Abstract
The freshwater cyanobacteria, Microcystis sp., commonly form large colonies with bacteria embedded in their mucilage. Positive and negative interactions between Microcystis species and their associated bacteria have been reported. However, the potential role of bacteria in the production and degradation of cyanobacterial secondary metabolites has not been investigated. In this study, a Microcystis‐associated bacterial community was isolated and added to the axenic M. aeruginosa PCC7806 liquid culture. After 3 years of cocultivation, we studied the bacterial genetic diversity adapted to the PCC7806 strain and compared the intra‐ and extracellular concentration of major cyanopeptides produced by the cyanobacterial strain under xenic and axenic conditions. Mass spectrometric analyses showed that the intracellular concentration of peptides was not affected by the presence of bacteria. Interestingly, the produced peptides were detected in the axenic media but could not be found in the xenic media. This investigation revealed that a natural bacterial community, dominated by Alpha‐proteobacteria, was able to degrade a wide panel of structurally varying cyclic cyanopeptides.
Keywords: Associated bacteria, biodegradation, cyanopeptides, Microcystis, phycosphere
Introduction
In the natural freshwater environment, cyanobacteria are always associated with heterotrophic bacteria that become embedded in their mucilage (Whitton 1973; Brunberg 1999; Berg et al. 2009). This habitat, in which intense interactions between cyanobacteria and bacteria occur, is referred to as the “phycosphere” by analogy of the rhizosphere of plants (Bell and Mitchell 1972). Several studies have focused on the interplay occurring between freshwater cyanobacteria and their associated bacteria, including competition or exchange of nutrients (Steppe et al. 1996; Fuks et al. 2005; Yuan et al. 2009), inhibition (Paerl 1996; Rashidan and Bird 2001; Ozaki et al. 2008) or enhancement of cyanobacterial growth (Paerl 1996; Casamatta and Wickstrom 2000; Eiler et al. 2006), degradation of cyanobacterial toxins (Bourne et al. 2001; Rapala et al. 2005; Ho et al. 2012), and finally formation of aggregates (Shen et al. 2011). Free‐living and attached bacterial communities have been shown to be distinct (Shi et al. 2012; Parveen et al. 2013) and marked changes occur in the structure and composition of attached bacterial communities during the course of a Microcystis bloom (Parveen et al. 2013), suggesting that the physiological status of cyanobacteria could have direct impacts on the associated bacterial community. Using a metatranscriptomic approach, Penn et al. (2014) recently highlighted the importance of functionally active co‐occurring bacteria in the metabolism of cyanobacterial exudates which can in turn help sustain cyanobacterial growth by nutrient and energy recycling.
It is well‐known that cyanobacteria release by exudation or cell lysis a variety of organic molecules such as organic acids, carbohydrates, proteins, and lipids (Amemiya et al. 1990; Tonietto et al. 2014; Kehr and Dittmann 2015), including bioactive compounds (Sivonen and Börner 2008). Indeed, freshwater cyanobacteria are able to produce a variety of secondary metabolites (aeruginosins: Ersmark et al. 2008; anabaenopeptins: Itou et al. 1999; cyanobactins: Sivonen et al. 2010; cyanopeptolins: Bister et al. 2004; microginins: Ishida et al. 2000; microviridins: Ishitsuka et al. 1990) displaying various bioactivities including cytotoxic, antiviral, antimalarial, or allelopathic through the inhibition of vital eukaryotic enzymes (mostly serine proteases; Welker and Von Döhren 2006; Niedermeyer 2015 and references herein). Among them, the microcystins (MC) are the most widely distributed and studied cyanotoxins due to their detrimental impact on a variety of organisms, including humans (Carmichael 1992). Although much is known about MC‐degrading bacteria (for reviews see Edwards and Lawton 2009; Ho et al. 2012; Dziga et al. 2013; Kormas and Lymperopoulou 2013), there have been very few studies on the potential role of bacteria in the degradation of the other cyanobacterial secondary metabolites (Kato et al. 2007).
In this study, we investigated the model organism Microcystis aeruginosa by high‐throughput sequencing, targeting a fragment of the 16S rRNA gene, combined with mass spectrometry techniques (LC–MS/MS), to examine the role of bacteria in the production and degradation of cyanopeptides. Our aims were (1) to compare the intra‐ and extracellular concentrations of major cyanopeptides produced by a M. aeruginosa strain in coculture with or without the natural bacterial community, (2) to characterize the diversity of a natural Microcystis‐associated heterotrophic bacterial community adapted to a M. aeruginosa strain culture, and (3) to explore specific interactions between cyanobacteria/bacteria within the phycosphere through the production of cyanopeptides and their degradation by the associated bacteria.
Material and Methods
Cyanobacterial and bacterial culture condition
The freshwater M. aeruginosa PCC 7806 cyanobacterial strain used was purchased from the Pasteur Culture collection of Cyanobacteria (http://cyanobacteria.web.pasteur.fr/). The interest to use this model was the possibility to work under axenic condition. PCC strains are the only axenic strains available in the world. The culture was cultivated in BG110 medium supplemented with 1.8 mmol L‐1 of NaNO3 and 10 mmol L‐1 of NaHCO3 (hereafter modified BG110 medium, Rippka and Herdman 1992). The culture grown under a 12:12 h light:dark regime using daylight white fluorescent tubes (Toshiba, 15 W, FL15D) with 10 μmol m−2 sec‐1 illumination at a constant temperature of 25°C on an orbital shaker (90–100 rpm). The cultures were maintained in exponential growth phase by repeated dilution every 3 weeks in fresh culture medium, while the axenicity was regularly evaluated as described in Briand et al. (2012).
