Abstract
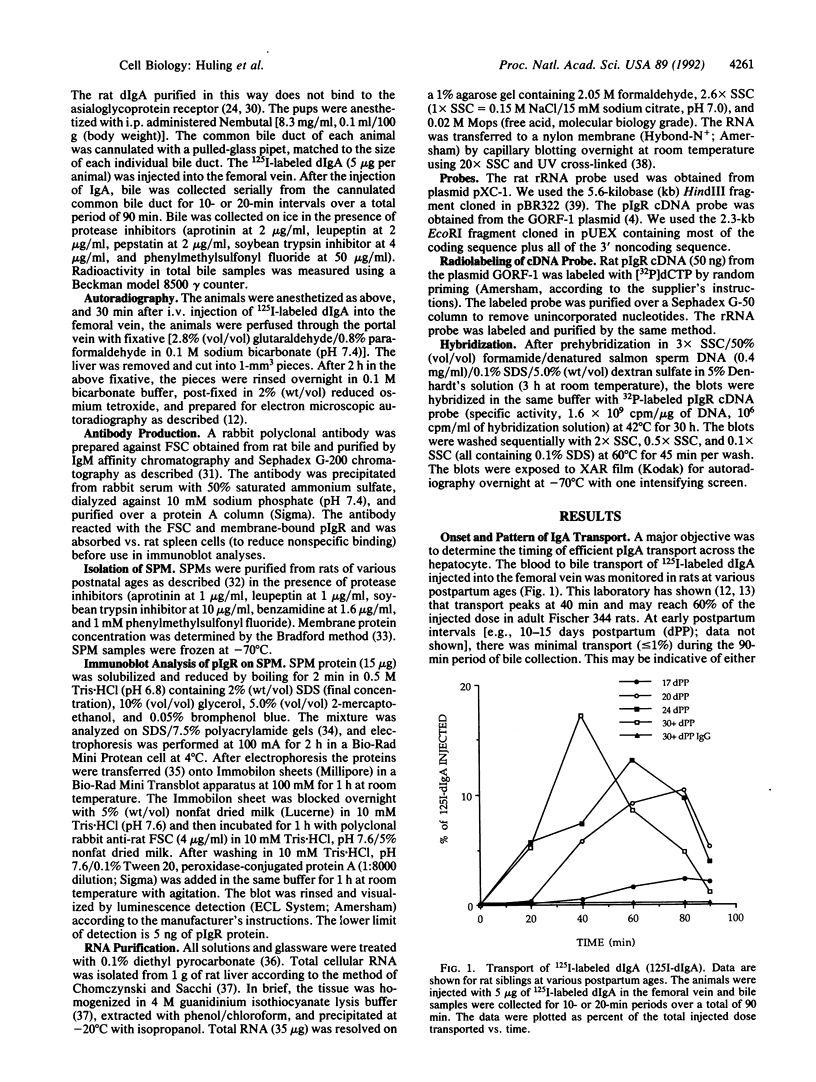
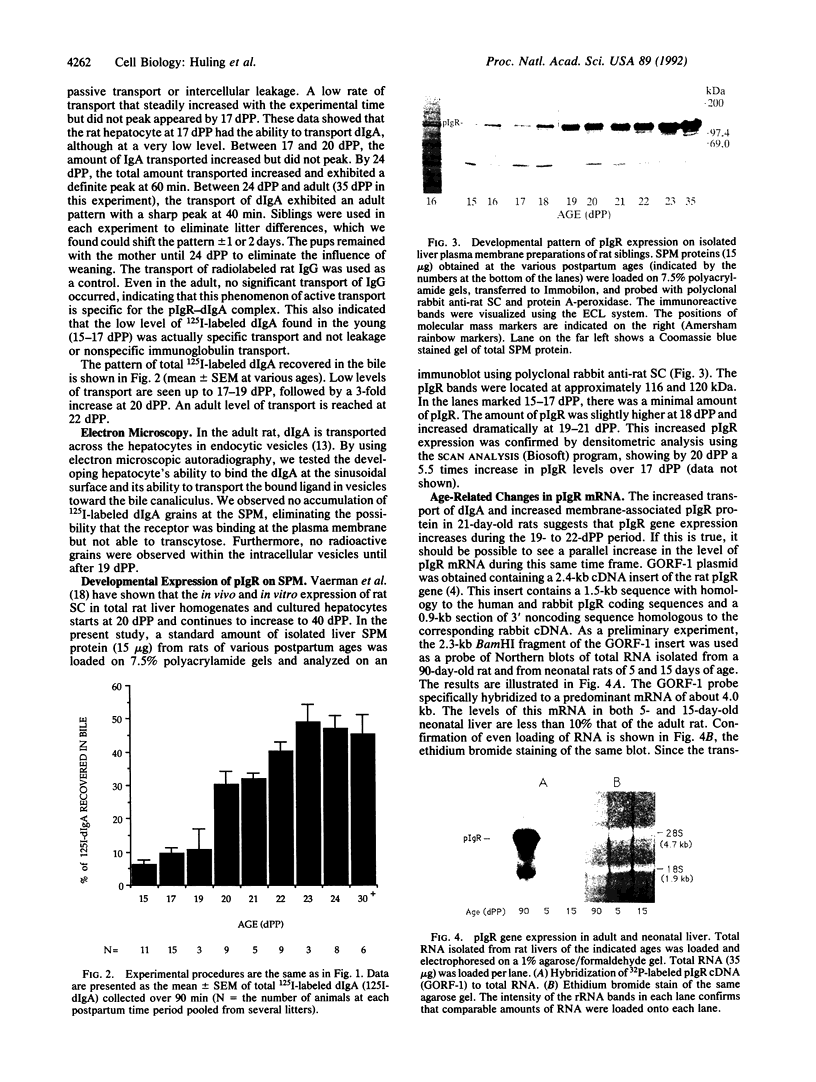
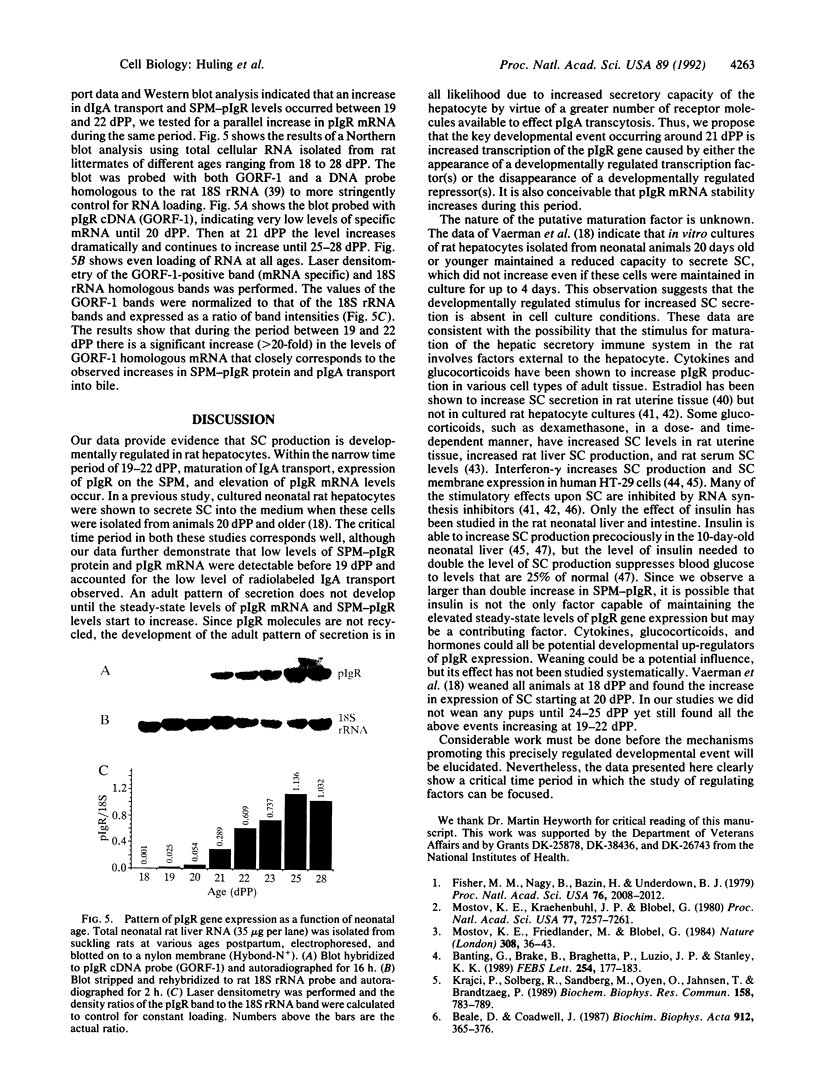
In the rat, secretion of polymeric IgA from serum into bile is dependent upon the presence of a functional polymeric immunoglobulin receptor (pIgR) that acts as a hepatocyte plasma membrane receptor for ligand binding and as a transcellular transport molecule. The objective of this study was to document the developmental maturation and regulation of functionally intact rat liver pIgR. An adult pattern of IgA secretion was not detected until after day 23 postpartum (dPP), by using intravenously injected 125I-labeled dimeric IgA. Radioactive dimeric IgA was not detectable in hepatocyte transport vesicles until 21 dPP by electron microscopy autoradiographic analysis. By using a rabbit polyclonal antibody against the rat secretory component domain of the pIgR, Western blot analysis demonstrated that the plasma-membrane-bound pIgR levels in hepatocytes from rats aged 19-22 dPP increased 10-fold during this period. To determine whether or not this increase in membrane-bound pIgR reflected increased pIgR gene expression, we probed Northern blots of total cellular RNA extracted from neonatal rat liver with pIgR cDNA [GORF-1; Banting, G., Brake, B., Braghetta, P., Luzio, J.P. & Stanley, K. K. (1989) FEBS Lett. 254, 177-183]. The pIgR RNA levels between 19 and 22 dPP rose more than 20-fold and paralleled the increased membrane-bound pIgR protein during this same interval. These data demonstrate a developmentally regulated process that controls the ontogeny of biliary dimeric IgA secretion at the termination of the third week postpartum. The process appears to depend on the up-regulation of pIgR gene expression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banting G., Brake B., Braghetta P., Luzio J. P., Stanley K. K. Intracellular targetting signals of polymeric immunoglobulin receptors are highly conserved between species. FEBS Lett. 1989 Aug 28;254(1-2):177–183. doi: 10.1016/0014-5793(89)81034-8. [DOI] [PubMed] [Google Scholar]
