Abstract
Ionizing radiation is an important treatment modality for a variety of malignant conditions. However, development of radiation-induced skin changes is a significant adverse effect of radiation therapy (RT). Cutaneous repercussions of RT vary considerably in severity, course, and prognosis. When they do occur, cutaneous changes to RT are commonly graded as acute, consequential-late, or chronic. Acute reactions can have severe sequelae that impact quality of life as well as cancer treatment. Thus, dermatologists should be informed about these adverse reactions, know how to assess their severity and be able to determine course of management. The majority of measures currently available to prevent these acute reactions are proper skin hygiene and topical steroids, which limit the severity and decrease symptoms. Once acute cutaneous reactions develop, they are treated according to their severity. Treatments are similar to those used in prevention, but incorporate wound care management that maintains a moist environment to hasten recovery. Chronic changes are a unique subset of adverse reactions to RT that may develop months to years following treatment. Chronic radiation dermatitis is often permanent, progressive, and potentially irreversible with substantial impact on quality of life. Here, we also review the etiology, clinical manifestations, pathogenesis, prevention, and management of late-stage cutaneous reactions to radiotherapy, including chronic radiation dermatitis and radiation-induced fibrosis.
Keywords: Acute, Chronic, Radiation dermatitis, Radiation burns, Radiation recall, Radiation skin toxicity
Introduction
Ionizing radiation (IR) is used to treat a variety of malignant conditions and is used to palliate metastatic disease. However, the development of radiation-induced skin changes is a significant adverse effect of radiation therapy (RT). Skin reactions to radiation are largely a function of technique, total dose, volume, and individual variations in treatment [1, 2]. While advances in technology and changes to therapeutic regimens have reduced the burden of cutaneous reactions to RT, radiation dermatitis remains a significant adverse effect of radiotherapy.
Cutaneous repercussions of RT vary considerably in severity, course, and prognosis. When they do occur, cutaneous changes to RT are commonly graded as acute, consequential-late, or chronic [3]. Acute changes include erythema and pain and occur within 90 days [3]. Even with modern radiotherapy techniques, approximately 85% of patients will experience a moderate to severe acute skin reaction in exposed areas [4]. Severe acute reactions may lead to blistering, erosions, and ulceration [5], which can lead to premature interruption of RT and potentially negatively influence cancer control and prognosis. Alternatively, the skin may appear relatively normal for months to years following RT, when chronic radiation dermatitis develops [3]. Chronic radiation dermatitis is permanent, progressive, and irreversible and has substantial impact on quality of life [5]. Thus, it is important for dermatologists to be able to recognize the adverse reactions to IR in order to assess the severity of disease and to assist in the management of these conditions. This review of cutaneous repercussions of RT is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by the authors.
Clinical Manifestations
Acute Radiation Dermatitis
Acute radiation dermatitis is one of the most common reactions to RT and usually occurs within 90 days of exposure. The severity of reaction ranges from mild erythema to moist desquamation and ulceration (Table 1) [6, 7]. The reaction typically starts within 1–4 weeks after starting radiation treatment and persists during the radiation treatment period [8]. Acute radiation dermatitis is likely to heal with mild cutaneous changes.
Table 1.
Dose-dependent acute cutaneous findings after local radiation exposure [7]
| Observed acute skin reaction | Radiation dose (Gy) | Onset of findings |
|---|---|---|
| Transient erythema | 2 | Hours |
| Faint erythema and epilation | 6–10 | 7–10 days |
| Defined erythema and hyperpigmentation | 12–20 | 2–3 weeks |
| Dry desquamation | 20–25 | 3–4 weeks |
| Moist desquamation | 30–40 | 4 weeks or more |
| Ulceration | >40 | 6 weeks or more |
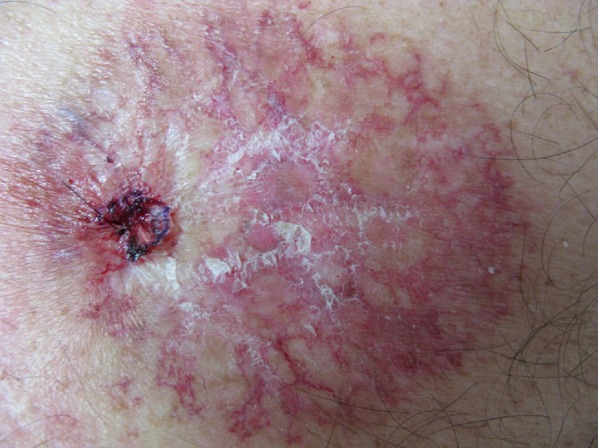
The severity of disease can be graded on a scale of 1–4 according to the National Cancer Institute (Table 2). Acute reactions start with erythema, edema, pigmentary changes and depilation that correlate with the amount of radiation exposure. Grade 1 changes include dry desquamation with a generalized erythema (Fig. 1). Pruritus, epilation, scaling and depigmentation can also occur. With grade 2, there is brisk erythema or localized focal sloughing of the epidermis (Fig. 2). These reactions lead to moist desquamation confined to the skin folds once the cumulative radiation dose reaches 40 Gy or more [9]. With moist desquamation, the epidermal layer is lost and there is a high propensity for infection. The reaction peaks in 1–2 weeks with subsequent healing. Patients can experience increased pain due to exposure of nerve endings. Grade 3 presents with extensive moist desquamation outside of skin folds (Fig. 3). With grade 4, ulcerations, hemorrhage and skin necrosis occur that in some cases does not resolve, leading to the late-consequential changes of acute dermatitis that include ulcerations and fibrosis.
Table 2.
Classification of acute radiation dermatitis
| Grade | |||
|---|---|---|---|
| 1 | 2 | 3 | 4 |
| Faint erythema or dry desquamation | Moderate to brisk erythema or patchy moist desquamation, mostly confined to skin folds and creases; moderate erythema | Moist desquamation other than skin folds; pitting edema, bleeding from minor trauma or abrasion | Skin necrosis or ulceration of full-thickness dermis; may have spontaneous bleeding from affected area |
According to National Cancer Institute Common Terminology Criteria for Adverse Events Version 3
Fig. 1.
Grade 1 acute radiation dermatitis. Reproduced from Mesía et al. [132] under open-access article distributed under the terms of the creative commons attribution license.
Copyright 2009
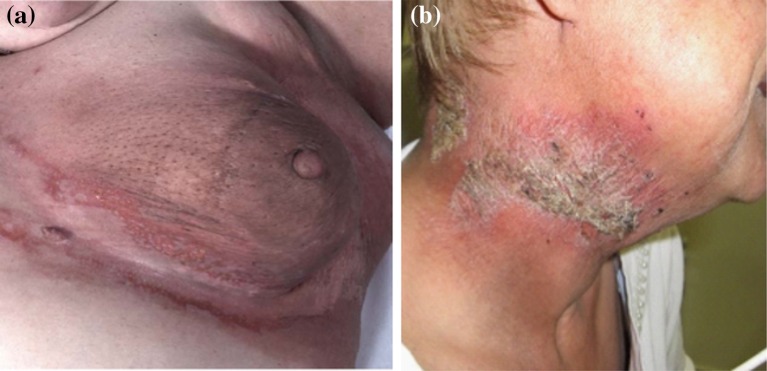
Fig. 2.
Grade 2 acute radiation dermatitis. a Radiation dermatitis of the breast with moist desquamation limited to the inframammary fold. b Radiation dermatitis with moderate erythema and scaly dry desquamation. a Reprinted from Journal of the American Academy of Dermatology, 54, Sharon R. Hymes, Eric A. Strom, Caroline Fife, Radiation dermatitis: Clinical presentation, pathophysiology, and treatment 2006, 28–46, Copyright (2006), with permission from Elsevier
Fig. 3.
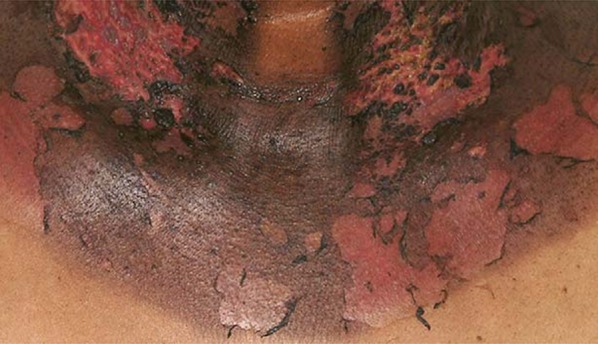
Grade 3 acute radiation dermatitis with confluent moist desquamation. Reprinted from Journal of the American Academy of Dermatology, 54, Sharon R. Hymes, Eric A. Strom, Caroline Fife, Radiation dermatitis: Clinical presentation, pathophysiology, and treatment 2006, 28–46, Copyright (2006), with permission from Elsevier
Reepithelialization usually starts within 10 days, but can be prolonged with exposure to radiosensitizing drugs especially platinum based chemotherapy. Additional findings that may occur with acute radiation dermatitis include comedo reactions of whiteheads and blackheads in head and neck cancers. Pseudorecidives (keratosis-like lesions) and transient hair loss may progress to permanent hair loss if follicular fibrosis occurs [10].
Radiation Burns
Radiation burns, although rare with current treatment modalities, can occur with high-dose exposure to x-rays during interventional radiology procedures or with RT [11]. Management of these lesions is difficult because of the inability to differentiate injured tissue from uninjured tissue [12, 13], the unpredictable inflammatory waves that can come weeks to years after tissue injury, and the occurrence of opiate-resistant pain. [9].