The natural bacterial community was isolated from the mucilage of M. aeruginosa colonies during a bloom in a French pond in October 2011 as described by Shen et al. (2011). Briefly, colonies were repeatedly washed in sterile Milli‐Q water and centrifuged (10 min, ×4000g at 4°C). The supernatant containing the natural bacterial community was filtered through sterile GF/C filter papers (Whatman, Buckinghamshire, UK) in order to avoid contamination by naturally occurring M. aeruginosa cells. Inoculation of 1 mL of the supernatant containing the natural bacterial community was immediately added to 40 mL of the axenic M. aeruginosa PCC 7806 culture. The xenic culture was scaled up with fresh modified BG110 medium under the conditions described above to obtain a culture volume of 1 L. The xenic culture was maintained during 3 years by addition of fresh medium every 4–5 months to maintain a constant volume due to the loss by evaporation.
Experiments setup
Four different treatments were established: an axenic Microcystis culture (M. aeruginosa axenic), a xenic Microcystis culture (M. aeruginosa xenic), a pure culture of heterotrophic bacteria (Bact), and an axenic M. aeruginosa cell‐free filtrate (M. aeruginoa filtrate). Each treatment was grown in 500‐mL Erlenmeyer flasks with a final volume of 300 mL. For the axenic and xenic treatments, 150 mL of exponentially growing axenic M. aeruginosa PCC 7806 strain were harvested by centrifugation (10 min, 4000g at 25°C) and transferred to 300 mL of modified BG110 medium for the axenic treatment, or transferred to a volume of 300 mL consisting of equal volumes of the natural bacterial community and 2× concentrated culture modified BG11 medium for the xenic treatment. The volume of the natural bacterial community added to the axenic M. aeruginosa PCC 7806 culture was harvested from the xenic culture maintained during 3 years in exponential growth phase after centrifugation (10 min, 4000g at 25°C) and filtration through sterile GF/C paper filter (Whatmann, UK). “Bact treatment” consisted of 150 mL of bacterial culture (harvested as described above) and 150 mL of cell‐free filtrate of the axenic M. aeruginosa PCC 7806 culture. M. aeruginosa PCC 7806 cell‐free filtrate was obtained from an exponential growing axenic cyanobacterial culture after centrifugation (10 min, 4000g at 25°C) and filtration through sterile 0.2 μm nitrocellulose filter (Millipore, Cork, Ireland). Treatment M. aeruginosa filtrate corresponded to 300 mL of axenic cyanobacterial filtrate. Each treatment was prepared in triplicate and was incubated under the conditions described above. The experiment lasted 3 weeks at which time the cultures were harvested for extraction and High‐performance liquid chromatography mass spectrometry (HPLC–MS/MS) analysis.
Sampling, extraction, and internal standard addition
After 3 weeks of experiment, volumes were harvested and centrifuged (10 min, 4000g at 25°C). Freeze‐dried biomass was extracted up to three times in 2:1 dichloromethane:methanol (DCM:MeOH, JT Baker, Center Valley, PA) and were evaporated to dryness. The supernatants were filtered through a sterile 0.2 μm nitrocellulose filter (Millipore) and extracted on SPE‐C18 cartridges (GracePureTM; 5000 mg, Columbia, MD). Samples were evaporated to dryness under N2 and kept frozen until analysis.
Dry intra‐ and extracellular extracts were dissolved with acetonitrile (ACN, EMD Chemicals, Gibbstown, NJ) and internal standard (BOC‐L‐protected Ornithine, 0.25 mg mL−1 in ACN, Chem‐Impex International, Wood Dale, IL) was added before HPLC–MS/MS analysis.
High‐performance liquid chromatography–electrospray ionization‐mass spectrometry (HPLC–ESI‐MS/MS) analysis
Each sample (20 μL) was injected twice into a reverse‐phase HPLC system using a Phenomenex Kinetex C18 column (5μ, 100 mm × 4.60 mm) with a gradient of 5–99% ACN in water with 0.1% formic acid over 12 min, held at 99% for 5 min, and then returned to 5% at 22 min for another 3 min. The solvents were LC–MS grade (JT Baker, Center Valley, PA). The flow rate was 0.7 mL min‐1.
The HPLC eluate was electrospray ionized (capillary temperature at 325°C, source voltage at 5 kV, and a sheath gas flow rate of 69 L min‐1) and analyzed in the positive mode in the mass range of m/z from 300 to 2000 using a Thermo‐Finnigan LCQ Advantage ion trap mass spectrometer (Thermo‐Finnigan, San Jose, CA). MS/MS spectra were obtained in a data‐dependent manner using collision‐induced dissociation at 35 eV.
Cyanopeptide identification and relative quantification
Secondary metabolites profiles of the M. aeruginosa PCC 7806 strain has been described in a previous experiment (Briand et al. 2015). Among those identified cyanopeptides, eight major compounds belonging to three known peptide classes were screened (Table 1): cyanobactins (Aer A, B, C, and D), cyanopeptolins (Cya A and Cya 963A), and microcystins (MC‐LR and Des‐MCLR).
Table 1.
Peptides produced by the studied strains
| Peptide class | m/z [M+H]+ | Peak number (retention time in min) | Assignment (Reference) |
|---|---|---|---|
| Microcystin (MC) | 981 | 2 (11.1) | Des‐MCLR (Mayumi et al. 2006) |
| 995 | 3 (11.2) | MC‐LR (Mayumi et al. 2006) | |
| Cyanopeptolin (Cya) | 946 [M‐H2O]+ | 5 (13.0) | Cyanopeptolin 963A (Bister et al. 2004) |
| 957 | 4 (11.6) | Cyanopeptolin A (Martin et al. 1993) | |
| Cyanobactin (Aer) | 517 | 7 (14.3) | Aerucyclamide C (Portmann et al. 2008b) |
| 533 | 8 (14.6) | Aerucyclamide B (Portmann et al. 2008a) | |
| 535 | 6 (14.0) | Aerucyclamide A (Portmann et al. 2008a) | |
| 603 [M+OH]+ | 1 (10.7) | Aerucyclamide D (Portmann et al. 2008b) |
In order to compare relative concentrations of specific metabolites between different treatments, the peak area was determined with the software Xcalibur (Thermo) using the Peak Detection Genesis Algorithm. The quantification was based on the ratio of the peak area of metabolites and the added internal standard, BOC‐L‐protected ornithine. Data were then normalized to the dry weight for intracellular metabolites and to the volume for extracellular metabolites as described in Winnikoff et al. (2014).