- Bartles J. R., Feracci H. M., Stieger B., Hubbard A. L. Biogenesis of the rat hepatocyte plasma membrane in vivo: comparison of the pathways taken by apical and basolateral proteins using subcellular fractionation. J Cell Biol. 1987 Sep;105(3):1241–1251. doi: 10.1083/jcb.105.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale D., Coadwell J. The sites of tryptic cleavage in bovine secretory component: structural and functional implications. Biochim Biophys Acta. 1987 Apr 30;912(3):365–370. doi: 10.1016/0167-4838(87)90041-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Breitfeld P. P., Harris J. M., Mostov K. E. Postendocytotic sorting of the ligand for the polymeric immunoglobulin receptor in Madin-Darby canine kidney cells. J Cell Biol. 1989 Aug;109(2):475–486. doi: 10.1083/jcb.109.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Buts J. P., De Keyser N., Dive C. Intestinal development in the suckling rat: effect of insulin on the maturation of villus and crypt cell functions. Eur J Clin Invest. 1988 Aug;18(4):391–398. doi: 10.1111/j.1365-2362.1988.tb01029.x. [DOI] [PubMed] [Google Scholar]
- Buts J. P., Delacroix D. L. Ontogenic changes in secretory component expression by villous and crypt cells of rat small intestine. Immunology. 1985 Jan;54(1):181–187. [PMC free article] [PubMed] [Google Scholar]
- Casanova J. E., Breitfeld P. P., Ross S. A., Mostov K. E. Phosphorylation of the polymeric immunoglobulin receptor required for its efficient transcytosis. Science. 1990 May 11;248(4956):742–745. doi: 10.1126/science.2110383. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Daniels C. K., Schmucker D. L., Jones A. L. Age-dependent loss of dimeric immunoglobulin A receptors in the liver of the Fischer 344 rat. J Immunol. 1985 Jun;134(6):3855–3858. [PubMed] [Google Scholar]
- Fisher M. M., Nagy B., Bazin H., Underdown B. J. Biliary transport of IgA: role of secretory component. Proc Natl Acad Sci U S A. 1979 Apr;76(4):2008–2012. doi: 10.1073/pnas.76.4.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R. A., Erlanger B. F., Guntaka R. V. Evidence for extensive methylation of ribosomal RNA genes in a rat XC cell line. Biochim Biophys Acta. 1983 Apr 15;739(3):258–264. doi: 10.1016/0167-4781(83)90099-4. [DOI] [PubMed] [Google Scholar]
- Krajci P., Solberg R., Sandberg M., Oyen O., Jahnsen T., Brandtzaeg P. Molecular cloning of the human transmembrane secretory component (poly-Ig receptor) and its mRNA expression in human tissues. Biochem Biophys Res Commun. 1989 Feb 15;158(3):783–789. doi: 10.1016/0006-291x(89)92790-3. [DOI] [PubMed] [Google Scholar]