Radiation Recall
Radiation recall is an acute inflammatory reaction confined to an area previously exposed to radiation after a chemotherapeutic agent or other medication. Clinically, radiation recall manifests with maculopapular eruptions, dry desquamation, pruritus, swelling and ulcerations. The incidence has been reported to occur in up to 6% of individuals undergoing RT, but reactions are drug-specific and can occur weeks to months after the original RT and subsequent chemotherapeutic administration [14]. However, the majority of reactions occur when the drug has been administered within 2 months of RT [15]. Radiation recall is most frequently associated with traditional chemotherapeutic agents including anthracyclines, taxanes, and antimetabolites [14], but reactions have been reported with EGFR inhibitors, BRAF tyrosine kinase inhibitors [16] and other non-chemotherapeutic agents (see Table 3) [14, 15].
Table 3.
| Chemotherapeutic agent |
|---|
| Doxorubicin |
| Docetaxel |
| Paclitaxel |
| Gemcitabine |
| Capecitabine |
| Trimetrexate |
| Methotrexate |
| Hydroxyurea |
| Tamoxifen |
| Dactinomycin |
| Vimblastine |
| Vemurafenib |
| Cetuximab |
Chronic Radiation Dermatitis
Rarely, acute radiation fails to heal and consequential-late changes of RT may develop, which include chronic wounds and skin necrosis [3]. In contrast, chronic radiation dermatitis is a true late-stage reaction that develops months to years after exposure to IR. The condition may develop in patients who only experienced minimal acute radiation dermatitis and so may develop in near-normal-appearing skin. Unlike acute radiation dermatitis, chronic radiation dermatitis is unlikely to self-repair and may remain indefinitely [3]. The defining features of the late-stage are fibrosis, atrophy, hypo- or hyperpigmentary changes and the development of cutaneous malignancies (Table 4).
Table 4.
Clinical manifestations of chronic radiation dermatitis and radiation-induced fibrosis
| Late reaction or complication | Clinical manifestations |
|---|---|
| Textural changes | Xerosis |
| Scale | |
| Hyperkeratosis | |
| Persistent poikilodermatous changes (indicate severe RT damage) | Dyspigmentation |
| Atrophy | |
| Telangiectasia | |
| Absence of hair follicles and sweat glands | Alopecia |
| Decreased or absent sweating | |
| Destruction or permanent loss of nail appendages | Friable nails |
| Longitudinal striations | |
| Cutaneous breakdown | Epidermal atrophy |
| Slow-healing, painful erosions and ulcerations | |
| Necrosis of soft tissue, cartilage, and bone | |
| Cutaneous and subcutaneous tissue fibrosis | Pain, limited range of motion, contractures |
| Secondary malignancy | Primarily SCC and BCC |
Post-inflammatory dyspigmentation is common, and, depending on the skin type of the patient and severity of the reaction, may slowly resolve or worsen over time [3]. The skin may become xerotic, scaly, and hyperkeratotic. Significant cutaneous injury is characterized by persistent dyspigmentation, atrophy, and telangiectasia (Fig. 4) [3]. Telangiectasia commonly results from boost dosing, acute radiation grade 3 injury, and moist desquamation [17, 18]. With severe cutaneous injury, there may be permanent loss of nail and skin appendages, absence of hair follicles and sebaceous glands with resultant alopecia, and absent or reduced sweating [3]. Small arteries and arterioles predisposed to thrombosis or obstruction may lead to skin breakdown and ulceration [3, 9]. Further, atrophied skin is fragile and is predisposed to erosions and ulcerations that are painful and slow to heal [3, 19].
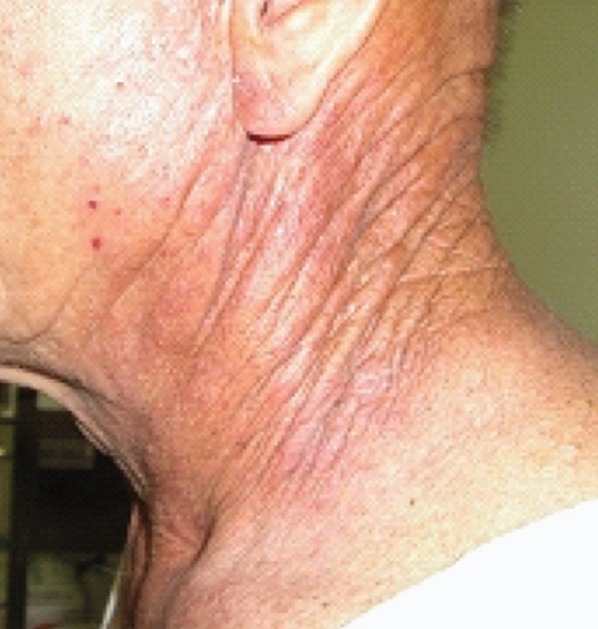
Fig. 4.
Chronic radiation dermatitis at the site of radiation beam entry. The lesion is an 8 × 6 well-demarcated erythematous atrophic plaque with telangiectasias and ulceration. Reprinted from The American Journal of Cardiology, 110, Alison Spiker, Zachary Zinn, William H. Carter, Roxann Powers, Rodney Kovach, Fluoroscopy-Induced Chronic Radiation Dermatitis, 1861–1863., Copyright (2012), with permission from Elsevier
Radiation-induced Fibrosis
When skin and subcutaneous tissue develops fibrosis, there can be a limited range of motion, contractures, and pain [3]. Radiation-induced fibrosis of the skin and subcutaneous tissues may develop at any RT treatment site; however, fibrosis most commonly occurs in breast cancer patients who were formerly treated with a combination of surgical intervention and RT. These patients may experience pain, skin retraction and induration, restricted arm and neck movement, lymphedema, and skin necrosis and ulcerations [20]. Boost dosing is an added risk factor for the development of fibrosis [21]. Fibrosis in the skin and subcutaneous tissue is usually diagnosed by palpation and inspection. Radiation-induced fibrosis is limited to the region treated with RT. If tumor recurrence is suspected, MRI may be obtained to differentiate [22, 23]. However, biopsy should be obtained to confirm fibrosis.
Secondary Cutaneous Malignancies
Individuals treated with IR are also at risk for the long-term development of secondary cutaneous malignancies. Increased risk for skin cancers may last a lifetime following radiation, is dose-related, and increases over the patient’s lifespan [24–26]. Patients who are exposed to radiation at younger ages are at greater risk for the development of basal cell carcinoma (BCC) than those who are exposed as adults [24, 25, 27, 28]. BCCs that do present following RT are often more aggressive or unusual variants [3]. The link between cancer treatment with RT and the development of melanoma and other non-melanoma skin cancers later in life is less clear [24, 28].
Pathophysiology
Acute Cutaneous Reactions
Radiation-induced tissue injury occurs on a functional, cellular, and gross level [3]. The susceptibility of the skin to radiation is due to the rapid rate of proliferation and maturation of cells, so that the basal keratinocytes, hair follicle stem cells and melanocytes are the most susceptible [29]. RT interferes with normal production and maturation of epithelial and hair matrix cells and also leads to the development of atypical fibroblasts and cutaneous vasculature [30]. With the first dose of RT, there is immediate tissue damage, generation of short-lived free radicals, irreversible breaks in cellular DNA, and generation of an inflammatory response [3, 31–33]. The early inflammatory response to radiation is principally caused by a proinflammatory cytokine cascade (IL-1, IL-3, IL-5, IL-6, TNF-a), chemokines (IL-8, eotaxin, CCR receptor), receptor tyrosine kinase, and adhesions molecules (ICAM-1, VCAM, E-selectin). These factors create a local inflammatory reaction of eosinophils and neutrophils that leads to self-perpetuating tissue damage and loss of the protective barrier [34]. Wound healing is impaired by the destruction of the basal keratinocytes, so that repeated exposures do not allow time for tissue or cellular repair. Each additional exposure to RT results in further direct tissue injury, inflammation, and impaired epithelial regeneration, all of which contribute to the development of acute radiation injury [35].
Chronic Cutaneous Reactions
The development of chronic radiation dermatitis is intricately related to the cytokine TGF-β [3, 36]. TGF-β is a regulatory protein that controls proliferation and differentiation of many cell types, wound healing, and synthesis of extracellular matrix proteins in the normal tissue inflammatory response [37]. Importantly, TGF-β activates fibroblasts, which are key cells in the development of late radiation-induced fibrotic changes [36]. TGF-β has been found to be upregulated in fibrotic tissue of irradiated patients, but not in non-irradiated controls [38].
Once the skin has had sufficient opportunity to “heal” from radiation-induced injury, long-lasting cellular dysfunction and stromal changes remain that impair cutaneous integrity [3, 35]. Permanently atypical fibroblasts may lead to cutaneous atrophy, contraction, and fibrosis [39, 40]. These late effects are more dependent on the type of radiation, area, volume, fraction size and schedule rather than total radiation dose [41]. The pathogenesis of telangiectasia development is unknown; however, it is thought to be in part due to acutely damaged microvasculature and production of platelet-derived growth factor (PDGF) and fibroblast growth factor by damaged cells [35]. Leukocyte infiltration at sites of irradiation is also likely to lead to atrophy, fibrosis, and necrosis in surrounding normal tissues [42].
