, et al. Implications for blood-brain-barrier permeability, in vitro oxidative stress and neurotoxicity potential induced by mesoporous silica nanoparticles: effects of surface modification. RSC Adv. 2016;6(4):2800–2809. doi: 10.1039/C5RA17517H. [DOI] [Google Scholar]
- 92.Kim TH, Kim M, Park HS, Shin US, Gong MS, Kim HW. Size-dependent cellular toxicity of silver nanoparticles. J Biomed Mater Res Part A. 2012;100A(4):1033–1043. doi: 10.1002/jbm.a.34053. [DOI] [PubMed] [Google Scholar]
- 93.Kim SH, Ko JW, Koh SK, Lee IC, Son JM, Moon C, et al. Silver nanoparticles induce apoptotic cell death in cultured cerebral cortical neurons. Mol Cell Toxicol. 2014;10(2):173–179. doi: 10.1007/s13273-014-0019-6. [DOI] [Google Scholar]
- 94.Bulcke F, Thiel K, Dringen R. Uptake and toxicity of copper oxide nanoparticles in cultured primary brain astrocytes. Nanotoxicology. 2014;8(7):775–785. doi: 10.3109/17435390.2013.829591. [DOI] [PubMed] [Google Scholar]
- 95.Xu PJ, Xu J, Liu SC, Ren GG, Yang Z. In vitro toxicity of nanosized copper particles in PC12 cells induced by oxidative stress. J Nanopart Res. 2012;14(6):9. doi: 10.1007/s11051-012-0906-5. [DOI] [Google Scholar]
- 96.Wang JY, Rahman MF, Duhart HM, Newport GD, Patterson TA, Murdock RC, et al. Expression changes of dopaminergic system-related genes in PC12 cells induced by manganese, silver, or copper nanoparticles. Neurotoxicology. 2009;30(6):926–933. doi: 10.1016/j.neuro.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 97.Imam SZ, Lantz-McPeak SM, Cuevas E, Rosas-Hernandez H, Liachenko S, Zhang YB, et al. Iron oxide nanoparticles induce dopaminergic damage: in vitro pathways and in vivo imaging reveals mechanism of neuronal damage. Mol Neurobiol. 2015;52(2):913–926. doi: 10.1007/s12035-015-9259-2. [DOI] [PubMed] [Google Scholar]
- 98.Hohnholt MC, Geppert M, Dringen R. Treatment with iron oxide nanoparticles induces ferritin synthesis but not oxidative stress in oligodendroglial cells. Acta Biomater. 2011;7(11):3946–3954. doi: 10.1016/j.actbio.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 99.Soenen SJ, Manshian B, Montenegro JM, Amin F, Meermann B, Thiron T, et al. Cytotoxic effects of gold nanoparticles: a multiparametric study. ACS Nano. 2012;6(7):5767–5783. doi: 10.1021/nn301714n. [DOI] [PubMed] [Google Scholar]
- 100.Mytych J, Lewinska A, Zebrowski J, Wnuk M. Gold nanoparticles promote oxidant-mediated activation of NF-kappa B and 53BP1 recruitment-based adaptive response in human astrocytes. Biomed Res Int. 2015:9. [DOI] [PMC free article] [PubMed]
- 101.Sruthi S, Mohanan PV. Investigation on cellular interactions of astrocytes with zinc oxide nanoparticles using rat C6 cell lines. Colloid Surf B-Biointerfaces. 2015;133:1–11. doi: 10.1016/j.colsurfb.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 102.Asadpour E, Sadeghnia HR, Ghorbani A, Sedaghat M, Boroushaki MT. Oxidative stress-mediated cytotoxicity of zirconia nanoparticles on PC12 and N2a cells. J Nanopart Res. 2016;18(1):13. doi: 10.1007/s11051-015-3316-7. [DOI] [Google Scholar]
- 103.Huerta-Garcia E, Perez-Arizti JA, Marquez-Ramirez SG, Delgado-Buenrostro NL, Chirino YI, Iglesias GG, et al. Titanium dioxide nanoparticles induce strong oxidative stress and mitochondrial damage in glial cells. Free Radic Biol Med. 2014;73:84–94. doi: 10.1016/j.freeradbiomed.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 104.Mates JM, Segura JA, Alonso FJ, Marquez J. Intracellular redox status and oxidative stress: implications for cell proliferation, apoptosis, and carcinogenesis. Arch Toxicol. 2008;82(5):273–299. doi: 10.1007/s00204-008-0304-z. [DOI] [PubMed] [Google Scholar]
- 105.Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Curr Med Chem. 2004;11(9):1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 106.Verbon EH, Post JA, Boonstra J. The influence of reactive oxygen species on cell cycle progression in mammalian cells. Gene. 2012;511(1):1–6. doi: 10.1016/j.gene.2012.08.038. [DOI] [PubMed] [Google Scholar]
- 107.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48(9):1121–1132. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spagnuolo G, D’Anto V, Cosentino C, Schmalz G, Schweikl H, Rengo S. Effect of N-acetyl-L-cysteine on ROS production and cell death caused by HEMA in human primary gingival fibroblasts. Biomaterials. 2006;27(9):1803–1809. doi: 10.1016/j.biomaterials.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 109.Fu AL, Dong ZH, Sun MJ. Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res. 2006;1109:201–206. doi: 10.1016/j.brainres.2006.06.042. [DOI] [PubMed] [Google Scholar]
