Abstract
Introduction
Interleukin (IL)-17 inhibitors are the most recent class of monoclonal antibodies approved by the FDA for psoriasis treatment. Preclinical and phase II studies of brodalumab, a high-affinity IL-17 receptor monoclonal antibody, have been encouraging.
Methods
We conducted a literature search using the PubMed database in order to assess the efficacy and safety profile of brodalumab. The search included the following key words: “psoriasis” and “IL-17” or “brodalumab.” We also reviewed citations within articles to identify relevant sources.
Results
At week 12, the proportion of patients attaining a 75% improvement from the baseline Psoriasis Area and Severity Index (PASI 75) was similar among the three phase III trials (AMAGINE-1, 83%; AMAGINE-2, 86%; AMAGINE-3, 85%). Brodalumab remained efficacious through 52 weeks of treatment. It maintained a satisfactory safety profile; the most frequently reported adverse events consisted of nasopharyngitis, headache, upper respiratory tract infection, and arthralgia.
Conclusion
Use of brodalumab revealed prompt clinical improvement and a favorable short-term safety profile in phase III trials, although further extension studies are needed to assess long-term safety. Based on the results, brodalumab appears to be a potent therapeutic option for patients with moderate-to-severe plaque-type psoriasis.
Keywords: AMAGINE, Anti-interleukin-17, Biologics, Brodalumab, Interleukin 17, Phase III, Psoriasis
Introduction
Psoriasis is a common chronic immune-mediated skin disease that occurs in 3–4% of the adult US population [1]. Symptoms of psoriasis, which include redness, scaling, flaking, pruritus, skin tightness, pain, and bleeding, have a significantly negative impact on patients’ physical and mental functioning [2]. Psoriasis also leads to impairment of quality of life, psychological well-being, and work productivity [2, 3]. Despite the rapid development of novel treatment modalities over the past 2 decades, surveys conducted by the National Psoriasis Foundation reveal that a significant portion of patients with psoriasis remain undertreated relative to the severity of their disease [3]. These patients will require new medications with superior long-term efficacy and safety for the treatment of moderate-to-severe psoriasis.
Brodalumab is one of three biologic agents, in addition to secukinumab and ixekizumab, which targets the interleukin (IL)-17 cytokine pathway, which has been implicated in the pathogenesis of psoriasis. IL-17A is one of six subsets of IL-17 and is regarded as the most significant subtype in psoriasis development [4, 5]. The cytokine applies its effect by binding the IL-17 receptor type A (IL-17RA) [6]. TH17 cells are increased in psoriatic lesions and are activated by IL-23 to release IL-17 [7–11]. IL-17 plays a part in the stimulation and recruitment of neutrophils, the defense of neutrophil apoptosis, and the provocation of psoriasis angiogenesis [5, 12–14]. Proof of increased levels of IL-17 mRNA [15] and IL-17 in psoriatic lesions and in the serum of psoriasis patients further supports the role of IL-17 in the pathogenesis of psoriasis [8, 16–19]. Brodalumab is a human immunoglobulin G (IgG) 2 monoclonal antibody that acts by specifically inhibiting IL-17RA, thus decreasing the downstream effect of IL-17 [20]. It binds with high affinity to IL-17RA and blocks the biological activity of IL-17A, IL-17F, and IL-25 (IL-17E) [20].
In the following article, we review the results of the clinical phase III trials establishing the efficacy and safety of brodalumab in patients with moderate-to-severe plaque psoriasis. Two co-primary end points were examined: a 75% reduction in the Psoriasis Area and Severity Index (PASI 75) and static physician global assessment (sPGA) of 0 (clear) or 1 (almost-clear) on a five-point scale by week 12 of treatment. sPGA is a method employed by investigators to document their assessment of disease severity, with scores ranging from 0 (clear) to 4 (severe disease). We also report long-term efficacy results up to 52 weeks in the clinical trials that have been completed to date.