DNA extraction and high‐throughput sequencing
The natural bacterial community was sequenced after 3 years of coculture with the M. aeruginosa strain. The cell pellet of the xenic culture was centrifuged (10 min, 4000 g, 25°C), freeze‐dried overnight, and kept frozen until DNA extraction. The DNA extraction procedure was based on mechanical and chemical extraction and was adapted from the procedure described in Massana et al. (1997). A fragment of the 16S rRNA gene, including the variable V4–V5 region, was amplified by PCR from the DNA using primers 515F (5′‐GTGYCAGCMGCCGCGGTA‐3′) and 909R (5′‐CCCCGYCAATTCMTTTRAGT‐3′) (Tamaki et al. 2011). The sequencing was performed by Research and Testing Laboratory (Lubbock, Texas) on IlluminaMiSeq. The forward and reverse reads were merged together using the PEAR Illumina paired‐end read merger (Zhang et al. 2014). Chimera detection and removal by executing UCHIME (Edgar et al. 2011) and sequences were clustered at a 4% divergence into OTUs using the UPARSE algorithm (Edgar 2013). The centroid sequence from each cluster was then run against either the USEARCH global alignment algorithm (Edgar 2010) or the RDP Classifier against a database of high‐quality sequences derived from the NCBI database.
Statistical analysis
Statistical differences in normalized relative concentrations were evaluated using two‐tailed t‐test. The analyses were conducted with the software Graphpad Prism 4.00 (San Diego, CA). Differences were accepted as statistically significant when P < 0.05. Values are given as mean ± standard deviation (SD).
Results and Discussion
Comparison of the intra‐ and extracellular concentrations of major cyanopeptides produced by M. aeruginosa under xenic and axenic conditions
In a first experiment, we compared the secondary metabolic profile of the axenic M. aeruginosa strain PCC 7806 with the profile of the same strain after the addition of a natural bacterial community. As described in a previous experiment (Briand et al. 2015), the axenic strain produced eight major compounds (Table 1) belonging to three known peptide classes: cyanobactins (Aer A, B, C, and D), cyanopeptolins (Cya A and 963A), and microcystins (MC‐LR and Des‐MCLR).
No significant difference was found in the intracellular concentration of the eight cyanopeptides of the axenic and xenic PCC 7806 strain (Fig. 1A, P values >0.05), suggesting that the associated bacteria do not directly influence the production of secondary metabolites by M. aeruginosa PCC 7806. Conflicting findings have been reported concerning this bacterial–cyanobacterial interaction in previous studies. Sivonen (1990) has shown that MC production by Planktothrix agardhii strains was not influenced by the presence of bacteria whereas Dziallas and Grossart (2011) reported a strong impact of heterotrophic bacteria on the quantity and nature of MC variants produced by a M. aeruginosa strain. It has also been widely reported that bacteria influence the metabolic profile of marine diatoms; for example, the rate of biosynthesis of the neurotoxin domoic acid increases in xenic cultures of Pseudo‐nitzschia multiseries (Bates et al. 1995, 2004; Kobayashi et al. 2009; Stewart 2008). The regulation of production of such secondary metabolites, even for the most widespread and studied MC, is still unclear and requires further investigation concerning their regulation in response to biotic and abiotic conditions.
Figure 1.
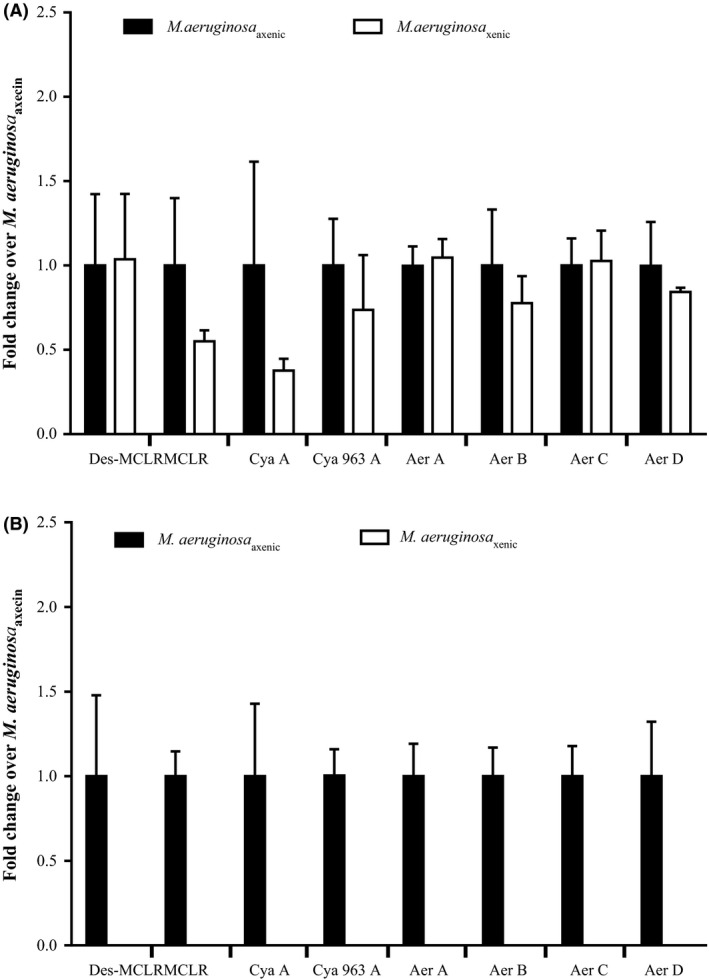
Relative concentrations of the main cyanopeptides produced by M. aeruginosa PCC 7806 strain (A: intracellular and B: extracellular) under axenic (black histograms) or xenic conditions (white histograms). Aer: Aerucyclamide, Cya: Cyanopeptolin, Des‐MCLR: Desmethyl Microcystin LR, MC‐LR: Microcystin LR.
