Abstract
For construction of the bacterial flagellum, flagellar proteins are exported via its specific export apparatus from the cytoplasm to the distal end of the growing flagellar structure. The flagellar export apparatus consists of a transmembrane (TM) export gate complex and a cytoplasmic ATPase complex consisting of FliH, FliI, and FliJ. FlhA is a TM export gate protein and plays important roles in energy coupling of protein translocation. However, the energy coupling mechanism remains unknown. Here, we performed a cross‐complementation assay to measure robustness of the energy transduction system of the export apparatus against genetic perturbations. Vibrio FlhA restored motility of a Salmonella ΔflhA mutant but not that of a ΔfliH‐fliI flhB(P28T) ΔflhA mutant. The flgM mutations significantly increased flagellar gene expression levels, allowing Vibrio FlhA to exert its export activity in the ΔfliH‐fliI flhB(P28T) ΔflhA mutant. Pull‐down assays revealed that the binding affinities of Vibrio FlhA for FliJ and the FlgN–FlgK chaperone–substrate complex were much lower than those of Salmonella FlhA. These suggest that Vibrio FlhA requires the support of FliH and FliI to efficiently and properly interact with FliJ and the FlgN–FlgK complex. We propose that FliH and FliI ensure robust and efficient energy coupling of protein export during flagellar assembly.
Keywords: Bacterial flagella, cross‐complementation, FlhA, mutational robustness, Type III protein export
Introduction
The bacterial flagellum is a macromolecular assembly made of about 30 different proteins with their copy numbers ranging from a few to tens of thousands and consists of the basal body rings and a tubular axial structure. For assembly of the flagellar axial structure beyond the cell membranes, flagellar axial proteins are exported by a type III export apparatus from the cytoplasm to the distal end of the growing structure. The export apparatus consists of a transmembrane (TM) export gate complex made of FlhA, FlhB, FliO, FliP, FliQ, and FliR, and a cytoplasmic ATPase complex consisting of FliH, FliI, and FliJ (Macnab 2003; Chevance and Hughes 2008; Minamino et al. 2008; Minamino 2014). The export apparatus requires both ATP and proton motive force (PMF) across the cytoplasmic membrane as the fuels for rapid and efficient protein export (Minamino and Namba 2008; Paul et al. 2008). These component proteins are highly homologous to those of the type III secretion systems of pathogenic bacteria, which inject virulence effector proteins into their eukaryotic host cells for invasion (Cornelis 2006).
FlhA consists of an N‐terminal integral membrane domain with eight predicted TM helices (FlhATM), a flexible linker (FlhAL), and a C‐terminal cytoplasmic domain (FlhAC) (Fig. S1) (Minamino et al. 1994). FlhATM is responsible for the interaction with the basal body MS ring protein FliF (Kihara et al. 2001). A highly conserved hydrophilic cytoplasmic loop between TM‐4 and TM‐5 is responsible for the interaction of FlhA with FliR (Hara et al. 2011). FlhAC provides binding sites for FliH, FliI, FliJ, flagellar type III secretion chaperones, and export substrates (Minamino and Macnab 2000a; Minamino et al. 2003, 2009, 2010, 2011, 2012a; Bange et al. 2010; Kinoshita et al. 2013). FlhAC consists of four subdomains, D1, D2, D3, and D4 (Saijo‐Hamano et al. 2010). A well‐conserved hydrophobic dimple located at the interface of domains D1 and D2 of FlhAC is responsible for interactions of FlhA with the FlgN/FlgK, FlgN/FlgL, FliT/FliD, and FliS/FliC chaperone–substrate complexes (Bange et al. 2010; Minamino et al. 2012a; Kinoshita et al. 2013). A highly conserved Phe‐459 residue in this hydrophobic dimple of FlhAC is critical for the interaction with a well‐conserved Tyr residue of FlgN, FliT, and FliS chaperones (Minamino et al. 2012a; Kinoshita et al. 2013). The G368C mutation in domain D1 of FlhAC induces a large conformational change in domain D2 at the restrictive temperature of 42°C, thereby blocking the export process after the FliH–FliI–FliJ–export substrate complex binds to the FlhA–FlhB docking platform (Minamino et al. 2010; Shimada et al. 2012). This suggests that the D2 domain is directly involved in the translocation of the export substrate into the central channel of the growing flagellar structure (Shimada et al. 2012). FlhAL is involved in an interaction between FlhA and FliJ (Bange et al. 2010; Minamino et al. 2011). FliH and FliI help FliJ to efficiently and properly interact with FlhAL, thereby fully activating the PMF‐driven export gate (Minamino et al. 2011; Ibuki et al. 2013). ATP hydrolysis by FliI is linked to the gate‐activation process (Minamino et al. 2014). These observations suggest that FlhA plays important roles in energy coupling of flagellar type III protein export. However, the energy coupling mechanism remains unclear.
To examine robustness of the energy coupling mechanism of flagellar type III protein export against genetic perturbations, we replaced the Salmonella enterica flhA gene (StflhA) by the flhA gene of Vibrio alginolyticus (VaflhA). We show that VaFlhA exerts its export activity in S. enterica in the presence of FliH and FliI, but not in their absence. We also show that VaFlhA requires FliH and FliI for efficient interactions with FliJ and the FlgN–FlgK chaperone–substrate complex.
Experimental Procedures
Bacteria, plasmids, P22‐mediated transduction, DNA manipulations, and media
Salmonella strains and plasmids used in this study are listed in Table 1. P22‐mediated transduction was carried out as described (Yamaguchi et al. ). L‐broth (LB) and soft tryptone agar plates were prepared as described previously (Minamino and Macnab 1999, 2000a). To construct a Salmonella Vibrio flhA strain, the flhA gene on the chromosome was replaced by the Vibrio flhA allele using the λ Red homologous recombination system (Datsenko and Wanner 2000). Ampicillin and kanamycin were added at a final concentration of 100 and 15 μg/mL, respectively, if needed.
Table 1.
Strains and Plasmids used in this study
| Strains and plasmids | Relevant characteristics | Source or reference |
|---|---|---|
| E. coli | ||
| BL21(DE3) | Overexpression of proteins | Novagen |
| Salmonella | ||
| SJW1103 | Wild‐type for motility and chemotaxis | Yamaguchi et al. (1984) |
| SJW1368 | ∆cheW‐flhD | Ohnishi et al. (1992, 1994) |
| NH001 | ∆flhA | Hara et al. (2011) |
| NH002 | ∆flhA flhB(P28T) | Hara et al. (2011) |
| NH004 | ∆fliH‐fliI ∆flhA flhB(P28T) | Hara et al. (2011) |
| MMHI0117 | ∆fliH‐fliI flhB(P28T) | Minamino and Namba (2008) |
| MMB017 | flhB(P28T) | Minamino and Namba (2008) |
| MMA2001 | VaflhA | This study |
| MMA2002 | ∆fliH‐fliI ∆flhA flhB(P28T) flgM/pNY101 | This study |
| MMA2003 | ∆fliH‐fliI ∆flhA flhB(P28T) flgM/pNY101 | This study |
| MMA2004 | ∆fliH‐fliI ∆flhA flhB(P28T) flgM/pNY101 | This study |
| Plasmids | ||
| pGEX‐6p‐1 | Expression vector | GE Healthcare |
| pSU41 | Expression vector | Bartolomé et al. (1991) |
| pTrc99AFF4 | Expression vector | Ohnishi et al. (1997) |
| pHMK215 | pET3c/FlgN | Minamino et al. (2012b) |
| pMKGK2 | pTrc99A/FlgK | Furukawa et al. (2002) |
| pMM130 | pTrc99AFF4/FlhA | Kihara et al. (2001) |
| pMMHA1001 | pGEX6p‐1/GST‐FlhAC | Minamino et al. (2009) |
| pMM306 | pTrc99A/His‐FliH | Minamino and Macnab (2000a, 2000b) |
| pMM406 | pTrc99A/His‐FliJ | Minamino and Macnab (2000a, 2000b) |
| pMM1702 | pTrc99A/His‐FliI | Minamino and Macnab (2000a, 2000b) |
| pMKMHA003 | pTrc99AFF4/VaFlhATM‐L‐StFlhC | This study |
| pMKMHA004 | pTrc99AFF4/VaFlhATM‐StFlhAL‐C | This study |
| pMKM005 | pGEX6p‐1/GST‐VaFlhAC | This study |
| pNY101 | pSU41/VaFlhA | This study |
DNA manipulations
DNA manipulations were carried out as described (Saijo‐Hamano et al. 2004). DNA sequencing reactions were carried out using BigDye v3.1 as described in the manufacturer's instructions (Applied Biosystems, Tokyo, Japan), and then the reaction mixtures were analyzed by a 3130 Genetic Analyzer (Applied Biosystems).