The development of radiation-induced fibrosis is mediated by inflammation that begins immediately following RT and continues for months to years. TNF-α, IL-6, and IL-1 have been implicated in the inflammatory response, while TGF-β and PDGF modulate and enhance fibroblast activity and encourage production of extracellular matrix proteins [36, 43–45]. These changes in addition to radiation-induced alterations of the vascular system contribute significantly to late toxicity of RT.
Epidemiology
Skin injuries occur in about 95% of patients who receive RT [4]. Any body site treated with RT is susceptible to cutaneous injury; however, the face, neck, trunk, and extremities are particularly vulnerable [46]. Patients with breast cancer, head and neck cancer, lung cancer, and sarcoma are most often affected because of the higher radiation doses to the skin [4, 29, 41, 47]. RT was formerly used by dermatologists in the treatment of benign conditions such as acne, eczema and psoriasis [3]. These patients are also at risk for the development of chronic radiation dermatitis. In addition to RT, radiation dermatitis may occur as a result of accidental or occupational exposures to radiation [5].
Risk Factors
A variety of factors that increase the risk of developing acute cutaneous reactions to IR have been identified (see Table 5). The severity of the reaction is related to both intrinsic and extrinsic factors. Extrinsic factors include the total dosage of radiation, fractioned delivery schedules, volume of irradiated tissue and the intrinsic radiosensitivity of the involved tissue [48]. However, in general, moist intertriginous skin folds of the body are most susceptible. These areas include the skin under the breast, axilla, head and neck, and the groin due to the “bolus effect”, i.e. the propensity for higher doses of radiation to reach the skin folds [49].
Table 5.
Risk factors for development of acute skin reactions to radiation
| Extrinsic |
|---|
| Total radiation dose |
| Fractionation schedule |
| Type of radiation |
| Quality of radiation beam |
| Concurrent chemotherapy |
| Antibiotics |
| Anti-tuberculosis medications |
| Intrinsic |
|---|
| Advanced age |
| Female sex |
| Obesity |
| Comorbidities (diabetes mellitus, connective tissue disease) |
| Chronic sun exposure |
| Radiosensitive disorders |
| Ataxia telangiectasia |
| Xeroderma pigmentosa |
| Previous breast reconstruction/implants |
| Nutritional status |
| Immunocompromised |
| Smoking |
Extrinsic Factors
The total dose, dose/fraction, characteristics of the beam, volume and surface area of exposure to radiation all influence the degree of tissue damage [2, 3, 50–53]. For example, the total radiation dose is an important factor in the development of skin toxicity. However, the total dose that leads to cutaneous skin reactions varies depending on the dosing schedule. For instance, single doses of 16–22 Gy can result in the development of skin toxicity. However, if the dose is fractionated into 2-Gy fractions the total dose can be increased to 30–40 Gy before skin toxicity develops [54]. Thus, there is an increase in radiation tolerance with hyperfractionated treatments. This strategy allows for delivery of a higher total radiation dose with similar cutaneous toxicity to lower single-dose treatments. Interestingly, the time before clinical manifestations present is independent of the radiation dose, and is actually related to the timing of normal cell turnover. However, the total dose does affect the time required for the skin to clinically heal [55]. The use of boost doses, which intentionally create overlapping treatment fields, as well as bolus material are methods of RT that increase radiation dose and therefore increase risk of cutaneous reactions [53, 56].
The quality of radiation beam also influences the development of acute skin toxicity. In general, modern RT techniques have improved substantially so that normal tissue should be spared [57, 58]. New external beam radiation modalities such as intensity-modulated radiotherapy (IMRT) reduce radiation hot spots in the skin by delivering more homogenous radiation than traditional wedge beam radiation. Studies have shown as much as a 20% reduction in the development of moist desquamation from this modality alone [59, 60]. IMRT also shows promising reduction in the incidence of late radiation-induced cutaneous effects, such as induration and telangiectasia, in breast cancer patients [61–63]. Additionally, the type of particle that is emitted by the radiation source affects the depth of penetration and extent of damage that can occur (Table 6). The volume of the area being treated is proportional to the risk of developing skin reactions due to the higher radiation doses needed to treat larger areas.
Table 6.
Radiation particle and effect on skin [11]
| Particle type | Penetration | Effect on skin |
|---|---|---|
| Alpha | Large amount of ionization, but minimal skin penetration | Not able to penetrate stratum corneum when emitted |
| Beta | Greater penetration than alpha particle, but less ionization | Shallow penetration of skin |
| Gamma | Low ionization, but high penetration | More penetration in skin with damage inversely proportional to the energy |
| X-ray | Similar to Gamma ray; longer wave length providing more penetration | Effect on skin is proportional to energy of X-ray |
| Neutron | High penetration due to size and neutral charge | Can be lethal; high energy transfer destroying basal layer of skin leading to necrosis |
Certain drugs increase sensitivity to RT, so that the timing and dose of these agents is critical [3]. These drugs increase cellular damage that occurs with RT and hinder tissue repair. Conventional chemotherapeutic agents as well as anticancer therapies with EGFR inhibitors increase the risk of developing severe radiation dermatitis [64]. Commonly cited agents include dactinomycin, doxorubicin, methotrexate, 5-fluorouracil, hydroxyurea and bleomycin [56, 65]. New BRAF inhibitors such as vemurafenib have also produced severe skin and oral mucosal reactions when given with concurrent radiotherapy [66]. In the treatment of breast cancers, paclitaxel and docetaxel in conjunction with RT synergistically create cutaneous damage [67, 68]. Timing of adjuvant drugs also influences the development of chronic cutaneous changes to RT. In an RCT comparing sequential versus concurrent chemotherapy with RT in breast cancer patients, the risk for the development of late subcutaneous fibrosis was greater in those receiving both therapies at the same time [69]. Tamoxifen is also suspected to increase subcutaneous fibrosis when used in conjunction with RT [70].
Intrinsic Factors
Intrinsic factors such as general skin condition, nutritional status, age, comorbid disease (diabetes mellitus and connective tissue disorders) and ethnicity all modulate the risk of acute skin reactions [71, 72]. Moreover, smoking, actinic damage, and obesity have also been implicated [73]. In addition, patients with implants and breast reconstruction have a higher risk of radiation dermatitis due to the skin’s inability to dissipate heat [74, 75]. Furthermore, patients who are immunocompromised secondary to HIV infection who are treated with IR for cancers of the head and neck, abdomen, or pelvis have an increased risk of developing mucosal reactions [76].
Genetics influences the development of acute cutaneous reactions from radiation, particularly conditions resulting from mutations in DNA repair mechanisms. The most well-known example is ataxia telangiectasia, a rare autosomal-recessive disorder that results from mutations in both ATM genes. Patients with this disorder have a high propensity to develop severe complications after RT due to the inability to repair DNA. An estimated 1% of the population is heterozygous for the ATM gene [77], which predisposes patients to develop cutaneous reactions [78]. Modified treatment protocols with lower radiation dose and volumes can be utilized in these patients to avoid skin reactions and decrease the risk of skin toxicity. Other conditions that lead to chromosomal breakage includes Fanconi’s anemia, Bloom syndrome and xeroderma pigmentosum. Patients with these conditions develop gaps in skin fibroblasts after irradiation [79]. Moreover, specific genetic polymorphisms have been identified in DNA repair and oxidative stress response genes that confer a higher risk for acute skin reactions after radiotherapy [80].
Prevention
General Preventive Measures
Prevention of radiation dermatitis is an important consideration in the pre- and post-RT period. General measures, such as maintaining proper skin hygiene by washing with lukewarm water and mild soaps, and the use of unscented, lanolin-free water-based moisturizers, decreases the risk for acute radiation dermatitis [81, 82]. Avoiding metallic and/or oil based topical products, wearing loose-fitting clothes, and avoiding sun exposure may help prevent post-RT complications. However, to date, there are few randomized controlled trials (RCTs) that assess preventive measures for acute radiation-induced skin toxicity (Table 7). Topical moisturizers, gels, emulsions, or dressings can cause a bolus effect and so should not be applied shortly before radiation [83]. Careful positioning of the patient and appropriate placement of skin shields may decrease radiation-induced skin problems. Following RT sessions, exposure to ultraviolet light in treatment areas and temperature extremes should be avoided [3]. Patients undergoing RT treatment should avoid metallic compounds including magnesium in talcs and aluminum in antiperspirants [19].
Table 7.