When comparing the extracellular fractions between the axenic and xenic strains, all the cyanopeptides present in the intracellular fraction were detected in the axenic media but they were completely absent in the xenic media (Fig. 1B). The first result provides evidence that the major cyanopeptides detected in the intracellular fraction are released out of the cells due to lysis and/or active transport. The second finding showed that degradation and/or transformation of these natural products by bacteria might cause the observed result, although we cannot rule out the possibility of inhibition of excretion by the bacterial community.
Characterization of the natural bacterial community associated to M. aeruginosa
In order to characterize the bacteria potentially involved in degrading these metabolites, we sequenced the bacterial community from the xenic PCC 7806 strain. This community was dominated by Proteobacteria (88%), in particular by Alpha‐proteobacteria (82%), and all other phyla were present at very low abundance (Fig. 2). The global structure of this community differs significantly from bacterial communities occupying freshwater lakes that are characterized by a codominance of Actinobacteria, and Alpha‐ and Betaproteobacteria (Humbert et al. 2009; Newton et al. 2011). However, Proteobacteria have previously been found to dominate Microcystis‐associated bacterial communities (Li et al. 2011; Shi et al. 2012; Parveen et al. 2013). Moreover, a recent metatranscriptomic study of Microcystis‐associated bacterial communities revealed the prevalence of Proteobacteria interacting closely with cyanobacteria as indicated by the highest transcription levels being observed for uptake of branched‐chain amino acids and organic phosphorus compounds (Penn et al. 2014). Interestingly, when looking at the OTU level, it appears that the dominant OTUs belong to the order of Rhizobiales (Table S1) display a high sequence similarity with OTUs found in the rhizosphere of plants and/or in environments displaying high quantities of organic matter (OM) such as sludge (Agrobacterium tumefaciens, Rhizobium sp., Hyphomicrobium sp., and Mezorhizobium sp.). Similarly, the only Actinobacteria OTU found displays a high sequence similarity with Rhodococcus erythropolis, which is known for its ability to degrade organic pollutants (De Carvalho et al. 2014). The genus Sphingomonas, accounting for 2% of the total bacterial diversity in this study, has been pointed by most studies to be closely associated with Microcystis (Shi et al. 2009, 2012; Dziallas and Grossart 2011; Shen et al. 2011; Zhu et al. 2014). Sphingomonadales species are well adapted to the cyanobacterial phycosphere, especially that of Microcystis with regard to their capacity to degrade cyanobacterial toxins and other problematic organic compounds (Wilkes et al. 1996; Zylstra and Kim 1997; Berg et al. 2009). With this regard, the bacterial community added to the xenic Microcystis strain is most likely adapted to degrade cyanobacterial‐derived OM exudates including cyanopeptides.
Figure 2.
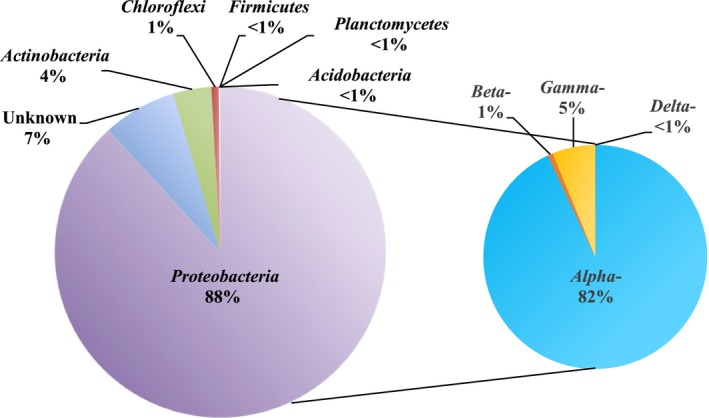
Relative abundance of major bacterial phyla.
Cyanopeptides‐degradation capability of the natural bacterial community associated to M. aeruginosa
With the goal to confirm the degradation of cyanopeptides by bacteria, we added the filtrate of the axenic PCC 7806 strain to two flasks either containing or not the natural bacterial community identified above (Fig. 3A).
Figure 3.

Chromatograms obtained for the extracellular cell‐free filtrate of the axenic M. aeruginosa PCC 7806 strain (M. aeruginosa filtrate) at the beginning (A) and at the end of the experiment (B), and that obtained for a pure culture of heterotrophic bacteria (Bact) treatment (C). Peaks legend: 1‐Aer D, Aerucyclamide D; 2‐Des‐MCLR, Desmethyl Microcystin LR; 3‐MC‐LR, Microcystin LR; 4‐Cya A, Cyanopeptolin A; 5‐Cya 963A, Cyanopeptolin 963A; 6‐Aer A, Aerucyclamide A; 7‐Aer C, Aerucyclamide C; 8‐Aer B, Aerucyclamide B; IS, Internal Standard.