Whole‐genome sequencing and data analysis
Genomic DNAs were isolated from the fliH‐fliI flhB(P28T) VaflhA strain and its pseudorevertants. Nextera XT kits (Illumina, Tokyo, Japan) were used to generate a MiSeq compatible library from the Salmonella genomic DNA. The constructed libraries were then loaded into a MiSeq 600‐Cycle v3 Reagent Kit (Illumina). The fastq files produced by the MiSeq were imported to a CLC Genomic work bench (Qiagen, Boston, MA, USA) and SNPs were detected using traditional variants detection tool by comparing the parent strain and their pseudorevertants.
Motility assay
Fresh transformants were inoculated onto soft tryptone agar plates and incubated at 30°C. At least seven independent assays were performed.
Secretion assay
Cells were grown at 30°C with shaking until the cell density had reached an OD600 of ca. 1.2–1.4. To test the effect of carbonyl cyanide m‐chlorophenylhydrazone (CCCP) on flagellar protein export, the cells were grown with shaking in 5 mL of LB containing ampicillin at 30°C until the cell density had reached an OD600 of ca. 0.6–0.7. After washing twice with LB, the cells were resuspended in 5 mL LB with or without CCCP and incubated at 30°C for 1 h. Cultures were centrifuged to obtain the cell pellets and culture supernatants. To test the effect of 100 mmol/L NaCl on flagellar protein export, the cells were grown with shaking in 5 mL of LB with or without 100 mmol/L NaCl at 30°C until the cell density had reached an OD600 of ca. 1.2–1.4. After centrifugation, the whole cellular and culture supernatant fractions were collected separately. Cell pellets were resuspended in sodium dodecyl sulfate (SDS)‐loading buffer normalized by the cell density to give a constant amount of cells. The proteins in the culture supernatants were precipitated by 10% trichloroacetic acid, suspended in a Tris‐SDS loading buffer, and heated at 95°C for 3 min. After SDS‐polyacrylamide gel electrophoresis (PAGE), immunoblotting with polyclonal anti‐FlgD, anti‐FlgE, anti‐FlgK, anti‐FlgL, anti‐FliD, and anti‐FliK antibodies was carried out as described previously (Minamino and Macnab 1999). Detection was done with an ECL plus immunoblotting detection kit (GE Healthcare, Tokyo, Japan). At least three independent experiments were carried out.
Observation of flagellar filaments with a fluorescent dye
The flagellar filaments produced by Salmonella cells were labeled using anti‐FliC antiserum and anti‐rabbit IgG conjugated with Alexa Fluor® 594 (Invitrogen) as described (Minamino et al. 2014). The cells were observed by fluorescence microscopy as described previously (Morimoto et al. 2010). Fluorescence images were analyzed using ImageJ software version 1.48 (National Institutes of Health, USA).
Protein expression and purification
The soluble fractions prepared from SJW1368 carrying pMMHA1001 or pMKMHA005 were loaded onto a glutathione Sepharose 4B column (GE Healthcare). After washing with PBS (8 g of NaCl, 0.2 g of KCl, 3.63 g of Na2HPO4·12H2O, 0.24 g of KH2PO4, pH 7.4 per liter), proteins were eluted with 50 mmol/L Tris‐HCl, pH 8.0, 10 mmol/L reduced glutathione. Fractions containing glutathione‐S‐transferase (GST)‐tagged proteins were pooled and dialyzed overnight against phosphate buffered saline (PBS) at 4°C with three changes of PBS.
His‐FliH, His‐FliI, and His‐FliJ were overproduced in SJW1368 transformed with pMM306, pMM1702, and pMM406, respectively, and purified by Ni‐NTA affinity chromatography as described previously (Minamino and Macnab 2000a).
FlgK and FlgN were overproduced in BL21 (DE3) transformed with pMKGK2, and pHMK215, respectively, and purified as described previously (Furukawa et al. 2002; Minamino et al. 2012a).
Pull‐down assay using GST affinity chromatography
Purified His‐FliI, His‐FliH, His‐FliJ, and FlgN/FlgK complex was mixed GST‐Salmonella FlhAC or GST‐Vibrio FlhAC, and then the mixtures were dialyzed overnight against PBS at 4°C with three changes of PBS. These mixtures were loaded onto a glutathione Sepharose 4B column (bed volume, 1 mL) pre‐equilibrated with 20 mL of PBS and washed with 10 mL of PBS at a flow rate of ca. 0.5 mL/min. Bound proteins were eluted with 5 mL of 50 mmol/L Tris‐HCl, pH 8.0, 10 mmol/L reduced glutathione. Eluted fractions were analyzed by SDS‐PAGE with Coomassie Brilliant blue (CBB) staining and immunoblotting. At least three independent experiments were carried out.
Sequence alignment
Sequence alignment was carried out by Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/).
Results
Vibrio FlhA restores motility of a Salmonella flhA null mutant
It has been shown that FlhA plays important roles in an energy coupling mechanism of flagellar type III protein export (Minamino et al. 2011). To measure robustness of the energy transduction system of the flagellar type III export apparatus, we performed a cross‐complementation assay of FlhA. V. alginolyticus is a γ‐proteobacterium that has two circular chromosomal DNAs. Interestingly, V. alginolyticus has two distinct sets of the flagellar systems, a polar flagellar system, and a lateral flagellar system, of which genes are encoded on chromosomal DNA I and II, respectively. The polar flagellum is a Na+‐driven rotary motor, whereas the lateral flagellum is a H+‐driven rotary motor (Zhu et al. 2013). It has been reported that V. alginolyticus also has two distinct SecDF complexes, SecDF1 and SecDF2, of which genes are encoded on chromosomal DNA I and II, respectively and that SecDF1 utilizes Na+ as the coupling ion to facilitate protein translocation, whereas SecDF2 requires H+ instead of Na+ (Ishii et al. 2015). Salmonella utilizes PMF across the cytoplasmic membrane as the energy source for flagellar motility as well as flagellar type III protein export. Because structural and functional diversities of the bacterial flagellum have been observed among bacterial species although its core structure is highly conserved (Minamino and Imada 2015), we investigated whether the FlhA protein of the Na+‐driven polar flagellar system of V. alginolyticus (VaFlhA) would be functional in the proton‐driven Salmonella flagellar system. The amino acid sequence of VaFlhA has 73.2% similarity and 52.9% identity with the FlhA protein of S. enterica serovar Typhimurium (StFlhA) (Fig. S1). We first transformed a Salmonella flhA null mutant (ΔflhA) with a pSU41‐based plasmid encoding VaFlhA and analyzed the motility of the resulting transformants in soft agar (Fig. 1A). VaFlhA restored motility of the ΔflhA mutant in soft agar although not to the Salmonella wild‐type level. In agreement with this, immunoblotting analyses revealed that FlgD, FlgE, FlgK, FlgL, and FliC were detected in the culture supernatants (Fig. 1B).
Figure 1.