Methods
We conducted a systematic English-language literature search of PubMed, MEDLINE, and Cochrane databases from January 2003 to May 2016 to identify and evaluate all phase III randomized clinical trials on efficacy and safety of brodalumab for plaque psoriasis. Key search terms included “brodalumab,” “psoriasis,” and “phase III” or “IL-17,” “psoriasis,” and “phase III.” We also reviewed citations within articles to identify relevant resources. Studies that were not in English, were not phase III, or did not conduct a randomized controlled trial for plaque psoriasis on humans were excluded from the review. This left three phase III clinical trials that were included in this assessment: AMAGINE-1, AMAGINE-2, and AMAGINE-3. Pooled measures of efficacy and incidence of adverse events were calculated by tabulating values from independently conducted studies. This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
Three major phase III clinical trials, AMAGINE-1, AMAGINE-2, and AMAGINE-3 (ClinicalTrials.gov identifiers: NCT01708590, NCT01708603, and NCT01708629, respectively), were conducted to determine the efficacy and safety of different doses of brodalumab compared to placebo [20, 21]. AMAGINE-2 and AMAGINE-3 also compared brodalumab to ustekinumab. The comparisons in baseline demographics are shown in Table 1, while the results from each of these trials are displayed in Table 2. All three trials were eventually terminated prematurely by the study sponsor (Amgen) prior to obtaining long-term extension data.
Table 1.
Baseline demographics and clinical characteristics for all patients enrolled in the AMAGINE-2 and AMAGINE-3 studies
| AMAGINE-1 | AMAGINE-2 | AMAGINE-3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 220) | Brodalumab 140 mg Q2W (n = 219) | Brodalumab 210 mg Q2W (n = 222) | Placebo (n = 309) | Ustekinumab (n = 300) | Brodalumab 140 mg Q2W (n = 610) | Brodalumab 210 mg Q2W (n = 612) | Placebo (n = 315) | Ustekinumab (n = 313) | Brodalumab 140 mg Q2W (n = 629) | Brodalumab 210 mg Q2W (n = 624) | |
| Age, year | 47 ± 13 | 46 ± 13 | 46 ± 12 | 44 ± 13 | 45 ± 13 | 45 ± 13 | 45 ± 13 | 44 ± 13 | 45 ± 13 | 45 ± 13 | 45 ± 13 |
| Male sex, no. (%) | 161 (73) | 162 (74) | 161 (73) | 219 (71) | 205 (68) | 413 (68) | 421 (69) | 208 (66) | 212 (68) | 437 (70) | 431 (69) |
| White race, no. (%)a | 202 (92) | 196 (90) | 2013 (91) | 273 (88) | 271 (90) | 557 (91) | 551 (90) | 294 (93) | 280 (90) | 569 (91) | 565 (91) |
| Weight, kg | 90.4 ± 20.1 | 90.6 ± 21.5 | 91.4 ± 23.4 | 92 ± 23 | 91 ± 24 | 92 ± 22 | 91 ± 23 | 89 ± 22 | 90 ± 22 | 89 ± 21 | 90 ± 23 |
| Body-mass indexb | N/A | N/A | N/A | 30.5 ± 7.0 | 30.6 ± 7.1 | 30.8 ± 7.4 | 30.5 ± 7.2 | 29.9 ± 6.7 | 30.4 ± 6.8 | 29.9 ± 6.7 | 30.3 ± 7.3 |