Cyanopeptides were detected at the beginning of the experiment in the cell‐free filtrate of the axenic strain (Fig. 3A) and also after 3 weeks in the flasks without a bacterial community (Fig. 3B). On the other hand, chromatograms obtained for the extracellular cell‐free filtrate of the bacterial pure culture (Fig. 3C) were completely flat except for the peak corresponding to the internal standard. None of the eight compounds were detected by scanning chromatograms for their molecular ion masses at their respective retention time, suggesting that the natural bacterial community was able to completely remove the cyanopeptides to concentrations below that required for analytical detection. To our knowledge, this is the first study showing that a natural bacterial community is able to degrade not only MC but also other cyanobacterial secondary metabolites (cyanopeptolins and aerucyclamides in this study). Of all the cyanotoxins biodegradation studies, most have focused on the MC, as from a practical perspective, such as cyanotoxin‐degrading bacteria could be implemented as a low‐cost biological treatment option in water or sewage treatment plants. So far, the majority of isolated organisms reported as having the ability to degrade MC or other cyanotoxins appears to belong to the class Alpha‐proteobacteria and members of the Sphingomonadaceae are the most prevalent family among this class (for reviews see Edwards and Lawton 2009; Ho et al. 2012; Dziga et al. 2013; Kormas and Lymperopoulou 2013). Kato et al. (2007) showed the more or less effective degradation of different cyclic cyanopeptides (anabaenopeptin A, aeruginopeptin 95A, microcyclamide, MC‐LR, microviridin I, nodularin, and nostophycin) by cell extracts of the Sphingomonas sp. B‐9, first isolated for its MC‐degradation ability (Imanishi et al. 2005). In this study, Shingomonas sp. accounted for only 2% of the total bacterial diversity suggesting that several species of the natural bacterial community were probably also involved in the degradation of the full spectrum of naturally occurring peptides. Further studies using the RNA stable isotope probing (RNA‐SIP, Manefield et al. 2002) technique would be an effective approach for specifically identifying active microorganisms that assimilate particular 13C‐labeled substrates (e.g., cyanobacterial cell extracts or specific cyanopeptides) into their cellular biomass. Such knowledge would be instrumental in employing their cyanopeptide‐degrading capability for bioremediation, reducing the costs associated with water or environmental treatments.
Therefore, our results demonstrate that the bacterial community associated with a Microcystis bloom and exposed to Microcystis exudates was able to degrade effectively dissolved cyanopeptides as one of its DOC and nitrogen sources required for growth. Interestingly, the growth of xenic Microcystis cultures was self‐sufficient over 3 years (fresh medium was added every 4–5 months to maintain a constant volume due to the loss by evaporation), whereas the axenic culture needed repeated dilution every 3 weeks in fresh culture medium in order to be maintained in the exponential growth phase. Although we did not measure the bacterial growth, our observations suggest that Microcystis exudates containing dissolved cyanopeptides were able to provide the bulk of dissolved organic carbon (DOC) and nutrients needed for bacterial growth. The impact of DOC released by cyanobacteria on growth and activity of associated bacteria has been shown in previous studies (Robarts and Zohary 1986; Christoffersen et al. 2002; Kirkwood et al. 2006). In turn, the bacterial community may contribute to support cyanobacterial growth by remineralizing this portion of the dissolved organic matter (DOM) back to CO2 (Cho and Azam 1988). Coupling isotopic tracers with imaging mass spectrometry analysis (NanoSIMS) would be a powerful approach to highlight and quantify the relative role of cyanopeptides among the pool of DOM in providing carbon and nutrients from cyanobacteria to heterotrophic bacteria and vice versa. Previous studies have been performed to visualize and quantify the nitrogen transfer from N2‐fixing symbionts to their hosts [e.g., bacteria/shipworm symbiosis (Lechene et al. 2007) and cyanobacteria/diatom symbiosis (Foster et al. 2011)], or more recently, sulfur cycling within a bacterial consortium (Wilbanks et al. 2014). The isotope tracer/NanoSIMS approach would also provide valuable information on the degradation rate of cyanopeptides by associated bacteria and on the flux of cyanopeptide‐derived carbon and nutrients within the cyanobacterial phycosphere. Hence, mutualistic interactions through the production, degradation, and remineralization of cyanopeptides within the tightly coupled microbial consortia may contribute to the ecological success of Microcystis in freshwater ecosystems.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Table S1. Relative abundance at the OTU level.
Acknowledgments
This work was supported by a Marie Curie IOF Fellowship within the 7th European Community Framework Programme (FP7‐ PEOPLE‐2011‐IOF, grant number 301244‐CYANOMIC), NIH grant GM107550 and the PHYCOCYANO project in the Jeunes Chercheurs‐Jeunes Chercheuses program of the French ANR (Agence Nationale de la Recherche; ANR‐11‐JSV7‐014‐01). Alexandrine Pannard and Marion Lengronne are acknowledged for their help for field sampling of the natural bacterial community.