- Kvale D., Brandtzaeg P., Løvhaug D. Up-regulation of the expression of secretory component and HLA molecules in a human colonic cell line by tumour necrosis factor-alpha and gamma interferon. Scand J Immunol. 1988 Sep;28(3):351–357. doi: 10.1111/j.1365-3083.1988.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larkin J. M., Sztul E. S., Palade G. E. Phosphorylation of the rat hepatic polymeric IgA receptor. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4759–4763. doi: 10.1073/pnas.83.13.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Breitfeld P., Harris J. M. An anchor-minus form of the polymeric immunoglobulin receptor is secreted predominantly apically in Madin-Darby canine kidney cells. J Cell Biol. 1987 Nov;105(5):2031–2036. doi: 10.1083/jcb.105.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K. E., Deitcher D. L. Polymeric immunoglobulin receptor expressed in MDCK cells transcytoses IgA. Cell. 1986 Aug 15;46(4):613–621. doi: 10.1016/0092-8674(86)90887-1. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Friedlander M., Blobel G. The receptor for transepithelial transport of IgA and IgM contains multiple immunoglobulin-like domains. Nature. 1984 Mar 1;308(5954):37–43. doi: 10.1038/308037a0. [DOI] [PubMed] [Google Scholar]
- Mostov K. E., Kraehenbuhl J. P., Blobel G. Receptor-mediated transcellular transport of immunoglobulin: synthesis of secretory component as multiple and larger transmembrane forms. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7257–7261. doi: 10.1073/pnas.77.12.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock B. M., Hinton R. H., Dobrota M., Peppard J., Orlans E. Endocytic vesicles in liver carry polymeric IgA from serum to bile. Biochim Biophys Acta. 1979 Oct 18;587(3):381–391. doi: 10.1016/0304-4165(79)90442-2. [DOI] [PubMed] [Google Scholar]
- Mullock B. M., Jones R. S., Hinton R. H. Movement of endocytic shuttle vesicles from the sinusoidal to the bile canalicular face of hepatocytes does not depend on occupation of receptor sites. FEBS Lett. 1980 May 5;113(2):201–205. doi: 10.1016/0014-5793(80)80591-6. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Baenziger J. U. Cleavage of membrane secretory component to soluble secretory component occurs on the cell surface of rat hepatocyte monolayers. J Cell Biol. 1987 Jun;104(6):1725–1733. doi: 10.1083/jcb.104.