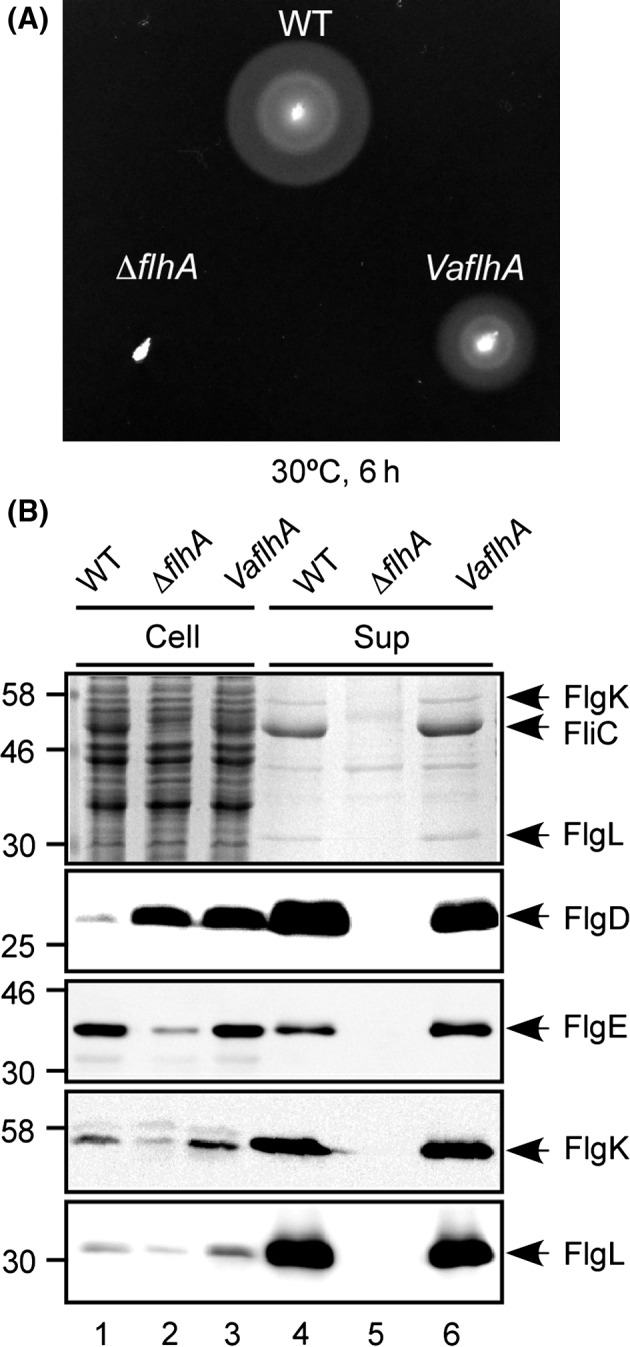
Cross‐complementation assay of Vibrio FlhA. (A) Motility of NH001 transformed with pNY101 (VaflhA) in soft agar. SJW1103 carrying pSU41 (WT) and NH001 harboring pSU41 (∆flhA) are used as the positive and negative controls. Plates were incubated at 30°C for 6 h. (B) Secretion assays. Whole‐cell proteins (Cell) and culture supernatant fractions (Sup) were prepared from the above transformants, and then analyzed by Coomassie Brilliant blue (CBB) staining (first row) and immunoblotting, using polyclonal anti‐FlgD (second row), anti‐FlgE (third row), anti‐FlgK (fourth row), or anti‐FlgL (fifth row) antibody. The positions of molecular mass markers are indicated on the left.
To test if the complementation ability of VaFlhA could be a consequence of its multicopy effect on the motility, the StflhA gene on the chromosomal DNA was replaced with the VaflhA gene using the λ Red homologous recombination system (Datsenko and Wanner 2000). The motility of the Salmonella VaflhA strain was lower than that of the Salmonella wild‐type strain and essentially the same as that of the Salmonella ΔflhA mutant transformed with pSU41‐VaFlhA (Fig. S2A). FlgD and FlgE were secreted to the culture supernatant at the wild‐type levels, and FliC, FlgK, and FlgL were secreted less than the wild‐type levels (Fig. S2B, lanes 4 and 6). Consistently, most of the VaflhA cells produced flagella at the wild‐type level although the length of flagellar filaments produced by the VaflhA cells was shorter than the wild‐type level (Fig. 2). Because the localization of FlhA to the basal body depend on the MS ring protein FliF, a basal body C ring protein FliG, and export gate proteins FliO, FliP, FliQ, and FliR, but not on an export gate protein FlhB, cytoplasmic proteins FliH, FliI, FliJ, and the remaining C ring proteins FliM and FliN (Morimoto et al. 2014), these results indicate that VaFlhA can assemble into the export gate complex within the MS ring with other Salmonella gate component proteins to exert its export activity in Salmonella.
Figure 2.
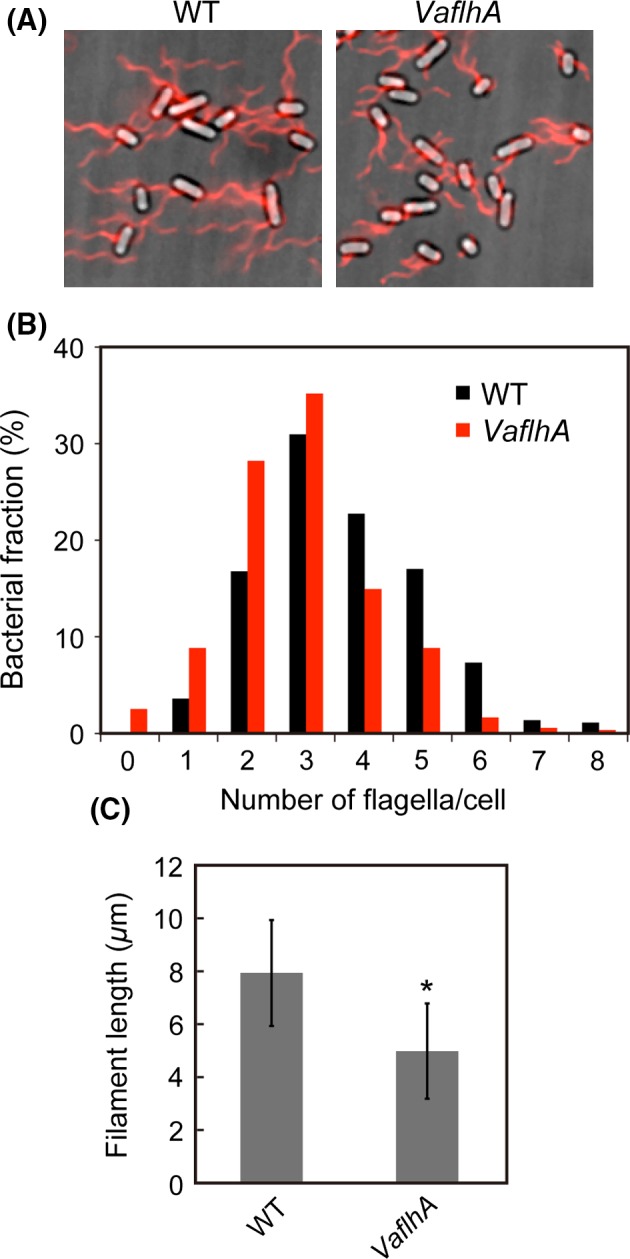
Measurements of the number and length of the flagellar filaments produced by the VaflhA cells. (A) Fluorescent images of SJW1103 (WT) and MMA2001 (VaflhA). Flagellar filaments were labeled with Alexa Fluor 594. The fluorescence images of the filaments labeled with Alexa Fluor 594 (red) were merged with the bright‐field images of the cell bodies. (B) Distribution of the number of the flagellar filaments in the wild‐type (black) and VaflhA cells (red). More than 400 cells for each transformants were counted. (C) Measurements of the length of the flagellar filaments. Filament length is the average of more than 150 cells, and vertical lines are standard deviations. Statistical analysis using Student's t‐test shows a significant difference with an asterisk (P < 0.001).
Vibrio FlhA forms a PMF‐driven export engine
The TM export gate complex made of FlhA, FlhB, FliO, FliP, FliQ, and FliR is powered by PMF across the cytoplasmic membrane (Minamino and Namba 2008; Paul et al. 2008). Therefore, we next tested whether treatment with a protonophore, CCCP, affects flagellar protein export by the VaflhA cells (Fig. S3A). The cellular levels of FlgD were maintained even in the presence of 50 μmol/L CCCP (lanes 1–6). However, the levels of FlgD secretion by the wild‐type and VaflhA cells both diminished (lanes 8 and 12), indicating that PMF is absolutely essential for FlgD export in the VaflhA cells.
The SecDF complex of Escherichia. coli utilizes H+ as the coupling ion to function in an ATP‐independent stage of protein translocation, whereas the SecDF1 complex of V. alginolyticus utilizes Na+ as the coupling ion instead of H+ even in E. coli cells (Tsukazaki et al. 2011; Ishii et al. 2015). Therefore, we tested whether Na+ ion affects the secretion rate of FlgD by the VaflhA cells (Fig. S3B). The FlgD secretion levels by the wild‐type and VaflhA cells both showed no Na+ dependence (lanes 7, 8, 11 and 12). Therefore, we conclude that VaFlhA forms the PMF‐driven export gate complex along with the Salmonella FlhB, FliO, FliP, FliQ, and FliR at the flagellar base.