MicrobiologyOpen 2016; 5(3): 469–478
References
- Amemiya, Y. , Kato K., Okino T., and Nakayama O.. 1990. Changes in the chemical composition of carbohydrates and proteins in surface water during a bloom of Microcystis in lake Suwa. Ecol. Res. 5:153–162. [Google Scholar]
- Bates, S. S. , Douglas D. J., Doucette G. J., and Léger C.. 1995. Enhancement of domoic acid production by reintroducing bacteria to axenic cultures of the diatom Pseudo‐nitzschia multiseries . Nat. Toxins 3:428–435. [DOI] [PubMed] [Google Scholar]
- Bates, S. S. , Gaudet J., Kaczmarska I., and Ehrman J. M.. 2004. Interaction between bacteria and the domoic‐acid‐producing diatom Pseudo‐nitzschia multiseries (Hasle) Hasle; can bacteria produce domoic acid autonomously? Harmful Algae 3:11–20. [Google Scholar]
- Bell, W. , and Mitchell R.. 1972. Chemotactic and growth responses of marine bacteria to algal extra cellular products. Biol. Bull. 143:265–277. [Google Scholar]
- Berg, K. A. , Lyra C., Sivonen K., Paulin L., Suomalainen S., Tuomi P., et al. 2009. High diversity of cultivable heterotrophic bacteria in association with cyanobacterial water blooms. ISME J. 3:314–325. [DOI] [PubMed] [Google Scholar]
- Bister, B. , Keller S., Baumann H. I., Nicholson G., Weist S., Jung G., et al. 2004. Cyanopeptolin 963A, a chymotrypsin of Microcystis PCC 7806. J. Nat. Prod. 67:1755–1757. [DOI] [PubMed] [Google Scholar]
- Bourne, D. G. , Riddles P., Jones G. J., Smith W., and Blakelay R. L.. 2001. Characterisation of a gene cluster involved in bacterial degradation of the cyanobacterial toxin microcystin LR. Environ. Toxicol. 16:523–534. [PubMed] [Google Scholar]
- Briand, E. , Bormans M., Quiblier C., Salencon M. J., and Humbert J.‐F.. 2012. Evidence of the cost of the production of microcystins by Microcystis aeruginosa under differing light and nitrate environmental conditions. PLoS ONE 7:e29981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand, E. , Bormans M., Gugger M., Dorrestein P. C., and Gerwick W. H.. 2015. Changes in secondary metabolic profiles of Microcystis aeruginosa strains in response to intraspecific interactions. Environ. Microbiol. doi:10.1111/1462‐2920.12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunberg, A. K. 1999. Contribution of bacteria in the mucilage of Microcystis spp. (Cyanobacetria) to benthic and pelagic bacterial production in a Hypereutrophic lake. FEMS Microbiol. Ecol. 29:13–22. [Google Scholar]
- Carmichael, W. W. 1992. Cyanobacteria secondary metabolites – the cyanotoxins. J. Appl. Bacteriol. 72:445–459. [DOI] [PubMed] [Google Scholar]
- Casamatta, D. A. , and Wickstrom C. E.. 2000. Sensitivity of two bacterioplankton communities to exudates from the cyanobacterium Microcystis aeruginosa Kützing. Microb. Ecol. 41:64–73. [DOI] [PubMed] [Google Scholar]
- Cho, B. C. , and Azam F.. 1988. Major role of bacteria in biogeochemical fluxes in the ocean's interior. Nature 332:441–443. [Google Scholar]
- Christoffersen, K. , Lyck S., and Winding A.. 2002. Microbial activity and bacterial community structure during degradation of microcystins. Aquat. Microb. Ecol. 27:125–136. [Google Scholar]
- De Carvalho, C. C. C. R. , Costa S. S., Fernandes P., Couto I., and Viveiros M.. 2014. Membrane transport systems and the biodegradation potential and pathogenicity of genus Rhodococcus . Front. Physiol. 5:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziallas, C. , and Grossart H. P.. 2011. Increasing oxygen radicals and water temperature select for toxic Microcystis sp. PLoS ONE 6:e25569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziga, D. , Wasylewski M., Wladyka B., Nybom S., and Meriluoto J.. 2013. Microbial degradation of microcystins. Chem. Res. Toxicol. 26:841–852. [DOI] [PubMed] [Google Scholar]
- Edgar, R. C. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. [DOI] [PubMed] [Google Scholar]
- Edgar, R. C. 2013. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10:996–998. [DOI] [PubMed] [Google Scholar]
- Edgar, R. C. , Haas B. J., Clemente J. C., Quince C., and Knight R.. 2011. UCHIME improves sensitivity and speed of chimera detection. Oxford J. Bioinf. 27:2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, C. , and Lawton L. A.. 2009. Bioremediation of cyanotoxins. Adv. Appl. Microbiol. 67:109–129. [DOI] [PubMed] [Google Scholar]
- Eiler, A. , Olsson J. A., and Bertilsson S.. 2006. Diurnal variations in the auto‐ and heterotrophic activity of cyanobacterial phycospheres (Gloeotrichia echinulata) and the identity of attached bacteria. Freshw. Biol. 51:289–311. [Google Scholar]
- Ersmark, K. , Del Valle J. R., and Hanessian S.. 2008. Chemistry and biology of the aeruginosin family of serine protease inhibitors. Angew. Chem. Int. Ed. 47:1202–1223. [DOI] [PubMed] [Google Scholar]
- Foster, R. A. , Kuypers M. M. M., Vagner T., Paerl R. W., Musat N., and Zehr J. P.. 2011. Nitrogen fixation and transfer in open ocean diatom‐cyanobacterial symbioses. ISME J. 5:1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks, D. , Radic J., Radic T., Najdek M., Blazina M., Degobbis D., et al. 2005. Relationships between heterotrophic bacteria and cyanobacteria in the northern Adriatic in relation to the mucilage phenomenon. Sci. Total Environ. 353:178–188. [DOI] [PubMed] [Google Scholar]