- 110.Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M. Effect of antioxidant N-acetyl-L-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain Res. 2004;1016(1):90–95. doi: 10.1016/j.brainres.2004.04.072. [DOI] [PubMed] [Google Scholar]
- 111.Ostrovsky S, Kazimirsky G, Gedanken A, Brodie C. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009;2(11):882–890. doi: 10.1007/s12274-009-9089-5. [DOI] [Google Scholar]
- 112.Kim JH, Jeong MS, Kim DY, Her S, Wie MB. Zinc oxide nanoparticles induce lipoxygenase-mediated apoptosis and necrosis in human neuroblastoma SH-SY5Y cells. Neurochem Int. 2015;90:204–214. doi: 10.1016/j.neuint.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 113.Prabakaran D, Ashokkumar N. Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie. 2013;95(2):366–373. doi: 10.1016/j.biochi.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 114.Mabalirajan U, Dinda AK, Sharma SK, Ghosh B. Esculetin restores mitochondrial dysfunction and reduces allergic asthma features in experimental murine model. J Immunol. 2009;183(3):2059–2067. doi: 10.4049/jimmunol.0900342. [DOI] [PubMed] [Google Scholar]
- 115.Kim SH, Kang KA, Zhang R, Piao MJ, Ko DO, Wang ZH, et al. Protective effect of esculetin against oxidative stress-induced cell damage via scavenging reactive oxygen species. Acta Pharmacol Sin. 2008;29(11):1319–1326. doi: 10.1111/j.1745-7254.2008.00878.x. [DOI] [PubMed] [Google Scholar]
- 116.Zhang YL, Guan L, Wang XF, Wen T, Xing JJ, Zhao JY. Protection of chlorophyllin against oxidative damage by inducing HO-1 and NQO1 expression mediated by PI3K/Akt and Nrf2. Free Radic Res. 2008;42(4):362–371. doi: 10.1080/10715760801993076. [DOI] [PubMed] [Google Scholar]
- 117.Kumar SS, Shankar B, Sainis FB. Effect of chlorophyllin against oxidative stress in splenic lymphocytes in vitro and in vivo. Biochim Biophys Acta-Gen Subj. 2004;1672(2):100–111. doi: 10.1016/j.bbagen.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 118.Suryavanshi S, Sharma D, Checker R, Thoh M, Gota V, Sandur SK, et al. Amelioration of radiation-induced hematopoietic syndrome by an antioxidant chlorophyllin through increased stem cell activity and modulation of hematopoiesis. Free Radic Biol Med. 2015;85:56–70. doi: 10.1016/j.freeradbiomed.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 119.El-Ghor AA, Noshy MM, Galal A, Mohamed HRH. Normalization of nano-sized TiO2-induced clastogenicity, genotoxicity and mutagenicity by chlorophyllin administration in mice brain, liver, and bone marrow cells. Toxicol Sci. 2014;142(1):21–32. doi: 10.1093/toxsci/kfu157. [DOI] [PubMed] [Google Scholar]
- 120.Niska K, Santos-Martinez MJ, Radomski MW, Inkielewicz-Stepniak I. CuO nanoparticles induce apoptosis by impairing the antioxidant defense and detoxification systems in the mouse hippocampal HT22 cell line: protective effect of crocetin. Toxicol Vitro. 2015;29(4):663–671. doi: 10.1016/j.tiv.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 121.Yoshino F, Yoshida A, Umigai N, Kubo K, Lee MCI. Crocetin reduces the oxidative stress induced reactive oxygen species in the stroke-prone spontaneously hypertensive rats (SHRSPs) brain. J Clin Biochem Nutr. 2011;49(3):182–187. doi: 10.3164/jcbn.11-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen XC, Qian ZY. Effects of crocetin on antioxidant enzymatic activities in cardiac hypertrophy induced by norepinephrine in rats. Pharmazie. 2006;61(4):348–352. [PubMed] [Google Scholar]
- 123.Ahmad AS, Ansari MA, Ahmad M, Saleem S, Yousuf S, Hoda MN, et al. Neuroprotection by crocetin in a hemi-parkinsonian rat model. Pharmacol Biochem Behav. 2005;81(4):805–813. doi: 10.1016/j.pbb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 124.Yum S, Park H, Hong S, Jeong S, Kim W, Jung Y. N-(2-mercaptopropionyl)-glycine, a diffusible antioxidant, activates HIF-1 by inhibiting HIF prolyl hydroxylase-2: implication in amelioration of rat colitis by the antioxidant. Biochem Biophys Res Commun. 2014;443(3):1008–1013. doi: 10.1016/j.bbrc.2013.12.081. [DOI] [PubMed] [Google Scholar]
- 125.Liu SC, Xu LJ, Zhang T, Ren GG, Yang Z. Oxidative stress and apoptosis induced by nanosized titanium dioxide in PC12 cells. Toxicology. 2010;267(1-3):172–177. doi: 10.1016/j.tox.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 126.Zhao JX, Yao Y, Liu SC, Zhang T, Ren GG, Yang Z. Involvement of reactive oxygen species and high-voltage-activated calcium currents in nanoparticle zinc oxide-induced cytotoxicity in vitro. J Nanopart Res. 2012;14(11):14. doi: 10.1007/s11051-012-1238-1. [DOI] [Google Scholar]
- 127.Leonard SS, Harris GK, Shi XL. Metal-induced oxidative stress and signal transduction. Free Radic Biol Med. 2004;37(12):1921–1942. doi: 10.1016/j.freeradbiomed.2004.09.010. [DOI] [PubMed] [Google Scholar]