Vibrio FlhA fails to exert its export activity in the absence of FliH and FliI
Most of mutations at conserved charged residues within FlhATM were tolerated in the presence of FliH and FliI, but resulted in loss‐of‐function in their absence (Hara et al. 2011, 2012), suggesting that FliH and FliI are critical for the robustness of FlhA against genetic perturbations. To test whether VaFlhA is still functional in the absence of FliH and FliI, we used a ΔfliH‐fliI flhB(P28T) bypass mutant whose second‐site P28T mutation in FlhB considerably increases the probability of flagellar protein export in the absence of FliH and FliI (Minamino and Namba 2008). VaFlhA did not restore motility of the Salmonella ΔfliH‐fliI flhB(P28T) ΔflhA mutant (Fig. 3A). In agreement with this, immunoblotting with anti‐FlgD antibody revealed that the ΔfliH‐fliI flhB(P28T) VaflhA cells did not secrete FlgD into the culture supernatant (Fig. 3B, lane 6). These results indicate that VaFlhA cannot work in this bypass mutant background.
Figure 3.
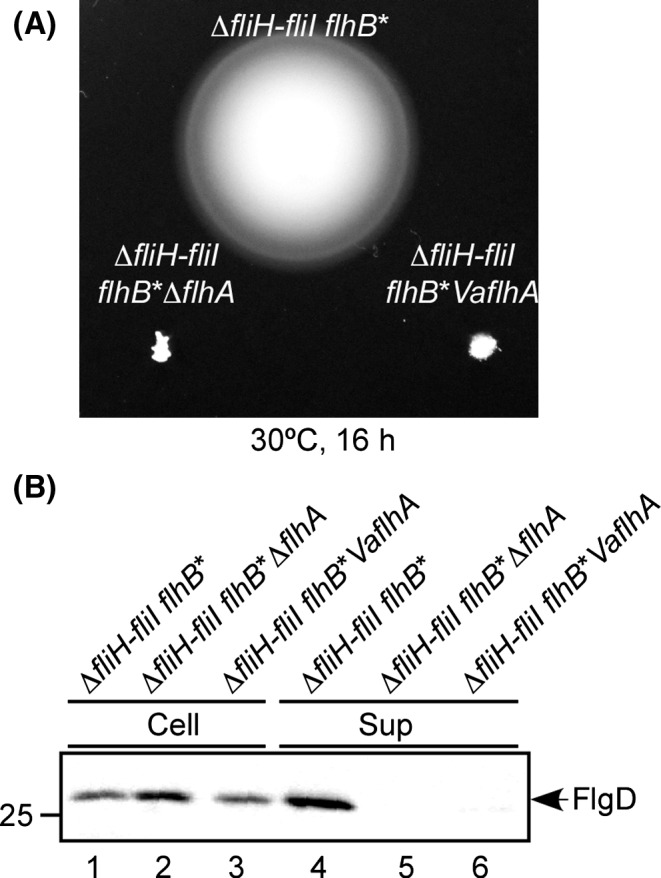
Cross‐complementation assay of Vibrio FlhA in the absence of FliH and FliI. (A) Motility of NH004 transformed with pNY101 (∆fliH‐fliI flhB* VaflhA) in soft agar. MMHI0117 carrying pSU41 (∆fliH‐fliI flhB*) and NH004 harboring pSU41 (∆fliH‐fliI flhB* ∆flhA) were used as the positive and negative controls. Plates were incubated at 30°C for 16 h. (B) Immunoblotting, using polyclonal anti‐FlgD antibody, of whole‐cell proteins (Cell) and culture supernatant fractions (Sup) prepared from the above strains.
We next investigated the effect of the flhB(P28T) mutation by itself on the function of VaFlhA (Fig. S4). VaFlhA fully restored motility of a Salmonella flhB(P28T) ΔflhA mutant (Fig. S4A). Consistently, FlgD was detected in the culture supernatant of the flhB(P28T) VaflhA strain (Fig. S4B, lane 6). These results suggest that the presence of FliH and FliI allows VaFlhA to exert its export function considerably even in the presence of the FlhB(P28T) bypass mutation.
Isolation of pseudorevertants from the ΔfliH‐fliI flhB(P28T) VaflhA strain
To understand why VaFlhA requires the support of FliH and FliI for its PMF‐driven export activity in Salmonella, pseudorevertants were isolated from the ΔfliH‐fliI flhB(P28T) VaflhA strain by streaking an overnight culture out on soft agar plates, incubating them at 30°C for 2 days and looking for motility halos emerging from the streak. In total, seven motile colonies were purified from such halos. The motility of these pseudorevertants was considerably better than that of the parent strain (Fig. 4A). In agreement with this, FlgD was detected in the culture supernatants of these pseudorevertants (Fig. 4B). DNA sequencing revealed that all suppressor mutations are located in the flgM gene (Fig. 4C). They can be divided into two categories. The first category consists of missense mutations: P8S and P11L (isolated four times). The other category is a frameshift at codon 45 (isolated twice), resulting in truncation of the C‐terminal region of FlgM. FlgM acts as an anti‐sigma factor of the flagellar regulon (Gillen and Hughes 1991; Ohnishi et al. 1992). C‐terminal truncations of FlgM cause a loss of its anti‐sigma factor activity (Iyoda and Kutsukake 1995) and hence result in a two‐ to threefold increase in the number of the flagella per cell (Kutsukake and Iino 1994). Because it has been shown that deletions of the cytoplasmic FliH‐FliI‐FliJ ATPase complex can be bypassed by a significant increment in flagellar gene expression levels (Erhardt et al. 2014), we suggest that these flgM suppressor mutations considerably increased the cytoplasmic levels of both export substrates and cytoplasmic export proteins, allowing the ΔfliH‐fliI flhB(P28T) VaflhA strain to export flagellar proteins to produce flagella to considerable degree.
Figure 4.
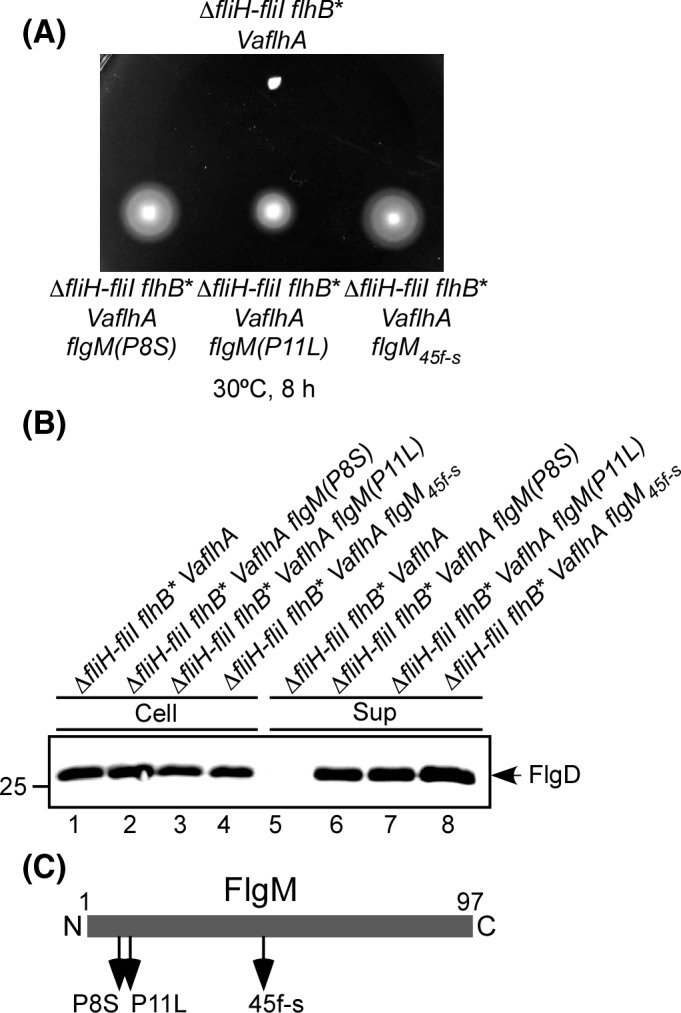
Isolation of pseudorevertants from the ∆ fliH‐fliI flhB(P28T) VaflhA strain. (A) Motility of NH004 transformed with pNY101 (∆fliHI flhB* VaflhA) and its pseudorevertants, MMA2002 (∆fliH‐fliI flhB* VaflhA flgM(P8S)), MMA2003 (∆fliH‐fliI flhB* VaflhA flgM(P11L)), and MMA2004 (∆fliH‐fliI flhB* VaflhA flgM 45f‐s) at 30°C for 8 h. (B) Immunoblotting, using polyclonal anti‐FlgD antibody, of whole‐cell proteins (Cell) and culture supernatant fractions (Sup) prepared from the above strains. (C) Location of extragenic flgM suppressor mutations. FlgM consists of 97 amino acid resides. The sites of suppressor mutations in FlgM are shown by arrows. Missence mutations are indicated as P8S and P11L. A frameshift mutation is indicated by f‐s. The N and C termini of FlgM are labeled as 1 and 97, respectively.