- Ho, L. , Sawade E., and Newcombe G.. 2012. Biological treatment options for cyanobacteria metabolite removal – A review. Water Res. 46:1536–1548. [DOI] [PubMed] [Google Scholar]
- Humbert, J.‐F. , Dorigo U., Cecchi P., Le Berre B., Debroas D., and Bouvy M.. 2009. Comparison of the structure and composition of bacterial communities from temperate and tropical freshwater ecosystems. Environ. Microbiol. 11:2339–2350. [DOI] [PubMed] [Google Scholar]
- Imanishi, S. , Kato H., Mizuno M., Tsuji K., and Harada K. I.. 2005. Bacterial degradation of microcystins and nodularin. Chem. Res. Toxicol. 18:591–598. [DOI] [PubMed] [Google Scholar]
- Ishida, K. , Kato T., Murakami M., Watanabe M., and Watanabe M. F.. 2000. Microginins, zinc metalloproteases inhibitors from the cyanobacterium Microcystis aeruginosa . Tetrahedron 56:8643–8656. [Google Scholar]
- Ishitsuka, M. O. , Kusumi T., Kakisawa H., Kaya K., and Watanabe M. F.. 1990. Microviridin, a novel tricyclic depsipeptide from the toxic cyanobacterium Microcystis viridis . J. Am. Chem. Soc. 112:8180–8182. [Google Scholar]
- Itou, Y. , Suzuki S., Ishida K., and Murakami M.. 1999. Anabaenopeptins G and H, potent carboxypeptidase A inhibitors from the cyanobacterium Oscillatoria agardhii (NIES‐595). Bioorg. Med. Chem. Lett. 9:1243–1246. [DOI] [PubMed] [Google Scholar]
- Kato, H. , Imanishi S. Y., Tsuji K., and Harada K.. 2007. Microbial degradation of cyanobacterial peptides. Water Res. 41:1754–1762. [DOI] [PubMed] [Google Scholar]
- Kehr, J. C. , and Dittmann E.. 2015. Biosynthesis and function of extracellular glycans in cyanobacteria. Life 5:164–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood, A. E. , Nalewajko C., and Fulthorpe R. R.. 2006. The effects of cyanobacterial exudates on bacterial growth and biodegradation of organic contaminants. Microb. Ecol. 51:4–12. [DOI] [PubMed] [Google Scholar]
- Kobayashi, K. , Takata Y., and Kodama M.. 2009. Direct contact between Pseudo‐nitzschia multiseries and bacteria is necessary for the diatom to produce a high level of domoic acid. Fish. Sci. 75:771–776. [Google Scholar]
- Kormas, K. A. , and Lymperopoulou D. S.. 2013. Cyanobacterial toxin degrading bacteria: who are they? Biomed. Res. Int. 2013:463893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechene, C. P. , Luyten Y., McMahon G., and Distel D. L.. 2007. Quantitative imaging of nitrogen fixation by individual bacteria within animal cells. Science 317:1563–1566. [DOI] [PubMed] [Google Scholar]
- Li, N. , Zhang L., Li F., Wang Y., Zhu Y., Kang H., et al. 2011. Metagenome of microorganisms associated with the toxic cyanobacteria Microcystis aeruginosa analyzed using the 454 sequencing platform. Chin. J. Oceanol. Limnol. 29:505–513. [Google Scholar]
- Manefield, M. , Whiteley A. S., Griffiths R. I., and Bailey M. J.. 2002. RNA stable isotope probing, a novel means of linking microbial community function to phylogeny. Appl. Environ. Microbiol. 68:5367–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, C. , Oberer L., Ino T., König W. A., Busch M., and Weckesser J.. 1993. Cyanopeptolins, new depsipeptides from the cyanobacterium Microcystis sp. PCC 7806. J. Antibiot. 46:1550–1556. [DOI] [PubMed] [Google Scholar]
- Massana, R. , Murray A. E., Preston C. M., and DeLong E. F.. 1997. Vertical distribution and phylogenetic characterization of marine planktonic Archaea in the Santa Barbara Channel. Appl. Environ. Microbiol. 63:50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayumi, T. , Kato H., Imanishi S., Kawasaki Y., Hasegawa M., and Harada K.. 2006. Structural characterization of microcystins by LC/MS/MS under ion trap conditions. J. Antibiot. 59:710–719. [DOI] [PubMed] [Google Scholar]
- Newton, R. J. , Jones S. E., Eiler A., McMahon K. D., and Bertilsson S.. 2011. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. 75:14–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedermeyer, T. H. 2015. Anti‐infective natural products from Cyanobacteria. Planta Med. 81:1309–1325. [DOI] [PubMed] [Google Scholar]
- Ozaki, K. , Ohta A., Iwata C., Horikawa A., Tsuji K., Ito E., et al. 2008. Lysis of cyanobacteria with volatile organic compounds. Chemosphere 71:1531–1538. [DOI] [PubMed] [Google Scholar]
- Paerl, H. W. 1996. Microscale physiological and ecological studies of aquatic cyanobacteria: macroscale implications. Microsc. Res. Tech. 33:47–72. [DOI] [PubMed] [Google Scholar]
- Parveen, B. , Ravet V., Djediat C., Mary I., Quiblier C., Debroas D., et al. 2013. Bacterial communities associated with Microcystis colonies differ from free‐living communities living the same ecosystem. Environ. Microbiol. Rep. 5:716–724. [DOI] [PubMed] [Google Scholar]
- Penn, K. , Wang J., Fernando S. C., and Thompson J. R.. 2014. Secondary metabolite gene expression and interplay of bacterial functions in a tropical freshwater cyanobacterial bloom. ISME J. 8:1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portmann, C. , Blom J. F., Gademann K., and Jüttner F.. 2008a. Aerucyclamides A and B: isolation and synthesis of toxic ribosomal heterocyclic peptides from the cyanobacterium Microcystis aeruginosa PCC 7806. J. Nat. Prod. 71:1193–1196. [DOI] [PubMed] [Google Scholar]
- Portmann, C. , Blom J. F., Kaiser M., Brun R., Jüttner F., and Gademann K.. 2008b. Isolation of aerucyclamides C and D and structure revision of microcyclamide 7806A: heterocyclic ribosomal peptides from Microcystis aeruginosa PCC 7806 and their antiparasite evaluation. J. Nat. Prod. 71:1891–1896. [DOI] [PubMed] [Google Scholar]