| Duration of psoriasis, year | 21 ± 12 | 19 ± 13 | 20 ± 13 | 18 ± 12 | 19 ± 13 | 19 ± 12 | 19 ± 12 | 18 ± 12 | 18 ± 12 | 17 ± 12 | 18 ± 12 |
| Psoriatic arthritis (yes), no. (%) | 63 (29) | 60 (27) | 58 (26) | 51 (17) | 50 (17) | 125 (21) | 114 (19) | 59 (19) | 64 (20) | 134 (21) | 127 (20) |
| Body-surface area involved, % | 26.9 ± 17.1 | 27.4 ± 17.1 | 25.1 ± 15.3 | 28 ± 17 | 27 ± 19 | 27 ± 17 | 26 ± 16 | 28 ± 17 | 28 ± 18 | 29 ± 18 | 28 ± 18 |
| PASI scorec | 19.7 ± 7.7 | 20.0 ± 7.4 | 19.4 ± 6.6 | 20.4 ± 8.2 | 20.0 ± 8.4 | 20.5 ± 8.2 | 20.3 ± 8.3 | 20.1 ± 8.7 | 20.1 ± 8.4 | 20.1 ± 8.5 | 20.4 ± 8.3 |
| sPGA, no. (%)d | |||||||||||
| 3 | 114 (52) | 129 (59) | 121 (55) | 167 (54) | 153 (51) | 358 (59) | 316 (52) | 192 (61) | 192 (61) | 412 (66) | 373 (60) |
| 4 | 91 (41) | 80 (37) | 87 (39) | 120 (39) | 132 (44) | 217 (36) | 254 (42) | 113 (36) | 103 (33) | 192 (31) | 226 (36) |
| 5 (very severe) | 15 (7) | 10 (5) | 14 (6) | 22 (7) | 15 (5) | 35 (6) | 42 (7) | 10 (3) | 18 (6) | 25 (4) | 25 (4) |
| PSI scoree | 19 ± 6.7 | 19.7 ± 7.3 | 18.9 ± 6.7 | 18.6 ± 7.1 | 18.9 ± 7.0 | 18.9 ± 7.0 | 18.6 ± 6.8 | 19.0 ± 6.7 | 18.7 ± 6.8 | 18.1 ± 7.1 | 18.7 ± 7.2 |
| Previous systemic treatment or phototherapy, no. (%) | N/A | N/A | N/A | 230 (74) | 225 (75) | 471 (77) | 469 (77) | 206 (65) | 220 (70) | 439 (70) | 422 (68) |
| Previous biologic therapy, no. (%) | 101 (46) | 99 (45) | 105 (47) | 90 (29) | 84 (28) | 179 (29) | 177 (29) | 76 (24) | 75 (24) | 160 (25) | 157 (25) |
Plus-minus values are mean ± SD
aRace was self-reported
bThe body-mass index is the weight in kilograms divided by the square of the height in meters
cScores on the psoriasis area-and-severity index (PASI) range from 0 to 72, with higher scores indicating more severe disease
dScores on the static physician global assessment (sPGA) range from 0 (clear) to 5 (very severe); a score of 3 indicates moderate disease
eScores on the Psoriasis Symptom Inventory (PSI) range from 0 to 32, with higher scores indicating more severe disease
Table 2.
Primary and secondary end points at week 12 for brodalumab compared to placebo and ustekinumab
Disclaimer: these data were tabulated from independent studies that were not conducted in a head-to-head manner
| End point | Study | Brodalumab 210 mg | Brodalumab 140 mg | Placebo | Ustekinumab 45 mg or 90 mg |
|---|---|---|---|---|---|
| PASI 75 | AMAGINE-1, % (no./total), 95% CI | 83.3%† (185/222), 78–88 | 60.3%† (132/219), 54–67 | 2.7% (6/220), 1–6 | – |
| AMAGINE-2, % (no./total), 95% CI | 86.3%†, ¶ (528/612), 83–89 | 66.6%†, ¶ (406/610), 63–70 | 8.1% (25/309), 5–12 | 70% (210/300), 65–75 | |
| AMAGINE-3, % (no./total), 95% CI | 85.1%†, * (531/624), 82–88 | 69.2%†, ¶ (396/629), 65–73 | 6% (19/315), (4–9 | 69.3% (217/313), 64–74 | |