VaFlhAL‐C requires FliH and FliI for efficient interactions with FliJ and the chaperone–substrate complexes
FlhA consists of three regions: FlhATM, FlhAL, and FlhAC (Fig. 5A and Fig. S1). Cooperative interactions of FlhATM with FlhB, FliH, FliI, and FliR are required for the translocation of export substrates in a PMF‐dependent manner. FlhAL connecting FlhAC to FlhATM is responsible for the interaction with FliJ (Bange et al. 2010; Minamino et al. 2011), and the D1 and D2 domains of FlhAC are directly involved in interactions with the flagellar chaperone–substrate complexes (Bange et al. 2010; Minamino et al. 2012a; Kinoshita et al. 2013). To identify which regions of VaFlhA require support of FliH and FliI to interact with its binding partners, we replaced VaFlhAL and VaFlhAC by StFlhAL and StFlhAC, respectively, to create two FlhA chimeras, VaFlhATM‐L‐StFlhAC and VaFlhATM‐StFlhAL‐C (Fig. 5A). These two chimeric proteins conferred motility of the Salmonella ΔflhA mutant in a way similar to VaFlhA (Fig. 5B, left panel), indicating their capability of forming the export gate complex along with other Salmonella export gate proteins. The VaFlhATM‐StFlhAL‐C chimeric protein restored motility of the ΔfliH‐fliI flhB(P28T) ΔflhA mutant to some degree, whereas the VaFlhATM‐L‐StFlhAC did not at all (Fig. 5B, right panel). These results indicate that VaFlhAL‐C requires FliH and FliI to efficiently and properly interact with its binding partners.
Figure 5.
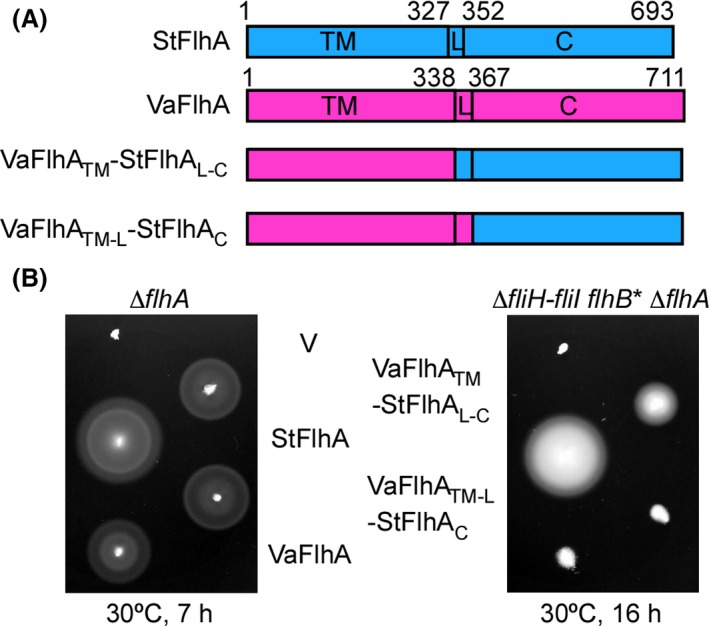
Complementation of FlhA chimera proteins. (A) Representation of the VaFlhATM‐StFlhAL ‐C and VaFlhATM ‐L‐StFlhAC chimera proteins comprising the N‐terminal region of VaFlhATM (residues 1– 338 of Vibrio FlhA) fused to the C‐terminal cytoplasmic region of StFlhAL ‐C (residues 328–693 of Salmonella FlhA) and the N‐terminal region of VaFlhATM ‐L (residues 1–366 of Vibrio FlhA) fused to the C‐ cytoplasmic domain of StFlhAC (residues 352–693 of Salmonella FlhA), respectively. (B) Motility of NH001 (∆flhA) and NH004 (∆fliH‐fliI flhB* ∆flhA) transformed with pSU41 (V), pMM130 (StFlhA), pNY101 (VaFlhA), pMKMHA003 (VaFlhATM‐StFlhAL ‐C), or pMKMHA004 (VaFlhATM ‐L‐StFlhAC) in soft agar at 30°C.
To analyze the binding affinities of VaFlhAL‐C for FliH, FliI, FliJ, and the FlgN–FlgK chaperone–substrate complexes, we carried out pull‐down assays by GST affinity chromatography (Fig. 6). GST‐VaFlhAL‐C bound to FliH and FliI at levels similar to GST‐StFlhAL‐C (Fig. 6A and B), indicating that the binding affinities of VaFlhAL‐C for FliH and FliI were essentially the same as those of StFlhAL‐C. The amount of FliJ co‐purified with GST‐VaFlhAL‐C was about 10‐fold lower than those co‐purified with GST‐StFlhAL‐C (Fig. 6C). The FlgN–FlgK complex co‐purified with GST‐StFlhAL‐C but not with GST‐VaFlhAL‐C (Fig. 6D). These observations indicate that the binding affinities of VaFlhAL‐C are weaker for FliJ and markedly weaker for the FlgN/FlgK complex than those of StFlhAL‐C. Neither FliH, FliI, FliJ nor the FlgN–FlgK complex co‐purified with GST (data not shown), in agreement with previous reports (Minamino et al. 2010, 2012a,b). These results confirmed that the low affinity of VaFlhAL‐C for FliJ and the FlgN/FlgK complex are somehow compensated by FliH and FliI.
Figure 6.