- Rapala, J. , Berg K. A., Lyra C., Niemi R. M., Manz W., S. Suomalainen , et al. 2005. Paucibacter toxinivorans gen. nov., sp. nov., a bacterium that degrades cyclic cyanobacterial hepatotoxins microcystins and nodularin. Int. J. Syst. Evol. Microbiol. 55:1563–1568. [DOI] [PubMed] [Google Scholar]
- Rashidan, K. K. , and Bird D. F.. 2001. Role of predatory bacteria in the termination of a cyanobacterial bloom. Microb. Ecol. 41:97–105. [DOI] [PubMed] [Google Scholar]
- Rippka, R. , and Herdman H.. 1992. Pasteur culture collection of cyanobacteria: catalogue and taxonomic handbook I. catalogue of strains. Institut Pasteur, Paris. [Google Scholar]
- Robarts, R. D. , and Zohary T.. 1986. Influence of cyanobacterial hyperscum on heterotrophic activity of planktonic bacteria in a hypertrophic lake. Appl. Environ. Microbiol. 51:609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, H. , Niu Y., Xie P., Tao M., and Yang X.. 2011. Morphological and physiological changes in Microcystis aeruginosa as a result of interactions with heterotrophic bacteria. Freshw. Biol. 56:1065–1080. [Google Scholar]
- Shi, L. , Cai Y., Yang H., Xing P., Li P., Kong L., et al. 2009. Phylogenetic diversity and specificity of bacteria associated with Microcystis aeruginosa and other cyanobacteria. J. Environ. Sci. (China) 21:1581–1590. [DOI] [PubMed] [Google Scholar]
- Shi, L. , Cai Y., Kong F., and Yu Y.. 2012. Specific association between bacteria and buoyant Microcystis colonies compared with other bulk bacterial communities in the eutrophic Lake Taihu, China. Environ. Microbiol. Rep. 4:669–678. [DOI] [PubMed] [Google Scholar]
- Sivonen, K. 1990. Effects of light, temperature, nitrate, orthophosphate, and bacteria on growth of and hepatotoxin production by Oscillatoria agardhii strains. Appl. Environ. Microbiol. 56:2658–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivonen, K. , and Börner T.. 2008. Bioactive compounds produced by cyanobacteria Pp. 159–197 in Herrero A. and Flores E., eds. The cyanobacteria: molecular biology, genomics and evolution. Caister Academic Press, Norfolk. [Google Scholar]
- Sivonen, K. , Leikoski N., Fewer D. P., and Jokela J.. 2010. Cyanobactins – ribosomal cyclic peptides produced by cyanobacteria. Appl. Microbiol. Biotechnol. 86:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steppe, T. F. , Olson J. B., Paerl H. W., Litaker R. W., and Belnap J.. 1996. Consortial N2 fixation: a strategy for meeting nitrogen requirements of marine and terrestrial cyanobacterial matts. FEMS Microbiol. Ecol. 21:149–156. [Google Scholar]
- Stewart, J. E. 2008. Bacterial involvement in determining domoic acid levels in Pseudo‐nitzschia multiseries cultures. Aquat. Microb. Ecol. 50:135–144. [Google Scholar]
- Tamaki, H. , Wright C. L., Li X., Lin Q., Hwang C., Thimmapuram J., et al. 2011. Analysis of 16S rRNA amplicon sequencing options on the roche/454 Next‐generation titanium sequencing platform. PLoS ONE 6:e25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonietto, A. E. , Lombardi A. T., Vieira A. A. H., Parrish C. C., and Choueri R. B.. 2014. Cylindrospermopsis raciborskii (Cyanobacteria) exudates: chemical characterization and complexation capacity for Cu, Zn, Cd and Pb. Water Res. 49:381–390. [DOI] [PubMed] [Google Scholar]
- Welker, M. , and Von Döhren H.. 2006. Cyanobacterial peptides – nature's own combinatorial biosynthesis. FEMS Microbiol. Rev. 30:530–563. [DOI] [PubMed] [Google Scholar]
- Whitton, B. A. . 1973. Interactions with other organisms Pp 415–433 In: Carr N. G., Whitton B. A. eds. The biology of blue‐green algae. Blackwell: Okford, United Kingdom. [Google Scholar]
- Wilbanks, E. G. , Jaekel U., Salman V., Humphrey P. T., Eisen J. A., Facciotti M. T., et al. 2014. Microscale sulfur cycling in the phototrophic pink berry consortia of the Sippewissett Salt Marsh. Env. Microbiol. 16:3398–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes, H. , Wittich R. M., Timmis K. N., Fortnagel P., and Francke W.. 1996. Degradation of chlorinated dibenzofurans and dibenzo‐p‐dioxins by Sphingomonas sp. strain RW1. Appl. Environ. Microbiol. 62:367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnikoff, J. R. , Glukhov E., Wartrous J., Dorrestein P. C., and Gerwick W. H.. 2014. Quantitative molecular networking to profile marine cyanobacterial metabolomes. J. Antibiot. 67:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, L. , Zhu W., Xiao L., and Yang L.. 2009. Phosphorus cycling between the colonial cyanobacterium Microcystis aeruginosa and attached bacteria, Pseudomonas . Aquat. Ecol. 43:859–866. [Google Scholar]
- Zhang, J. , Kobert K., Flouri T., and Stamatakis A.. 2014. PEAR: a fast and accurate illumina paired‐end reAd mergeR. Bioinformatics 30:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, L. , Wu Y., Song L., and Gan N.. 2014. Ecological dynamics of toxic Microcystis spp. and microcystin‐degrading bacteria in Dianchi Lake, China. Appl. Environ. Microbiol. 80:1874–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylstra, G. J. , and Kim E.. 1997. Aromatic hydrocarbon degradation by Sphingomonas yanoikuyae B1. J. Ind. Microbiol. Biotechnol. 19:408–414. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Relative abundance at the OTU level.