| PASI 90 | AMAGINE-1, % (no./total), 95% CI | 70.3%† (156/222), 64–76 | 42.5%† (93/219), 36–49 | 0.9% (2/220), 0–3 | – |
| AMAGINE-2, % (no./total), 95% CI | 69.9%†, * (428/612), N/A | 49.0%†, ¶ (299/610), N/A | 1.9% (6/309), N/A | 47.0% (141/300), N/A | |
| AMAGINE-3, % (no./total), 95% CI | 68.9%†, * (430/624), N/A | 52.0%†, ¶ (327/629), N/A | 2.9% (9/315), N/A | 47.9% (150/313), N/A | |
| PASI 100 | AMAGINE-1, % (no./total), 95% CI | 41.9%† (93/222), 36–49 | 23.3%† (51/219), 18–30 | 0.5% (1/220), 0–3 | – |
| AMAGINE-2, % (no./total), 95% CI | 44.4%†, * (272/612), 41–49 | 25.7%†, ¶ (157/610), 22–29 | 0.6% (2/309), 0–2 | 21.7% (65/300), 17–27 | |
| AMAGINE-3, % (no./total), 95% CI | 36.7%†, * (229/624), 33–41 | 27%†, * (170/629), 24–31 | 0.3% (1/315), 0–2 | 18.5% (58/313), 14–23 | |
| sPGA 0/1 | AMAGINE-1, % (no./total), 95% CI | 75.7%† (168/222), 70–81 | 53.9%† (118/219), 47–61 | 1.4% (3/220), 0–4 | – |
| AMAGINE-2, % (no./total), 95% CI | 78.6%†, * (481/612), 75–82 | 58.0%†, ¶ (354/610), 54–62 | 4% (12/309), 2–7 | 61% (183/300), 55–67 | |
| AMAGINE-3, % (no./total), 95% CI | 79.6%†, * (497/624), 76–83 | 59.9%†, ¶ (377/629), 56–64 | 4.1% (13/315), 2–7 | 57.2% (179/313), 52–63 | |
| PSI Response | AMAGINE-1, % (no./total), 95% CI | 60.8%† (135/222), 54–67 | 53.0%† (116/219), 46–60 | 4.1% (9/220), 2–8 | – |
| AMAGINE-2, % (no./total), 95% CI | 67.6%† (414/612), 64–71 | 51.5%a (314/610), 47–56 | 6.8% (21/309), 4– 0 | 55.3% (166/300), 50–61 | |
| AMAGINE-3, % (no./total), 95% CI | 61.2%† (382/624), 57–65 | 53.4%† (336/629), 49–57 | 6.3% (17/315), 4–10 | 51.8% (162/313), 46–57 |
sPGA static Physician Global Assessment, PASI Psoriasis Area and Severity Index, PSI Psoriasis Symptom Inventory, CI confidence interval
* P < 0.01 for the comparison with ustekinumab
† P < 0.001 for the comparison with placebo
¶ P > 0.05 for the comparison with ustekinumab
AMAGINE-1
Study Design
This was a randomized, double-blind, placebo-controlled multicenter trial that consisted of 661 patients randomly distributed in a 1:1:1 ratio to receive 210 mg brodalumab, 140 mg brodalumab, or placebo (all given as a subcutaneous injection on day 1 and weeks 1, 2, 4, 6, 8, and 10) [20]. The study incorporated the co-primary end points of PASI 75 and sPGA 0 or 1 at week 12. PASI 90, PASI 100, and Psoriasis Symptom Inventory (PSI) response were included as secondary end points in the study. The PSI is a patient-performed symptom self-assessment that measures psoriasis signs and symptoms. At 12 weeks, patients receiving brodalumab were re-randomized to receive placebo, brodalumab 210 mg, or brodalumab 140 mg every 2 weeks (Q2W) through week 52.