Preventions and treatments for acute cutaneous skin reactions to radiotherapy
| Prevention | Level of evidence |
|---|---|
| Proper skin hygiene | Wash with mild soaps and lukewarm water to help maintain skin barrier |
| Protection of skin from additional trauma |
Use of topical steroids; use before development of radiation dermatitis to slow progression to radiation dermatitis (Grade B) Oral Wobe-Mugus can decrease odds of developing radiation dermatitis (Grade C) Wear loose fitting clothing, avoid sun exposure, avoid metallic based topical products, use water based lanolin-free moisturizers |
| Treatment of | Level of evidence |
|---|---|
| Dry desquamation |
Low- to mid-potency topical steroids; decrease progression and severity of itching, burning and irritation (Grade C) Use of hydrophilic moisturizers |
| Moist desquamation | Wound care management with hydrogel and hydrocolloid dressings |
| Radiation burns |
Removal of necrotic debris and mesenchymal stem cell injections to area to increase healing Mesenchymal stem cell injections around lesions to enhance wound healing (Grade D) |
Topical Corticosteroids
Topical corticosteroids have long been used for the prevention and treatment of RT-induced skin toxicity due to the underlying inflammatory pathophysiology. However, the efficacy of topical corticosteroids in reducing the frequency and severity of radiation dermatitis has been evaluated in several small clinical trials, with inconsistent results [3]. Some studies show no statistically significant difference between steroid (mometasone furoate 0.1% cream [84]; 0.2% hydrocortisone valerate [85]) versus placebo, whereas other groups demonstrated decreased severity or frequency of acute radiation dermatitis in the topical steroid group [86–88]. Advocates of corticosteroid use recommend application of low to medium potency steroid to the treatment field 1–2 times a day after each RT session to reduce the severity of acute radiation dermatitis and decrease the severity of symptoms, including decreased itching, irritation, burning, and discomfort. Whether or not application of corticosteroids during periods of RT can impact the frequency or severity of eventual chronic radiation dermatitis remains to be seen. It is also not known whether corticosteroids may increase the incidence of infection, telangiectasia, or skin atrophy [3].
Other Adjuvants
Oral Wobe-Mugus (a proteolytic enzyme mixture of 100 mg papain, 40 mg trypsin and 40 mg chymotrypsin) has been shown in two nonblinded RCTs versus no medication to decrease the odds for developing RT-induced skin toxicity by as much as 87% [89, 90]. However, dosages and treatment schedule varied between studies. Other agents, including aloe vera, trolamine, sucralfate, and hyaluronic acid, do not have supportive evidence for use in the prevention of radiation dermatitis [91–96].
Management
Acute Cutaneous Reactions
Grade 1
Management is based principally on the severity of damaged skin. Patients with grade 1 radiation dermatitis are treated with nonspecific treatment similar to the aforementioned general prevention measures. Dry desquamation can be treated with hydrophilic moisturizers, while pruritus and irritation can be treated with low to mid potency steroids.
Grades 2 and 3
With more severe reactions involving moist desquamation (grades 2 and 3), treatment should be directed toward preventing secondary infection and dressing the areas of moist desquamation. Dressings are used in moist desquamation to maintain a wet environment over de-epithelialized skin, which allows for a higher rate of wound healing [97]. A variety of dressings have been employed in the treatment of these lesions, but results to date are inconclusive [98–100].
Two types of dressings commonly used in moist desquamation are hydrogel and hydrocolloid dressings. Hydrogel dressings do not adhere to wounds and allow for ease of cleaning and reapplication. Hydrocolloid dressings are absorbent, self-adhering, and can be left in place for several days to simplify wound care [101]. These dressings have been shown to speed wound healing and improve patient comfort, but no high-powered RCTs exist to date comparing these treatments [102].
Grade 4
In severe skin reactions to RT (grade 4), there is significant full-thickness skin necrosis and ulceration. Treatment requires a multidisciplinary approach and discontinuation of RT. In addition, surgical debridement of necrotic tissues and the utilization of full-thickness skin grafts or pedicle flaps may be indicated. These high-grade cutaneous skin toxicity reactions can lead to late-consequential changes including fibrosis and non-healing ulcers, which have potential for malignant transformation. Moreover, waves of inflammation can occur with radiation burns leading to the need for successive surgical excisions, reconstruction, and potential need for amputation [12, 13].
Chronic Cutaneous Reactions
Unlike the majority of cases of acute cutaneous reactions to RT, chronic radiation dermatitis and radiation-induced fibrosis are unlikely to be self-repairing. Management of late cutaneous reactions of RT is reviewed in Table 8.
Table 8.
Management of chronic radiation dermatitis and radiation-induced fibrosis
| Late reaction or complication | Management |
|---|---|
| Ulcers and erosions | Non-specific, follows general wound care guidelines, including |
| Hydrophilic or lipophilic barrier creams with or without hydrogel or hydrocolloid dressings | |
| Careful and selective debridement, eschar removal | |
| For infected or at-risk wounds, antibacterial agents as needed and silver-based dressings | |
| Surgical intervention for nonhealing ulcers with skin flaps, less commonly with staged skin-muscle or axial-pedical flaps | |
| Grade D | |
| Artificial or bioengineered skin | |
| Low-intensity helium laser | |
| Fibrosis | Supportive measures: physical therapy, massage, and pain management |
| Grade 2C | |
| Pentoxifylline with or without tocopherol | |
| Grade D | |
| Superoxide dismutase | |
| Interferon gamma (IFNγ) | |
| Hyperbaric oxygen therapy | |
| Laser therapy with epidermal grafting | |
| Telangiectasias |
Grade D Pulse dye laser |
| Secondary skin cancers and radiation-induced keratoses | Surgical excision preferred for skin cancers |
| Grade 2C | |
| Radiation-induced keratoses: | |
| Cryosurgery | |
| Mechanical destruction (peel, laser, or dermabrasion) | |
| Grade D | |
| Topical 5-fluorouracil | |
| Diclofenac | |
| Photodynamic therapy | |
| Imiquimod |
Chronic Ulcerations and Wounds
As in acute radiation dermatitis, the care of ulcerations and wounds resulting from chronic radiation dermatitis is non-specific and follows general wound care guidelines. Wound dressings protect the injured skin from environmental damage and infection and also serve to contain wound secretions [3]. Moisture helps with re-epithelialization of tissue as well as removal of necrotic tissue and bacteria [3, 9, 103, 104]. Hydrophilic and lipophilic creams and ointments may be used alone or with dressings to enhance barrier function. Similar to management of moist desquamation, hydrogel or hydrocolloid dressings may be utilized.
Chronic ulcers may require careful and selective debridement. Persistent eschars may be removed manually, or treated with enzymatic debridement or autolytic dressings [3]. Chronic, nonhealing ulcers are poorly vascularized, and may require surgical intervention with skin flaps or sometimes staged skin-muscle or axial-pedical flaps [105]. Less commonly, artificial and bioengineered skin have been used for nonhealing ulcerations [104]. Case reports show that laser therapy with low-intensity helium laser has benefitted some patients with chronic ulcerations [106]. For infected or at-risk wounds, antibacterial agents should be considered. Silver-based dressings may be effective for this purpose [3]. Chronic nonhealing ulcers and suspected lesions may need to be biopsied for histopathologic examination to exclude secondary skin cancers [3].
Radiation-induced Fibrosis
Radiation-induced fibrosis is one of the most difficult skin complications to treat [3]. A team approach with wound care, physical therapy, and pain management is needed to preserve quality of life [3]. Physical therapy may include active and passive range of motion exercises, which may help to improve range of motion and reduce contractures. Massage may also be beneficial [107]. Adequate pain control should be provided as pain from fibrosis can be significant.
Pentoxifylline (PTX) may be used alone or in combination with tocopherol (vitamin E) to treat radiation-induced fibrosis as well as to prevent pulmonary fibrosis. PTX is a methylxanthine derivative that is commonly used as an inhibitor of platelet aggregation, while vitamin E is a scavenger of reactive oxygen. PTX is thought to modulate the immune response by increasing polymorphonuclear leukocyte and monocyte phagocytic activity, antagonizing TNF-α and TNF-β [3], decreasing granulocyte–macrophage colony-stimulating factor and interferon gamma (IFNγ), among other effects [108, 119]. Combination with tocopherol may downregulate TGF-β expression and may even reverse alter the abnormal fibroblasts that perpetuate fibrosis [20, 110–112]. Multiple small randomized trials have suggested that PTX and/or tocopherol may reduce fibrosis [113–116]. However, the results of the largest of these trials have met with mixed results. In these studies, patients treated with PTX in combination with vitamin E demonstrated marginal improvement in their condition, but treatment had little to no benefit over placebo [115, 116]. However, longer-term therapy may be an important element in the treatment of fibrosis. In a study of 44 women with superficial radiation-induced fibrosis treated with PTX and tocopherol over a range of 6–48 months, regression of superficial fibrosis was seen [117]. An average of 68% regression of the radiation-induced fibrosis required an average of 24 months of treatment. Those who stopped treatment prior to 12 months saw a rebound in the fibrotic area after treatment. PTX and vitamin E can reverse superficial radiation-induced fibrosis, but the optimal dose and duration of therapy are unknown at this time.
Additional therapeutic agents that have been attempted in the treatment of fibrosis include superoxide dismutase (SOD), IFNγ, hyperbaric oxygen therapy, and laser therapy with epidermal grafting [3, 118]. Liposomal SOD is thought to downregulate TGF-β expression by myofibroblasts as well as function as an anti-inflammatory agent and anti-oxidant [3, 36]. In a clinical trial of 34 patients treated with 6 intramuscular injections of SOD over a 3-week period, clinical regression of fibrosis was seen at 2-month follow up [119]. IFNγ is an inflammatory cytokine that is thought to inhibit collagen production by fibroblasts [3]. Treatment with IFNγ in 5 patients over a 1-year period was shown to be useful in the treatment of cutaneous fibrosis [120].
Hyperbaric oxygen has been evaluated as a treatment for radiation-induced fibrosis; however, there is insufficient evidence to show efficacy at this time [121–123]. Treatment may result in less pain, swelling, redness, or lymphedema, but no effect on fibrosis has been found [3, 124]. However, hyperbaric oxygen improves neutrophil function and has anti-bacterial effects, and thus may be considered as a guard against infection [3].