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Baenziger J. U. Intracellular transport and processing of secretory component in cultured rat hepatocytes. Gastroenterology. 1987 Dec;93(6):1194–1204. doi: 10.1016/0016-5085(87)90244-7. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Baenziger J. U. Proteolytic processing of rat liver membrane secretory component. Cleavage activity is localized to bile canalicular membranes. J Biol Chem. 1988 Oct 25;263(30):15799–15808. [PubMed] [Google Scholar]
- Phillips J. O., Everson M. P., Moldoveanu Z., Lue C., Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by human epithelial cells. J Immunol. 1990 Sep 15;145(6):1740–1744. [PubMed] [Google Scholar]
- Renston R. H., Jones A. L., Christiansen W. D., Hradek G. T., Underdown B. J. Evidence for a vesicular transport mechanism in hepatocytes for biliary secretion of immunoglobulin A. Science. 1980 Jun 13;208(4449):1276–1278. doi: 10.1126/science.7375938. [DOI] [PubMed] [Google Scholar]
- Renston R. H., Maloney D. G., Jones A. L., Hradek G. T., Wong K. Y., Goldfine I. D. Bile secretory apparatus: evidence for a vesicular transport mechanism for proteins in the rat, using horseradish peroxidase and [125I]insulin. Gastroenterology. 1980 Jun;78(6):1373–1388. [PubMed] [Google Scholar]
- Salamero J., Sztul E. S., Howell K. E. Exocytic transport vesicles generated in vitro from the trans-Golgi network carry secretory and plasma membrane proteins. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7717–7721. doi: 10.1073/pnas.87.19.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaerer E., Verrey F., Racine L., Tallichet C., Reinhardt M., Kraehenbuhl J. P. Polarized transport of the polymeric immunoglobulin receptor in transfected rabbit mammary epithelial cells. J Cell Biol. 1990 Apr;110(4):987–998. doi: 10.1083/jcb.110.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff J. M., Fisher M. M., Jones A. L., Underdown B. J. Human IgA as a heterovalent ligand: switching from the asialoglycoprotein receptor to secretory component during transport across the rat hepatocyte. J Cell Biol. 1986 Mar;102(3):920–931. doi: 10.1083/jcb.102.3.