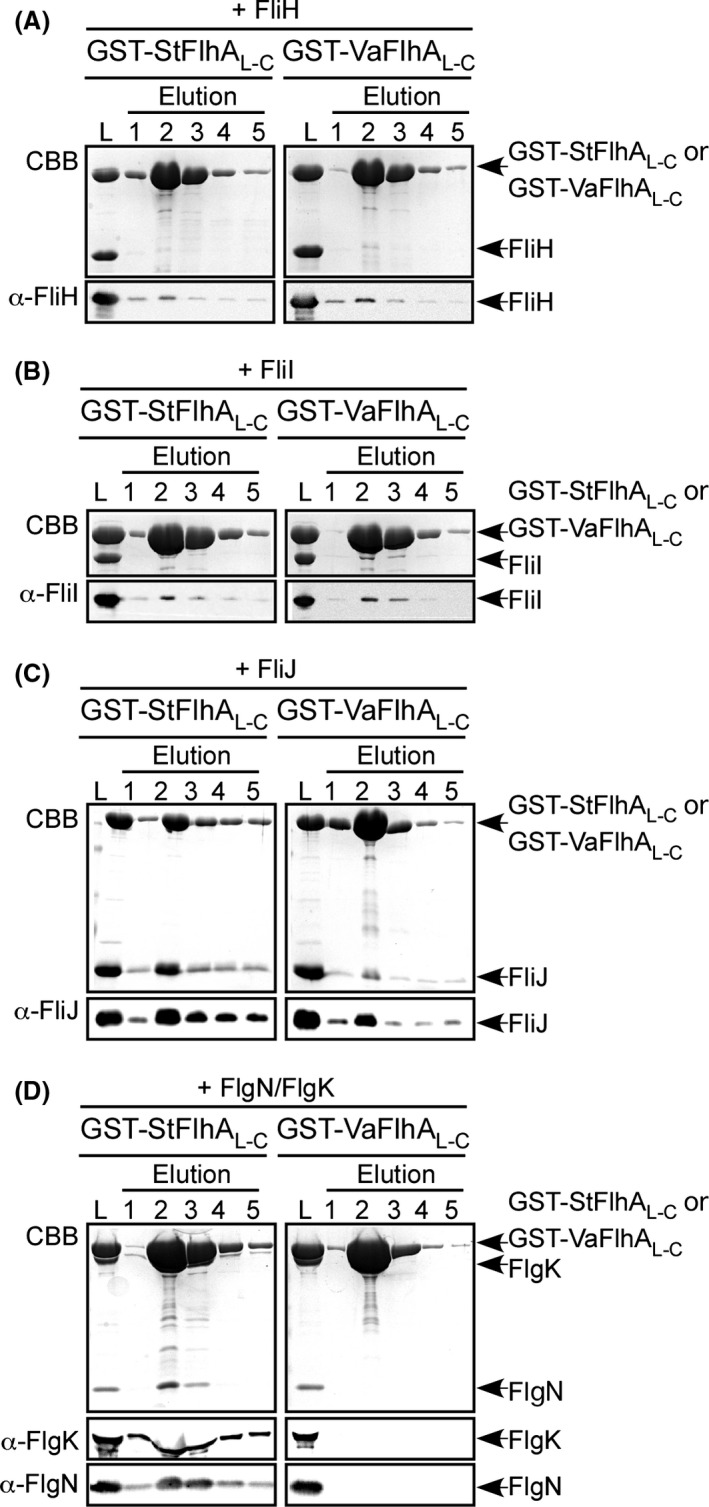
Interaction of Vibrio FlhA with FliH, FliI, FliJ, and the flagellar chaperone–substrate complex. Purified (A) FliH, (B) FliI, (C) FliJ, or (D) FlgN/FlgK complex was mixed with purified GST‐StFlhAL ‐C (left panel) or GST‐VaFlhAL ‐C (right panel), and dialyzed overnight against PBS. These mixtures (L) were loaded onto a GST column. After washing with 10 mL PBS, proteins were eluted with 10 mmol/L reduced glutathione. Elution fractions were analyzed by Coomassie Brilliant blue (CBB) staining (first rows) and immunoblotting by polyclonal anti‐FliH (A, second rows), anti‐FliI (B, second rows), anti‐FliJ (C, second rows), anti‐FlgK (D, second rows) or anti‐FlgN antibody (D, third rows).
Discussion
The flagellar type III export apparatus transports 14 flagellar proteins with their copy numbers ranging from a few to a few tens of thousands during flagellar assembly. A remarkable feature of type III protein export is that the export apparatus can coordinate protein export with assembly. Therefore, flagellar type III protein export is a complex process involving a substantial number of checkpoints to ensure the correct order of export (Chevance and Hughes 2008; Minamino 2014). A plausible export mechanism has been proposed. The cytoplasmic ATPase complexes consisting of FliH, FliI, and FliJ bind to export substrates and chaperone–substrate complexes in the cytoplasm and deliver the substrates and chaperone–substrate complexes to the base of the growing flagellar structure through interactions of FliH with FlhA and FliN (Bai et al. 2014). Once ATP hydrolysis by FliI ATPase activates a PMF‐driven TM export gate complex through an interaction between FliJ and FlhA, the export gate processively transports the substrates in a PMF‐dependent manner (Minamino et al. 2014).
The cytoplasmic ATPase complex shares an evolutionary relationship with F‐ and V‐type rotary ATPases (Pallen et al. 2006; Imada et al. 2007; Ibuki et al. 2011; Kishikawa et al. 2013). An increase in the cytoplasmic levels of export substrates and an increment in PMF are capable of bypassing the absence of the cytoplasmic FliH–FliI–FliJ ATPase complex (Erhardt et al. 2014). Biological systems can generally maintain their functional activities against internal and external perturbations. Since such robustness is a fundamental property that biological systems have evolved to gain by natural selection (Kitano 2004), it has been proposed that environmental pressures have driven the flagellar type III export apparatus to evolve via distinct evolutionary steps to develop robustness and efficiency by late addition of the cytoplasmic ATPase complex (Erhardt et al. 2014). However, it remained unknown how the ATPase complex ensures efficiency and robustness of the flagellar export system against various perturbations. In this study, we carried out a cross‐complementation assay to examine mutational robustness of an energy transduction system of the flagellar type III export apparatus. We found that VaFlhA is functional in Salmonella in the presence of FliH and FliI but not in their absence (Figs. 1, 3). We also showed that flgM mutations allow VaFlhA to function in the Salmonella export system even in the absence of FliH and FliI (Fig. 4). Because a loss of FlgM results in a considerable increment in the expression levels of all flagellar genes (Kutsukake and Iino 1994), we suggest that an increase in the cytoplasmic levels of FliJ, flagellar chaperones, and export substrates allows the export gate complex containing VaFlhA to transport export substrates into the distal end of the growing structure in the absence of FliH and FliI. The binding affinities of VaFlhAL‐C for FliJ and FlgN–FlgK chaperone substrate complex are much lower than those of StFlhAL‐C (Fig. 6). Therefore, we suggest that FliH and FliI ensure efficient recruitment of FliJ, flagellar chaperones, and export substrates to their binding sites of FlhAL‐C (Fig. 7).
Figure 7.
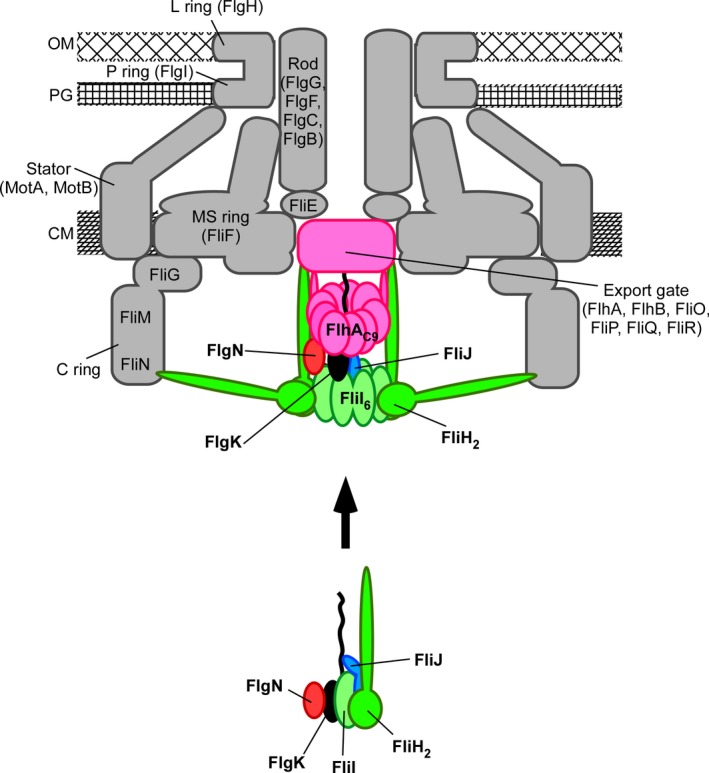
Schematic diagram of the flagellar type III protein export apparatus. The export gate is composed of six transmembrane proteins, FlhA, FlhB, FliO, FliP, FliQ, and FliR and is located within the MS ring. The C‐terminal cytoplasmic domain of FlhA (FlhAC) forms part of the docking platform for FliH, FliI ATPase, FliJ, flagellar type III secretion chaperones, and export substrates. FliI forms a hetero‐trimer with the FliH dimer in the cytoplasm. The FliH2–FliI complex acts as a dynamic carrier to deliver FliJ and the FlgN–FlgK chaperone‐export substrate complex to the FlhAC ring complex (FlhAC 9). Upon formation of the FliI6FliJ ring complex on the FlhAC 9 ring, the FliI6FliJ ring complex hydrolyses ATP and activates the export gate through an interaction between FliJ and FlhA, allowing the gate to translocate FlgK into the central channel of the growing flagellar structure. OM, outer membrane; PG, peptidoglycan layer; CM, cytoplasmic membrane.