Laser therapy with epidermal grafting has also been explored as a novel approach to the treatment of radiation-induced fibrosis. In one case series, three Vietnamese children who had developed significant chronic radiation dermatitis and fibrosis from RT for infantile hemangiomas were treated with pulse-dye laser and/or fractional laser with epidermal skin grafting. The study authors reported skin softening, increased flexibility, repigmentation of the skin, and improvement of the telangiectasias, suggesting that this treatment modality should be explored further [118].
Quercetin is a bioflavenoid with anti-inflammatory effects. A study performed in a mouse model of radiation-induced fibrosis demonstrated that oral administration reduced hind limb contracture, collagen expression, and TGF-β in irradiated skin [125]. However, quercetin has not yet been tested as a therapeutic agent for radiation-induced fibrosis in human trials.
Telangiectasias
Treatments of telangiectasias resulting from chronic radiation dermatitis are limited. Treatment with pulse dye laser has been shown in case series to be beneficial [126]. In a retrospective study of breast cancer patients with radiation-induced telangiectasias, all 11 patients experienced clinical improvement with pulse dye laser, with an average clearance of 72.7% [127].
Secondary Skin Cancers
Squamous cell carcinomas that arise in radiation fields exhibit aggressive behavior and more frequently metastasize, so surgical excision is the preferred modality for management [3]. Radiation-induced keratoses are pre-malignant and may be treated with cryosurgery when localized or with mechanical destruction with peels, laser, or dermabrasion when diffuse [3]. Topical 5-fluorouracil, diclofenac, photodynamic therapy, and imiquimod have also been used in the treatment of skin cancers and precancerous lesions [3, 128, 129].
Future Directions
The current advances in reducing cutaneous reactions have been primarily in the technological advancements of delivering increasingly targeted, homogenized RT utilizing fractionated schedules. The future will combine these advancements with targeted therapies for reducing the underlying inflammatory cascade, such as superoxide dismutase/catalase mimetics [130], to decrease reactive oxygen species and interleukin inhibitors. Anti-oxidant properties of curcumin could be used to reduce radiation skin toxicity [131]. In addition, stem cell treatments to replace necrotic tissue after radiation burns [12] and high-grade radiation dermatitis may become more readily available options.
Conclusions
Acute cutaneous skin reactions are common side effects of RT. Preventive measures for acute cutaneous skin reactions have proven elusive. However, progression and severity of reaction can be mitigated. After acute reactions to RT develop, they should be treated according to grade of severity, and RT treatment may be interrupted if necessary to allow for re-epithelialization and healing to occur. Moreover, proper wound management should be started promptly to decrease healing time and the risk of secondary infections. Similarly, therapeutic advancements in the treatment of chronic radiation dermatitis and radiation-induced fibrosis have been promising, however there is still great need for novel and developing therapies. Supportive care and appropriate wound care continue to be mainstays of treatment at this time.
Acknowledgments
No funding or sponsorship was received for this study or publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given the final approval for the version to be published.
Disclosures
Fleta N. Bray, Brian J. Simmons, Aaron H. Wolfson and Keyvan Nouri have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/21D4F0606B97A130.
Fleta N. Bray and Brian J. Simmons are co-first authors.
References
- 1.Ang K, Wilder R. The skin. In: Cox J, Ang K, editors. Radiation oncology. St. Louis: Mosby; 2003. [Google Scholar]
- 2.Hall E, Cox J. Physical and biological basis of radiation therapy. In: Cox J, Ang K, editors. Radiation oncology. St. Louis: Mosby; 2003. pp. 3–62. [Google Scholar]
- 3.Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54(1):28–46. doi: 10.1016/j.jaad.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 4.Salvo N, Barnes E, van Draanen J, et al. Prophylaxis and management of acute radiation-induced skin reactions: a systematic review of the literature. Curr Oncol. 2010;17(4):94–112. doi: 10.3747/co.v17i4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff K, Johnson R, Saavedra A. Skin reactions to ionizing radiation. Fitzpatrick’s color atlas and synopsis of clinical dermatology. New York: McGraw-Hill; 2013. [Google Scholar]
- 6.Bolderston A, Lloyd NS, Wong RK, Holden L, Robb-Blenderman L, Supportive Care Guidelines Group of Cancer Care Ontario Program in Evidence-Based C The prevention and management of acute skin reactions related to radiation therapy: a systematic review and practice guideline. Support Care Cancer. 2006;14(8):802–817. doi: 10.1007/s00520-006-0063-4. [DOI] [PubMed] [Google Scholar]
- 7.Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 Pt 2):985–993. doi: 10.1038/jid.2011.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuestion M. Evidence-based skin care management in radiation therapy. Semin Oncol Nurs. 2006;22(3):163–173. doi: 10.1016/j.soncn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Mendelsohn FA, Divino CM, Reis ED, Kerstein MD. Wound care after radiation therapy. Adv Skin Wound Care. 2002;15(5):216–224. doi: 10.1097/00129334-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Malkinson FD, Keane JT. Radiobiology of the skin: review of some effects on epidermis and hair. J Invest Dermatol. 1981;77(1):133–138. doi: 10.1111/1523-1747.ep12479347. [DOI] [PubMed] [Google Scholar]
- 11.Waghmare CM. Radiation burn—from mechanism to management. Burns. 2013;39(2):212–219. doi: 10.1016/j.burns.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Bey E, Prat M, Duhamel P, et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 2010;18(1):50–58. doi: 10.1111/j.1524-475X.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 13.Lataillade JJ, Doucet C, Bey E, et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen Med. 2007;2(5):785–794. doi: 10.2217/17460751.2.5.785. [DOI] [PubMed] [Google Scholar]
- 14.Burris HA, 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15(11):1227–1237. doi: 10.1634/theoncologist.2009-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hird AE, Wilson J, Symons S, Sinclair E, Davis M, Chow E. Radiation recall dermatitis: case report and review of the literature. Curr Oncol. 2008;15(1):53–62. doi: 10.3747/co.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boussemart L, Boivin C, Claveau J, et al. Vemurafenib and radiosensitization. JAMA Dermatol. 2013;149(7):855–857. doi: 10.1001/jamadermatol.2013.4200. [DOI] [PubMed] [Google Scholar]
- 17.Turesson I, Nyman J, Holmberg E, Oden A. Prognostic factors for acute and late skin reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys. 1996;36(5):1065–1075. doi: 10.1016/S0360-3016(96)00426-9. [DOI] [PubMed] [Google Scholar]
- 18.Bentzen SM, Overgaard J. Patient-to-patient variability in the expression of radiation-induced normal tissue injury. Semin Radiat Oncol. 1994;4(2):68–80. doi: 10.1016/S1053-4296(05)80034-7. [DOI] [PubMed] [Google Scholar]
- 19.Harper JL, Franklin LE, Jenrette JM, Aguero EG. Skin toxicity during breast irradiation: pathophysiology and management. South Med J. 2004;97(10):989–993. doi: 10.1097/01.SMJ.0000140866.97278.87. [DOI] [PubMed] [Google Scholar]
- 20.Delanian S, Balla-Mekias S, Lefaix JL. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol. 1999;17(10):3283–3290. doi: 10.1200/JCO.1999.17.10.3283. [DOI] [PubMed] [Google Scholar]
- 21.Chang DW, te Marvelde L, Chua BH. Prospective study of local control and late radiation toxicity after intraoperative radiation therapy boost for early breast cancer. Int J Radiat Oncol Biol Phys. 2014;88(1):73–79. doi: 10.1016/j.ijrobp.2013.09.049. [DOI] [PubMed] [Google Scholar]
- 22.Hoeller U, Bonacker M, Bajrovic A, Alberti W, Adam G. Radiation-induced plexopathy and fibrosis. Is magnetic resonance imaging the adequate diagnostic tool? Strahlentherapie Onkol. 2004;180(10):650–654. doi: 10.1007/s00066-004-1240-3. [DOI] [PubMed] [Google Scholar]
- 23.Oysu AS, Ayanoglu E, Kodalli N, Oysu C, Uneri C, Erzen C. Dynamic contrast-enhanced MRI in the differentiation of posttreatment fibrosis from recurrent carcinoma of the head and neck. Clin Imaging. 2005;29(5):307–312. doi: 10.1016/j.clinimag.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 24.Shore RE. Radiation-induced skin cancer in humans. Med Pediatr Oncol. 2001;36(5):549–554. doi: 10.1002/mpo.1128. [DOI] [PubMed] [Google Scholar]