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff J. M., Fisher M. M., Underdown B. J. Receptor-mediated biliary transport of immunoglobulin A and asialoglycoprotein: sorting and missorting of ligands revealed by two radiolabeling methods. J Cell Biol. 1984 Jan;98(1):79–89. doi: 10.1083/jcb.98.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solari R., Kraehenbuhl J. P. Biosynthesis of the IgA antibody receptor: a model for the transepithelial sorting of a membrane glycoprotein. Cell. 1984 Jan;36(1):61–71. doi: 10.1016/0092-8674(84)90074-6. [DOI] [PubMed] [Google Scholar]
- Solari R., Schaerer E., Tallichet C., Braiterman L. T., Hubbard A. L., Kraehenbuhl J. P. Cellular location of the cleavage event of the polymeric immunoglobulin receptor and fate of its anchoring domain in the rat hepatocyte. Biochem J. 1989 Feb 1;257(3):759–768. doi: 10.1042/bj2570759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Howell K. E., Palade G. E. Biogenesis of the polymeric IgA receptor in rat hepatocytes. I. Kinetic studies of its intracellular forms. J Cell Biol. 1985 Apr;100(4):1248–1254. doi: 10.1083/jcb.100.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underdown B. J., DeRose J., Koczekan K., Socken D., Weicker J. Isolation of human secretory component by affinity chromatography on IgM-sepharose. Immunochemistry. 1977 Feb;14(2):111–118. doi: 10.1016/0019-2791(77)90289-0. [DOI] [PubMed] [Google Scholar]
- Vaerman J. P., Buts J. P., Lescoat G. Ontogeny of secretory component in rat liver. Immunology. 1989 Nov;68(3):295–299. [PMC free article] [PubMed] [Google Scholar]
- Wira C. R., Bodwell J. E., Prabhala R. H. In vivo response of secretory component in the rat uterus to antigen, IFN-gamma, and estradiol. J Immunol. 1991 Mar 15;146(6):1893–1899. [PubMed] [Google Scholar]
- Wira C. R., Colby E. M. Regulation of secretory component by glucocorticoids in primary cultures of rat hepatocytes. J Immunol. 1985 Mar;134(3):1744–1748. [PubMed] [Google Scholar]
- Wira C. R., Rossoll R. M. Glucocorticoid regulation of the humoral immune system. Dexamethasone stimulation of secretory component in serum, saliva, and bile. Endocrinology. 1991 Feb;128(2):835–842. doi: 10.1210/endo-128-2-835. [DOI] [PubMed] [Google Scholar]
- Wira C. R., Stern J. E., Colby E. Estradiol regulation of secretory component in the uterus of the rat: evidence for involvement of RNA synthesis. J Immunol. 1984 Nov;133(5):2624–2628. [PubMed] [Google Scholar]