FliI ATPase exists not only as the FliI6 ring stably bound within the C ring to fully exert its ATPase activity but also as the FliH2FliI complex in the cytoplasm (Fig. 7) (Minamino and Macnab 2000b; Chen et al. 2011; Kawamoto et al. 2013; Bai et al. 2014). The chaperone–substrate complexes bind to the FliH2FliI complex through cooperative interactions between FliI and chaperone (Thomas et al. 2004; Imada et al. 2010; Minamino et al. 2012b). FliJ interacts not only with the central cavity of the FliI6 ring (Ibuki et al. 2011) but also with the FliH2FliI complex through cooperative interactions among FliH, FliI, and FliJ (González‐Pedrajo et al. 2002). Since FliI‐YFP shows dynamic turnovers between the basal body and the cytoplasmic pool as observed by fluorescence recovery after photobleaching of FliI‐YFP spots, the FliH2FliI complex is thought to act as a dynamic carrier to deliver FliJ and the chaperone–export substrate complexes to the docking platform formed by FlhAC (Bai et al. 2014). FliJ and the chaperone–substrate complexes bind to FlhAL and a well‐conserved hydrophobic dimple located at the interface between domains D1 and D2 of FlhAC, respectively (Bange et al. 2010; Minamino et al. 2011, 2012a; Kinoshita et al. 2013). Domain D2 of FlhAC is directly involved in the translocation of the export substrate (Minamino et al. 2010; Shimada et al. 2012). Because an interaction between FliJ and FlhA brought about by FliH and FliI allows the export gate to efficiently utilize PMF to facilitate flagellar protein export (Minamino et al. 2011; Ibuki et al. 2013), we propose that FliH and FliI contribute to an efficient and robust energy coupling mechanism of flagellar type III protein export during flagellar assembly by promoting and enforcing the interactions between FlhA and its partner proteins (Fig. 7).
Conflict of interest
None declared.
Supporting information
Figure S1. Sequence alignment of FlhA proteins of Salmonella enterica and Vibrio alginolyticus.Figure S2. Characterization of a Salmonella VaflhA strain.Figure S3. Effects of (A) carbonyl cyanide m‐chlorophenylhydrazone (CCCP) and (B) 100 mmol/L NaCl on the level of FlgD secreted by the VaflhA cells.Figure S4. Effect of the FlhB(P28T) mutation on motility of the VaflhA cells.
Acknowledgments
We acknowledge Masahiro Ueda for continuous support and encouragement. This work was supported in part by JSPS KAKENHI Grant Numbers 26293097 (to T. M.) and 21227006 and 25000013 (to K. N.) and MEXT KAKENHI Grant Numbers 23115008, 24117004, 25121718 and 15H01640 to T. M., 24117004 to M. H. and 26115720 and 15H01335 to Y. V. M
MicrobiologyOpen 2016; 5(3): 424–435
References
- Bai, F. , Morimoto Y. V., Yoshimura S. D. J., Hara N., N. Kami‐ike , Namba K., et al. 2014. Assembly dynamics and the roles of FliI ATPase of the bacterial flagellar export apparatus. Sci. Rep. 4:6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bange, G. , Kümmerer N., Engel C., Bozkurt G., Wild K., and Sinning I.. 2010. FlhA provides the adaptor for coordinated delivery of late flagella building blocks to the type III secretion system. Proc. Natl Acad. Sci. USA 107:11295–11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomé, B. , Jubete Y., Martínez E., and de la Cruz F.. 1991. Construction and properties of a family of pACYC184‐derived cloning vectors compatible with pBR322 and its derivatives. Gene 102:75–78. [DOI] [PubMed] [Google Scholar]
- Chen, S. , Beeby M., Murphy G. E., Leadbetter J. R., Hendrixson D. R., Briegel A., et al. 2011. Structural diversity of bacterial flagellar motors. EMBO J. 30:2972–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevance, F. F. , and Hughes K. T.. 2008. Coordinating assembly of a bacterial macromolecular machine. Nat. Rev. Microbiol. 6:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelis, G. R. 2006. The type III secretion injectisome. Nat. Rev. Microbiol. 4:811–825. [DOI] [PubMed] [Google Scholar]
- Datsenko, K. A. , and Wanner B. L.. 2000. One‐step inactivation of chromosomal genes in Escherichia coli K‐12 using PCR products. Proc. Natl Acad. Sci. USA 97:6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt, M. , Mertens M. E., Fabiani F. D., and Hughes K. T.. 2014. ATPase‐independent type‐III protein secretion in Salmonella enterica . PLoS Genet. 10:e1004800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa, Y. , Imada K., Vonderviszt F., Matsunami H., K. Sano , Kutsukake K., et al. 2002. Interactions between bacterial flagellar axial proteins in their monomeric state in solution. J. Mol. Biol. 318:889–900. [DOI] [PubMed] [Google Scholar]
- Gillen, K. L. , and Hughes K. T.. 1991. Molecular characterization of flgM, a gene encoding a negative regulator of flagellin synthesis in Salmonella typhimurium . J. Bacteriol. 173:6453–6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Pedrajo, B. , Fraser G. M., Minamino T., and Macnab R. M.. 2002. Molecular dissection of Salmonella FliH, a regulator of the ATPase FliI and the type III flagellar protein export pathway. Mol. Microbiol. 45:967–982. [DOI] [PubMed] [Google Scholar]
- Hara, N. , Namba K., and Minamino T.. 2011. Genetic characterization of conserved charged residues in the bacterial flagellar type III export protein FlhA. PLoS ONE 6:e22417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara, N. , Morimoto Y. V., Kawamoto A., Namba K., and Minamino T.. 2012. Interaction of the extreme N‐terminal region of FliH with FlhA is required for efficient bacterial flagellar protein export. J. Bacteriol. 194:5353–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibuki, T. , Imada K., Minamino T., Kato T., Miyata T., and Namba K.. 2011. Common architecture between the flagellar protein export apparatus and F‐ and V‐ATPases. Nat. Struct. Mol. Biol. 18:277–282. [DOI] [PubMed] [Google Scholar]
- Ibuki, T. Y. , Uchida Y., Hironaka K., Namba K. Imada, and Minamino T.. 2013. Interaction between FliJ and FlhA, components of the bacterial flagellar type III export apparatus. J. Bacteriol. 195:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada, K. , Minamino T., Tahara A., and Namba K.. 2007. Structural similarity between the flagellar type III ATPase FliI and F1‐ATPase subunits. Proc. Natl Acad. Sci. USA 104:485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada, K. , Minamino T., Kinoshita M., Furukawa Y., and Namba K.. 2010. Structural insight into the regulatory mechanisms of interactions of the flagellar type III chaperone FliT with its binding partners. Proc. Natl Acad. Sci. USA 107:8812–8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii, E. , Chiba S., Hashimoto N., Kojima S., Homma M., Ito K., et al. 2015. Nascent‐chain‐monitored remodeling of the sec machinery for salinity adaptation of marine bacteria. Proc. Natl Acad. Sci. USA 112:E5513–E5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyoda, S. , and Kutsukake K.. 1995. Molecular dissection of the flagellum‐specific anti‐sigma factor, FlgM, of Salmonella typhimurium . Mol. Gen. Genet. 249:417–424. [DOI] [PubMed] [Google Scholar]
- Kawamoto, A. , Morimoto Y. V., Miyata T., Minamino T., Hughes K. T., Kato T., et al. 2013. Common and distinct structural features of Salmonella injectisome and flagellar basal body. Sci. Rep. 3:3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara, M. , Minamino T., Yamaguchi S., and Macnab R. M.. 2001. Intergenic suppression between the flagellar MS ring protein FliF of Salmonella and FlhA, a membrane component of its export apparatus. J. Bacteriol. 183:1655–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita, M. , Hara N., Imada K., Namba K., and T. Minamino . 2013. Interactions of bacterial chaperone‐substrate complexes with FlhA contribute to coordinating assembly of the flagellar filament. Mol. Microbiol. 90:1249–1261. [DOI] [PubMed] [Google Scholar]
- Kishikawa, J. , Ibuki T., Nakamura S., Nakanishi A., Minamino T., Miyata T., et al. 2013. Common evolutionary origin for the rotor domain of rotary ATPases and flagellar protein export apparatus. PLoS ONE 8:e64695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano, H. 2004. Biological robustness. Nat. Rev. Genet. 5:826–837. [DOI] [PubMed] [Google Scholar]