- 25.Perkins JL, Liu Y, Mitby PA, et al. Nonmelanoma skin cancer in survivors of childhood and adolescent cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2005;23(16):3733–3741. doi: 10.1200/JCO.2005.06.237. [DOI] [PubMed] [Google Scholar]
- 26.Ron E, Modan B, Preston D, Alfandary E, Stovall M, Boice JD., Jr Radiation-induced skin carcinomas of the head and neck. Radiat Res. 1991;125(3):318–325. doi: 10.2307/3578117. [DOI] [PubMed] [Google Scholar]
- 27.Shore RE, Moseson M, Xue X, Tse Y, Harley N, Pasternack BS. Skin cancer after X-ray treatment for scalp ringworm. Radiat Res. 2002;157(4):410–418. doi: 10.1667/0033-7587(2002)157[0410:SCAXRT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Karagas MR, McDonald JA, Greenberg ER, et al. Risk of basal cell and squamous cell skin cancers after ionizing radiation therapy. For The Skin Cancer Prevention Study Group. J Natl Cancer Inst. 1996;88(24):1848–1853. doi: 10.1093/jnci/88.24.1848. [DOI] [PubMed] [Google Scholar]
- 29.McQuestion M. Evidence-based skin care management in radiation therapy: clinical update. Semin Oncol Nurs. 2011;27(2):e1–e17. doi: 10.1016/j.soncn.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Malkinson F, Hanson W. Radiobiology of the skin. In: Goldsmith L, editor. Physiology, biochemistry and molecular biology of the skin. Oxford: Oxford University Press; 1991. p. 976. [Google Scholar]
- 31.Lopez E, Guerrero R, Nunez MI, et al. Early and late skin reactions to radiotherapy for breast cancer and their correlation with radiation-induced DNA damage in lymphocytes. Breast Cancer Res. 2005;7(5):R690–R698. doi: 10.1186/bcr1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McBride WH, Chiang CS, Olson JL, et al. A sense of danger from radiation. Radiat Res. 2004;162(1):1–19. doi: 10.1667/RR3196. [DOI] [PubMed] [Google Scholar]
- 33.Williams JP, McBride WH. After the bomb drops: a new look at radiation-induced multiple organ dysfunction syndrome (MODS) Int J Radiat Biol. 2011;87(8):851–868. doi: 10.3109/09553002.2011.560996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peter RU. Diagnosis and Treatment of Cutaneous Radiation Injuries. In: Panizzon RG, Seegenschmiedt MH, editors. Radiation treatment and radiation reactions in dermatology. 2. Berlin: Springer; 2015. pp. 185–188. [Google Scholar]
- 35.Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound’. Radiother Oncol. 2002;63(2):129–145. doi: 10.1016/S0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 36.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47(2):277–290. doi: 10.1016/S0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 37.Pohlers D, Brenmoehl J, Loffler I, et al. TGF-beta and fibrosis in different organs—molecular pathway imprints. Biochim Biophys Acta. 2009;1792(8):746–756. doi: 10.1016/j.bbadis.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Canney PA, Dean S. Transforming growth factor beta: a promotor of late connective tissue injury following radiotherapy? Br J Radiol. 1990;63(752):620–623. doi: 10.1259/0007-1285-63-752-620. [DOI] [PubMed] [Google Scholar]
- 39.Tibbs MK. Wound healing following radiation therapy: a review. Radiother Oncol. 1997;42(2):99–106. doi: 10.1016/S0167-8140(96)01880-4. [DOI] [PubMed] [Google Scholar]
- 40.Tokarek R, Bernstein EF, Sullivan F, Uitto J, Mitchell JB. Effect of therapeutic radiation on wound healing. Clin Dermatol. 1994;12(1):57–70. doi: 10.1016/0738-081X(94)90257-7. [DOI] [PubMed] [Google Scholar]
- 41.Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. Int J Radiat Oncol Biol Phys. 1995;31(5):1171–1185. doi: 10.1016/0360-3016(94)00423-I. [DOI] [PubMed] [Google Scholar]
- 42.Quarmby S, Kumar P, Kumar S. Radiation-induced normal tissue injury: role of adhesion molecules in leukocyte-endothelial cell interactions. Int J Cancer. 1999;82(3):385–395. doi: 10.1002/(SICI)1097-0215(19990730)82:3<385::AID-IJC12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Haase O, Rodemann HP. Fibrosis and cytokine mechanisms: relevant in hadron therapy? Radiother Oncol. 2004;73(Suppl 2):S144–S147. doi: 10.1016/S0167-8140(04)80037-9. [DOI] [PubMed] [Google Scholar]
- 44.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nat Rev Cancer. 2006;6(9):702–713. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 45.Abdollahi A, Li M, Ping G, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201(6):925–935. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutreix J. Human skin: early and late reactions in relation to dose and its time distribution. Br J Radiol Suppl. 1986;19:22–28. [PubMed] [Google Scholar]
- 47.Hickok JT, Morrow GR, Roscoe JA, Mustian K, Okunieff P. Occurrence, severity, and longitudinal course of twelve common symptoms in 1129 consecutive patients during radiotherapy for cancer. J Pain Symptom Manag. 2005;30(5):433–442. doi: 10.1016/j.jpainsymman.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Porock D. Factors influencing the severity of radiation skin and oral mucosal reactions: development of a conceptual framework. Eur J Cancer Care (Engl). 2002;11(1):33–43. [PubMed] [Google Scholar]
- 49.Vuong T, Franco E, Lehnert S, et al. Silver leaf nylon dressing to prevent radiation dermatitis in patients undergoing chemotherapy and external beam radiotherapy to the perineum. Int J Radiat Oncol Biol Phys. 2004;59(3):809–814. doi: 10.1016/j.ijrobp.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 50.Hopewell JW, Nyman J, Turesson I. Time factor for acute tissue reactions following fractionated irradiation: a balance between repopulation and enhanced radiosensitivity. Int J Radiat Biol. 2003;79(7):513–524. doi: 10.1080/09553000310001600907. [DOI] [PubMed] [Google Scholar]
- 51.Fernando IN, Ford HT, Powles TJ, et al. Factors affecting acute skin toxicity in patients having breast irradiation after conservative surgery: a prospective study of treatment practice at the Royal Marsden Hospital. Clin Oncol. 1996;8(4):226–233. doi: 10.1016/S0936-6555(05)80657-0. [DOI] [PubMed] [Google Scholar]
- 52.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109–122. doi: 10.1016/0360-3016(91)90171-Y. [DOI] [PubMed] [Google Scholar]
- 53.Lee N, Chuang C, Quivey JM, et al. Skin toxicity due to intensity-modulated radiotherapy for head-and-neck carcinoma. Int J Radiat Oncol Biol Phys. 2002;53(3):630–637. doi: 10.1016/S0360-3016(02)02756-6. [DOI] [PubMed] [Google Scholar]
- 54.Seegenschmiedt H. Management of skin and related reactions to radiotherapy. Front Radiat Ther Oncol. 2006;39:102–119. doi: 10.1159/000090855. [DOI] [PubMed] [Google Scholar]
- 55.Dorr W. Skin and other reactions to radiotherapy—clinical presentation and radiobiology of skin reactions. Front Radiat Ther Oncol. 2006;39:96–101. doi: 10.1159/000090854. [DOI] [PubMed] [Google Scholar]
- 56.Sitton E. Early and late radiation-induced skin alterations. Part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19(5):801–807. [PubMed] [Google Scholar]
- 57.Eisbruch A, Ship JA, Dawson LA, et al. Salivary gland sparing and improved target irradiation by conformal and intensity modulated irradiation of head and neck cancer. World J Surg. 2003;27(7):832–837. doi: 10.1007/s00268-003-7105-6. [DOI] [PubMed] [Google Scholar]
- 58.Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52(5):1330–1337. doi: 10.1016/S0360-3016(01)02785-7. [DOI] [PubMed] [Google Scholar]
- 59.Freedman GM, Anderson PR, Li J, et al. Intensity modulated radiation therapy (IMRT) decreases acute skin toxicity for women receiving radiation for breast cancer. Am J Clin Oncol. 2006;29(1):66–70. doi: 10.1097/01.coc.0000197661.09628.03. [DOI] [PubMed] [Google Scholar]
- 60.Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26(13):2085–2092. doi: 10.1200/JCO.2007.15.2488. [DOI] [PubMed] [Google Scholar]
- 61.Donovan E, Bleakley N, Denholm E, et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol. 2007;82(3):254–264. doi: 10.1016/j.radonc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Barnett GC, Wilkinson JS, Moody AM, et al. Randomized controlled trial of forward-planned intensity modulated radiotherapy for early breast cancer: interim results at 2 years. Int J Radiat Oncol Biol Phys. 2012;82(2):715–723. doi: 10.1016/j.ijrobp.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 63.Shah C, Wobb J, Grills I, Wallace M, Mitchell C, Vicini FA. Use of intensity modulated radiation therapy to reduce acute and chronic toxicities of breast cancer patients treated with traditional and accelerated whole breast irradiation. Pract Radiat Oncol. 2012;2(4):e45–e51. doi: 10.1016/j.prro.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Tejwani A, Wu S, Jia Y, Agulnik M, Millender L, Lacouture ME. Increased risk of high-grade dermatologic toxicities with radiation plus epidermal growth factor receptor inhibitor therapy. Cancer. 2009;115(6):1286–1299. doi: 10.1002/cncr.24120. [DOI] [PubMed] [Google Scholar]
- 65.O’Rourke ME. Enhanced cutaneous effects in combined modality therapy. Oncol Nurs Forum. 1987;14(6):31–35. [PubMed] [Google Scholar]
- 66.Wallach JB, Rietschel P, Kalnicki S, Fox JL. BRAF inhibitor (vemurafenib) concurrent with radiation therapy for metastatic melanoma producing severe skin and oral cavity reactions. Pract Radiat Oncol. 2014;4(5):e213–e216. doi: 10.1016/j.prro.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 67.Coleman CN, Turrisi AT. Radiation and chemotherapy sensitizers and protectors. Crit Rev Oncol Hematol. 1990;10(3):225–252. doi: 10.1016/1040-8428(90)90033-O. [DOI] [PubMed] [Google Scholar]
- 68.Bentzen SM, Overgaard M, Thames HD, Christensen JJ, Overgaard J. Early and late normal-tissue injury after postmastectomy radiotherapy alone or combined with chemotherapy. Int J Radiat Biol. 1989;56(5):711–715. doi: 10.1080/09553008914551941. [DOI] [PubMed] [Google Scholar]
- 69.Toledano A, Garaud P, Serin D, et al. Concurrent administration of adjuvant chemotherapy and radiotherapy after breast-conserving surgery enhances late toxicities: long-term results of the ARCOSEIN multicenter randomized study. Int J Radiat Oncol Biol Phys. 2006;65(2):324–332. doi: 10.1016/j.ijrobp.2005.12.020. [DOI] [PubMed] [Google Scholar]
- 70.Azria D, Gourgou S, Sozzi WJ, et al. Concomitant use of tamoxifen with radiotherapy enhances subcutaneous breast fibrosis in hypersensitive patients. Br J Cancer. 2004;91(7):1251–1260. doi: 10.1038/sj.bjc.6602146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blackmar A. Radiation-induced skin alterations. Medsurg Nurs. 1997;6(3):172–175. [PubMed] [Google Scholar]
- 72.Porock D, Kristjanson L. Skin reactions during radiotherapy for breast cancer: the use and impact of topical agents and dressings. Eur J Cancer Care (Engl). 1999;8(3):143–153. doi: 10.1046/j.1365-2354.1999.00153.x. [DOI] [PubMed] [Google Scholar]
- 73.Morgan K. Radiotherapy-induced skin reactions: prevention and cure. Br J Nurs. 2014;23(16):S24, S26–32. [DOI] [PubMed]
- 74.Delfino S, Brunetti B, Toto V, Persichetti P. Burn after breast reconstruction. Burns. 2008;34(6):873–877. doi: 10.1016/j.burns.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 75.Vandeweyer E, Deraemaecker R. Radiation therapy after immediate breast reconstruction with implants. Plast Reconstr Surg. 2000;106(1):56–58. doi: 10.1097/00006534-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 76.Housri N, Yarchoan R, Kaushal A. Radiotherapy for patients with the human immunodeficiency virus: are special precautions necessary? Cancer. 2010;116(2):273–283. doi: 10.1002/cncr.24878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Swift M, Morrell D, Cromartie E, Chamberlin AR, Skolnick MH, Bishop DT. The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet. 1986;39(5):573–583. [PMC free article] [PubMed] [Google Scholar]
- 78.Iannuzzi CM, Atencio DP, Green S, Stock RG, Rosenstein BS. ATM mutations in female breast cancer patients predict for an increase in radiation-induced late effects. Int J Radiat Oncol Biol Phys. 2002;52(3):606–613. doi: 10.1016/S0360-3016(01)02684-0. [DOI] [PubMed] [Google Scholar]
- 79.Sanford KK, Parshad R, Gantt R, Tarone RE, Jones GM, Price FM. Factors affecting and significance of G2 chromatin radiosensitivity in predisposition to cancer. Int J Radiat Biol. 1989;55(6):963–981. doi: 10.1080/09553008914551001. [DOI] [PubMed] [Google Scholar]
- 80.Terrazzino S, La Mattina P, Masini L, et al. Common variants of eNOS and XRCC1 genes may predict acute skin toxicity in breast cancer patients receiving radiotherapy after breast conserving surgery. Radiother Oncol. 2012;103(2):199–205. doi: 10.1016/j.radonc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Campbell IR, Illingworth MH. Can patients wash during radiotherapy to the breast or chest wall? A randomized controlled trial. Clin Oncol. 1992;4(2):78–82. doi: 10.1016/S0936-6555(05)80971-9. [DOI] [PubMed] [Google Scholar]
- 82.Roy I, Fortin A, Larochelle M. The impact of skin washing with water and soap during breast irradiation: a randomized study. Radiother Oncol. 2001;58(3):333–339. doi: 10.1016/S0167-8140(00)00322-4. [DOI] [PubMed] [Google Scholar]
- 83.Bernier J, Bonner J, Vermorken JB, et al. Consensus guidelines for the management of radiation dermatitis and coexisting acne-like rash in patients receiving radiotherapy plus EGFR inhibitors for the treatment of squamous cell carcinoma of the head and neck. Ann Oncol. 2008;19(1):142–149. doi: 10.1093/annonc/mdm400. [DOI] [PubMed] [Google Scholar]
- 84.Miller RC, Schwartz DJ, Sloan JA, et al. Mometasone furoate effect on acute skin toxicity in breast cancer patients receiving radiotherapy: a phase III double-blind, randomized trial from the North Central Cancer Treatment Group N06C4. Int J Radiat Oncol Biol Phys. 2011;79(5):1460–1466. doi: 10.1016/j.ijrobp.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Potera ME, Lookingbill DP, Stryker JA. Prophylaxis of radiation dermatitis with a topical cortisone cream. Radiology. 1982;143(3):775–777. doi: 10.1148/radiology.143.3.7079509. [DOI] [PubMed] [Google Scholar]
- 86.Bostrom A, Lindman H, Swartling C, Berne B, Bergh J. Potent corticosteroid cream (mometasone furoate) significantly reduces acute radiation dermatitis: results from a double-blind, randomized study. Radiother Oncol. 2001;59(3):257–265. doi: 10.1016/S0167-8140(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 87.Ulff E, Maroti M, Serup J, Falkmer U. A potent steroid cream is superior to emollients in reducing acute radiation dermatitis in breast cancer patients treated with adjuvant radiotherapy. A randomised study of betamethasone versus two moisturizing creams. Radiother Oncol. 2013;108(2):287–292. doi: 10.1016/j.radonc.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 88.Omidvari S, Saboori H, Mohammadianpanah M, et al. Topical betamethasone for prevention of radiation dermatitis. Indian J Dermatol Venereol Leprol. 2007;73(3):209. doi: 10.4103/0378-6323.32755. [DOI] [PubMed] [Google Scholar]
- 89.Dale PS, Tamhankar CP, George D, Daftary GV. Co-medication with hydrolytic enzymes in radiation therapy of uterine cervix: evidence of the reduction of acute side effects. Cancer Chemother Pharmacol. 2001;47(Suppl):S29–S34. doi: 10.1007/s002800170006. [DOI] [PubMed] [Google Scholar]
- 90.Gujral MS, Patnaik PM, Kaul R, et al. Efficacy of hydrolytic enzymes in preventing radiation therapy-induced side effects in patients with head and neck cancers. Cancer Chemother Pharmacol. 2001;47(Suppl):S23–S28. doi: 10.1007/s002800170005. [DOI] [PubMed] [Google Scholar]
- 91.Elliott EA, Wright JR, Swann RS, et al. Phase III Trial of an emulsion containing trolamine for the prevention of radiation dermatitis in patients with advanced squamous cell carcinoma of the head and neck: results of Radiation Therapy Oncology Group Trial 99-13. J Clin Oncol. 2006;24(13):2092–2097. doi: 10.1200/JCO.2005.04.9148. [DOI] [PubMed] [Google Scholar]
- 92.Pommier P, Gomez F, Sunyach MP, D’Hombres A, Carrie C, Montbarbon X. Phase III randomized trial of Calendula officinalis compared with trolamine for the prevention of acute dermatitis during irradiation for breast cancer. J Clin Oncol. 2004;22(8):1447–1453. doi: 10.1200/JCO.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 93.Heggie S, Bryant GP, Tripcony L, et al. A Phase III study on the efficacy of topical aloe vera gel on irradiated breast tissue. Cancer Nurs. 2002;25(6):442–451. doi: 10.1097/00002820-200212000-00007. [DOI] [PubMed] [Google Scholar]
- 94.Sharp L, Finnila K, Johansson H, Abrahamsson M, Hatschek T, Bergenmar M. No differences between Calendula cream and aqueous cream in the prevention of acute radiation skin reactions—results from a randomised blinded trial. Eur J Oncol Nurs. 2013;17(4):429–435. doi: 10.1016/j.ejon.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Richardson J, Smith JE, McIntyre M, Thomas R, Pilkington K. Aloe vera for preventing radiation-induced skin reactions: a systematic literature review. Clin Oncol. 2005;17(6):478–484. doi: 10.1016/j.clon.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 96.Wells M, Macmillan M, Raab G, et al. Does aqueous or sucralfate cream affect the severity of erythematous radiation skin reactions? A randomised controlled trial. Radiother Oncol. 2004;73(2):153–162. doi: 10.1016/j.radonc.2004.07.032. [DOI] [PubMed] [Google Scholar]
- 97.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–294. doi: 10.1038/193293a0. [DOI] [PubMed] [Google Scholar]
- 98.Gollins S, Gaffney C, Slade S, Swindell R. RCT on gentian violet versus a hydrogel dressing for radiotherapy-induced moist skin desquamation. J Wound Care. 2008;17(6):268–270, 272, 274–265. [DOI] [PubMed]