- Kutsukake, K. , and Iino T.. 1994. Role of the FliA‐FlgM regulatory system on the transcriptional control of the flagellar regulon and flagellar formation in Salmonella typhimurium . J. Bacteriol. 176:3598–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab, R. M. 2003. How bacteria assemble flagella. Annu. Rev. Microbiol. 57:77–100. [DOI] [PubMed] [Google Scholar]
- Minamino, T. 2014. Protein export through the bacterial flagellar type III export pathway. Biochim. Biophys. Acta. 1843:1642–1648. [DOI] [PubMed] [Google Scholar]
- Minamino, T. , and Imada K.. 2015. The bacterial flagellar motor and its structural diversity. Trends Microbiol. 23:267–274. [DOI] [PubMed] [Google Scholar]
- Minamino, T. , and Macnab R. M.. 1999. Components of the Salmonella flagellar export apparatus and classification of export substrates. J. Bacteriol. 181:1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino, T. , and Macnab R. M.. 2000a. Interactions among components of the Salmonella flagellar export apparatus and its substrates. Mol. Microbiol. 35:1052–1064. [DOI] [PubMed] [Google Scholar]
- Minamino, T. , and Macnab R. M.. 2000b. FliH, a soluble component of the type III flagellar export apparatus of Salmonella, forms a complex with FliI and inhibits its ATPase activity. Mol. Microbiol. 37:1494–1503. [DOI] [PubMed] [Google Scholar]
- Minamino, T. , and Namba K.. 2008. Distinct roles of the FliI ATPase and proton motive force in bacterial flagellar protein export. Nature 451:485–488. [DOI] [PubMed] [Google Scholar]
- Minamino, T. , Iino T., and Kutuskake K.. 1994. Molecular characterization of the Salmonella typhimurium flhB operon and its protein products. J. Bacteriol. 176:7630–7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino, T. , González‐Pedrajo B., Kihara M., Namba K., and Macnab R. M.. 2003. The ATPase FliI can interact with the type III flagellar protein export apparatus in the absence of its regulator FliH. J. Bacteriol. 185:3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino, T. , Imada K., and Namba K.. 2008. Mechanisms of type III protein export for bacterial flagellar assembly. Mol. BioSyst. 4:1105–1115. [DOI] [PubMed] [Google Scholar]
- Minamino, T. , Yoshimura S. D. J., Morimoto Y. V., González‐Pedrajo B., Kami‐ike N., and Namba K.. 2009. Roles of the extreme N‐terminal region of FliH for efficient localization of the FliH‐FliI complex to the bacterial flagellar type III export apparatus. Mol. Microbiol. 74:1471–1483. [DOI] [PubMed] [Google Scholar]
- Minamino, T. , Shimada M., Okabe M., Saijo‐Hamano Y., Imada K., Kihara M., et al. 2010. Role of the C‐terminal cytoplasmic domain of FlhA in bacterial flagellar type III protein export. J. Bacteriol. 192:1929–1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino, T. , Morimoto Y. V., Hara N., and Namba K.. 2011. An energy transduction mechanism used in bacterial type III protein export. Nat. Commun. 2:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino, T. , Kinoshita M., Hara N., Takeuchi S., Hida A., Koya S., et al. 2012a. Interaction of a bacterial flagellar chaperone FlgN with FlhA is required for efficient export of its cognate substrates. Mol. Microbiol. 83:775–788. [DOI] [PubMed] [Google Scholar]
- Minamino, T. , Kinoshita M., Imada K., and Namba K.. 2012b. Interaction between FliI ATPase and a flagellar chaperone FliT during bacterial flagellar protein export. Mol. Microbiol. 83:168–178. [DOI] [PubMed] [Google Scholar]
- Minamino, T. , Morimoto Y. V., Kinoshita M., Aldridge P. D., and Namba K.. 2014. The bacterial flagellar protein export apparatus processively transports flagellar proteins even with extremely infrequent ATP hydrolysis. Sci. Rep. 4:7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto, Y. V. , Nakamura S., Kami‐ike N., Namba K., and Minamino T.. 2010. Charged residues in the cytoplasmic loop of MotA are required for stator assembly into the bacterial flagellar motor. Mol. Microbiol. 78:1117–1129. [DOI] [PubMed] [Google Scholar]
- Morimoto, Y. V. , Ito M., Hiraoka K. D., Che Y. S., Bai F., Kami‐Ike N., et al. 2014. Assembly and stoichiometry of FliF and FlhA in Salmonella flagellar basal body. Mol. Microbiol. 91:1214–1226. [DOI] [PubMed] [Google Scholar]
- Ohnishi, K. , Kutsukake K., Suzuki H., and Iino T.. 1992. A novel transcriptional regulation mechanism in the flagellar regulon of Salmonella typhimurium: anti‐sigma factor inhibits the activity of the flagellum‐specific sigma factor, σ F . Mol. Microbiol. 6:3149–3157. [DOI] [PubMed] [Google Scholar]
- Ohnishi, K. , Ohto Y., Aizawa S., Macnab R. M., and Iino T.. 1994. FlgD is a scaffolding protein needed for flagellar hook assembly in Salmonella typhimurium . J. Bacteriol. 176:2272–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi, K. , Fan F., Schoenhals G. J., Kihara M., and Macnab R. M.. 1997. The FliO, FliP, FliQ, and FliR proteins of Salmonella typhimurium: putative components for flagellar assembly. J. Bacteriol. 179:6092–6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallen, M. J. , Bailey C. M., and Beatson S. A.. 2006. Evolutionary links between FliH/YscL‐like proteins from bacterial type III secretion systems and second‐stalk components of the FoF1 and vacuolar ATPases. Protein Sci. 15:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, K. , Erhardt M., Hirano T., Blair D. F., and Hughes K. T.. 2008. Energy source of the flagellar type III secretion. Nature 451:489–492. [DOI] [PubMed] [Google Scholar]
- Saijo‐Hamano, Y. , Minamino T., Macnab R. M., and Namba K.. 2004. Structural and functional analysis of the C‐terminal cytoplasmic domain of FlhA, an integral membrane component of the type III flagellar protein export apparatus in Salmonella . J. Mol. Biol. 343:457–466. [DOI] [PubMed] [Google Scholar]
- Saijo‐Hamano, Y. , Imada K., Minamino T., Kihara M., Shimada M., Kitao A., et al. 2010. Structure of the cytoplasmic domain of FlhA and implication for flagellar type III protein export. Mol. Microbiol. 76:260–268. [DOI] [PubMed] [Google Scholar]
- Shimada, M. , Saijo‐Hamano Y., Furukawa Y., Minamino T., Imada K., and Namba K.. 2012. Functional defect and restoration of temperature‐sensitive mutants of FlhA, a subunit of the flagellar protein export apparatus. J. Mol. Biol. 415:855–865. [DOI] [PubMed] [Google Scholar]
- Thomas, J. , Stafford G. P., and Hughes C.. 2004. Docking of cytosolic chaperone‐substrate complexes at the membrane ATPase during flagellar type III protein export. Proc. Natl Acad. Sci. USA 101:3945–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukazaki, T. , Mori H., Echizen Y., Ishitani R., Fukai S., Tanaka T., et al. 2011. Structure and function of a membrane component SecDF that enhances protein export. Nature 474:235–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, S. , Fujita H., Sugata K., Taira T., and Iino T.. 1984. Genetic analysis of H2, the structural gene for phase‐2 flagellin in Salmonella . J. Gen. Microbiol. 130:255–265. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, S. , Aizawa S., Kihara M., Isomura M., Jones C. J., and Macnab R. M.. 1986. Genetic evidence for a switching and energy‐transducing complex in the flagellar motor of Salmonella typhimurium. J Bacteriol . 168:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, S. , Kojima S., and Homma M.. 2013. Structure, gene regulation and environmental response of flagella in Vibrio . Front. Microbiol. 4:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Sequence alignment of FlhA proteins of Salmonella enterica and Vibrio alginolyticus.Figure S2. Characterization of a Salmonella VaflhA strain.Figure S3. Effects of (A) carbonyl cyanide m‐chlorophenylhydrazone (CCCP) and (B) 100 mmol/L NaCl on the level of FlgD secreted by the VaflhA cells.Figure S4. Effect of the FlhB(P28T) mutation on motility of the VaflhA cells.