- 99.Macmillan MS, Wells M, MacBride S, Raab GM, Munro A, MacDougall H. Randomized comparison of dry dressings versus hydrogel in management of radiation-induced moist desquamation. Int J Radiat Oncol Biol Phys. 2007;68(3):864–872. doi: 10.1016/j.ijrobp.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 100.Mak SS, Molassiotis A, Wan WM, Lee IY, Chan ES. The effects of hydrocolloid dressing and gentian violet on radiation-induced moist desquamation wound healing. Cancer Nurs. 2000;23(3):220–229. doi: 10.1097/00002820-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 101.Margolin SG, Breneman JC, Denman DL, LaChapelle P, Weckbach L, Aron BS. Management of radiation-induced moist skin desquamation using hydrocolloid dressing. Cancer Nurs. 1990;13(2):71–80. doi: 10.1097/00002820-199004000-00001. [DOI] [PubMed] [Google Scholar]
- 102.Kedge EM. A systematic review to investigate the effectiveness and acceptability of interventions for moist desquamation in radiotherapy patients. Radiography. 2009;15(3):247–257. doi: 10.1016/j.radi.2008.08.002. [DOI] [Google Scholar]
- 103.Gray M. Preventing and managing perineal dermatitis: a shared goal for wound and continence care. J Wound Ostomy Cont Nurs. 2004;31(1 Suppl):S2–S9. doi: 10.1097/00152192-200401001-00002. [DOI] [PubMed] [Google Scholar]
- 104.Smith A, Fife C. Advanced therapeutics: the biochemistry and biophysical basis of wound products. In: Sheffield P, editor. Wound care practice. Flagstaff: Best; 2004. pp. 685–728. [Google Scholar]
- 105.Veness M, Richards S. Radiotherapy. In: Bolognia J, Jorizzo J, Schaffer J, editors. Dermatology. New York: Elsevier; 2012. [Google Scholar]
- 106.Schindl A, Schindl M, Pernerstorfer-Schon H, Mossbacher U, Schindl L. Low intensity laser irradiation in the treatment of recalcitrant radiation ulcers in patients with breast cancer–long-term results of 3 cases. Photodermatol Photoimmunol Photomed. 2000;16(1):34–37. doi: 10.1034/j.1600-0781.2000.160109.x. [DOI] [PubMed] [Google Scholar]
- 107.Bourgeois JF, Gourgou S, Kramar A, Lagarde JM, Guillot B. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14(1):71–76. doi: 10.1111/j.1600-0846.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- 108.Dion MW, Hussey DH, Doornbos JF, Vigliotti AP, Wen BC, Anderson B. Preliminary results of a pilot study of pentoxifylline in the treatment of late radiation soft tissue necrosis. Int J Radiat Oncol Biol Phys. 1990;19(2):401–407. doi: 10.1016/0360-3016(90)90549-Y. [DOI] [PubMed] [Google Scholar]
- 109.Samlaska CP, Winfield EA. Pentoxifylline. J Am Acad Dermatol. 1994;30(4):603–621. doi: 10.1016/S0190-9622(94)70069-9. [DOI] [PubMed] [Google Scholar]
- 110.Vozenin-Brotons MC, Gault N, Sivan V, et al. Histopathological and cellular studies of a case of cutaneous radiation syndrome after accidental chronic exposure to a cesium source. Radiat Res. 1999;152(3):332–337. doi: 10.2307/3580334. [DOI] [PubMed] [Google Scholar]
- 111.Lefaix JL, Delanian S, Vozenin MC, Leplat JJ, Tricaud Y, Martin M. Striking regression of subcutaneous fibrosis induced by high doses of gamma rays using a combination of pentoxifylline and alpha-tocopherol: an experimental study. Int J Radiat Oncol Biol Phys. 1999;43(4):839–847. doi: 10.1016/S0360-3016(98)00419-2. [DOI] [PubMed] [Google Scholar]
- 112.Lefaix JL, Delanian S, Leplat JJ, et al. Successful treatment of radiation-induced fibrosis using Cu/Zn-SOD and Mn-SOD: an experimental study. Int J Radiat Oncol Biol Phys. 1996;35(2):305–312. doi: 10.1016/0360-3016(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 113.Delanian S, Porcher R, Balla-Mekias S, Lefaix JL. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003;21(13):2545–2550. doi: 10.1200/JCO.2003.06.064. [DOI] [PubMed] [Google Scholar]
- 114.Jacobson G, Bhatia S, Smith BJ, Button AM, Bodeker K, Buatti J. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol Biol Phys. 2013;85(3):604–608. doi: 10.1016/j.ijrobp.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 115.Magnusson M, Hoglund P, Johansson K, et al. Pentoxifylline and vitamin E treatment for prevention of radiation-induced side-effects in women with breast cancer: a phase two, double-blind, placebo-controlled randomised clinical trial (Ptx-5) Eur J Cancer. 2009;45(14):2488–2495. doi: 10.1016/j.ejca.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 116.Gothard L, Cornes P, Earl J, et al. Double-blind placebo-controlled randomised trial of vitamin E and pentoxifylline in patients with chronic arm lymphoedema and fibrosis after surgery and radiotherapy for breast cancer. Radiother Oncol. 2004;73(2):133–139. doi: 10.1016/j.radonc.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 117.Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23(34):8570–8579. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 118.Tran TN, Hoang MV, Phan QA, et al. Fractional epidermal grafting in combination with laser therapy as a novel approach in treating radiation dermatitis. Semin Cutan Med Surg. 2015;34(1):42–47. doi: 10.12788/j.sder.2015.0141. [DOI] [PubMed] [Google Scholar]
- 119.Delanian S, Baillet F, Huart J, Lefaix JL, Maulard C, Housset M. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother Oncol. 1994;32(1):12–20. doi: 10.1016/0167-8140(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 120.Gottlober P, Steinert M, Bahren W, Weber L, Gerngross H, Peter RU. Interferon-gamma in 5 patients with cutaneous radiation syndrome after radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50(1):159–166. doi: 10.1016/S0360-3016(00)01542-X. [DOI] [PubMed] [Google Scholar]
- 121.Gothard L, Stanton A, MacLaren J, et al. Non-randomised phase II trial of hyperbaric oxygen therapy in patients with chronic arm lymphoedema and tissue fibrosis after radiotherapy for early breast cancer. Radiother Oncol. 2004;70(3):217–224. doi: 10.1016/S0167-8140(03)00235-4. [DOI] [PubMed] [Google Scholar]
- 122.Teas J, Cunningham JE, Cone L, et al. Can hyperbaric oxygen therapy reduce breast cancer treatment-related lymphedema? A pilot study. J Women’s Health. 2004;13(9):1008–1018. doi: 10.1089/jwh.2004.13.1008. [DOI] [PubMed] [Google Scholar]
- 123.Pritchard J, Anand P, Broome J, et al. Double-blind randomized phase II study of hyperbaric oxygen in patients with radiation-induced brachial plexopathy. Radiother Oncol. 2001;58(3):279–286. doi: 10.1016/S0167-8140(00)00319-4. [DOI] [PubMed] [Google Scholar]
- 124.Carl UM, Feldmeier JJ, Schmitt G, Hartmann KA. Hyperbaric oxygen therapy for late sequelae in women receiving radiation after breast-conserving surgery. Int J Radiat Oncol Biol Phys. 2001;49(4):1029–1031. doi: 10.1016/S0360-3016(00)01515-7. [DOI] [PubMed] [Google Scholar]
- 125.Horton JA, Li F, Chung EJ, et al. Quercetin inhibits radiation-induced skin fibrosis. Radiat Res. 2013;180(2):205–215. doi: 10.1667/RR3237.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lanigan SW, Joannides T. Pulsed dye laser treatment of telangiectasia after radiotherapy for carcinoma of the breast. Br J Dermatol. 2003;148(1):77–79. doi: 10.1046/j.1365-2133.2003.04861.x. [DOI] [PubMed] [Google Scholar]
- 127.Rossi AM, Nehal KS, Lee EH. Radiation-induced breast telangiectasias treated with the pulsed dye laser. J Clin Aesthet Dermatol. 2014;7(12):34–37. [PMC free article] [PubMed] [Google Scholar]
- 128.Bisht KS, Bradbury CM, Zoberi I, et al. Inhibition of cyclooxygenase-2 with NS-398 and the prevention of radiation-induced transformation, micronuclei formation and clonogenic cell death in C3H 10T1/2 cells. Int J Radiat Biol. 2003;79(11):879–888. doi: 10.1080/09553000310001621400. [DOI] [PubMed] [Google Scholar]
- 129.Guillen C, Sanmartin O, Escudero A, Botella-Estrada R, Sevila A, Castejon P. Photodynamic therapy for in situ squamous cell carcinoma on chronic radiation dermatitis after photosensitization with 5-aminolaevulinic acid. J Eur Acad Dermatol Venereol. 2000;14(4):298–300. doi: 10.1046/j.1468-3083.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 130.Rosenthal RA, Fish B, Hill RP, et al. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med Chem. 2011;11(4):359–372. doi: 10.2174/187152011795677490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okunieff P, Xu J, Hu D, et al. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 2006;65(3):890–898. doi: 10.1016/j.ijrobp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 132.Mesia R, Vilajosana E, Lozano A, Esteller L, Silvia V. Management of cutaneous toxicity and radiation dermatitis in patients with squamous cancer of the head and neck undergoing concurrent treatment with cetuximab and radiotherapy. J Cancer Sci Ther. 2009;1(1):28–33. doi: 10.4172/1948-5956.1000005. [DOI] [Google Scholar]