Efficacy
By week 12, the trial demonstrated statistically significant superiority of brodalumab 210 mg and brodalumab 140 mg over placebo. The proportions of patients achieving PASI 75 were 83.3% and 60.3% for brodalumab 210 mg and 140 mg, respectively, compared to 2.7% in those who took placebo (P < 0.001 compared to placebo). The percentage achieving sPGA 0 or 1 were 75.7% and 53.9% for brodalumab 210 mg and 140 mg, respectively, compared to 1.4% in those who took placebo (P < 0.001 compared to placebo). Both dosages of brodalumab were statistically superior to placebo in terms of PASI 90, PASI 100, and PSI (P < 0.001 compared to placebo) (Table 2). Data beyond week 12 revealed that this trend was sustained through 52 weeks with 83.1% and 70.2% of treatment responders maintaining sPGA 0 or 1 at week 52 while taking brodalumab 210 mg Q2W or brodalumab 140 mg Q2W, respectively. This is in contrast to 0% and 5.1% of patients maintaining sPGA 0/1 at week 52 when switching from brodalumab 210 mg and brodalumab 140 mg, respectively, to placebo (P < 0.001 compared to placebo).
Adverse Events
At 12 weeks, a greater proportion of patients receiving brodalumab 210 mg and brodalumab 140 mg experienced any treatment-related adverse events (59% and 57.5%, respectively) or serious adverse events (1.8% and 2.7%, respectively) compared to placebo (any adverse events, 50.9%; serious adverse events, 1.4%). The most common adverse events (occurring in ≥5% in any treatment group) were nasopharyngitis, upper respiratory tract infection, and headache. Most adverse events brought on by treatment were mild or moderate in severity with most patients continuing treatment with brodalumab. Depression was observed in 0.5% of individuals in each group taking brodalumab 210 mg, brodalumab 140 mg, and placebo at 12 weeks. There was one case each of neutropenia and oral candidiasis by 12 weeks, both cases in the brodalumab 140 mg group. Through 52 weeks on brodalumab, no evidence of a dose effect on adverse event rates was observed. Readers should interpret the data from this study cautiously, as results were obtained from the American Academy of Dermatology meeting poster session and have not yet been peer reviewed. Additionally, comparisons in adverse events are not statistically significant, as the studies are powered to detect differences in efficacy rather than rates of adverse events.
AMAGINE-2
Study Design
This was a randomized, double-blind, placebo-controlled, parallel group multicenter trial that consisted of 1831 patients randomly distributed in a 2:2:1:1 ratio to receive either brodalumab 210 mg or brodalumab 140 mg (both given as a subcutaneous injection on day 1 and weeks 1, 2, 4, 6, 8, and 10), ustekinumab [given as subcutaneous injection of 45 mg for patients with body weight ≤100 kg and 90 mg for patients with body weight >100 kg, on day 1 and week 4 and every 12 weeks (Q12W) thereafter], or placebo given as a subcutaneous dummy injection on day 1 and weeks 1, 2, 4, 6, 8, and 10), respectively [21]. At 12 weeks, patients in the brodalumab treatment arms were re-randomized in a 2:2:2:1 ratio to receive brodalumab at 210 mg Q2W, 140 mg Q2W, 140 mg every 4 weeks (Q4W), or 140 mg every 8 weeks (Q8W), respectively, through week 52. Patients originally taking placebo were switched to brodalumab 210 mg Q2W at 12 weeks through 52 weeks. Those taking ustekinumab continued to receive ustekinumab at the same dose Q12W. The trial incorporated the 12-week co-primary end points of PASI 75 and sPGA 0 or 1 in patients taking brodalumab versus placebo, and PASI 100 in patients taking brodalumab versus ustekinumab. PASI 90, PASI 100, and PSI response at week 12 and sPGA 0 or 1 at week 52 were included as secondary end points in the study.
Efficacy
At 12 weeks, the study demonstrated statistically significant superiority of brodalumab 210 mg and brodalumab 140 mg over placebo. The proportion of patients achieving PASI 75 were 86.3% and 66.6% for brodalumab 210 mg and brodalumab 140 mg, respectively, compared to 8.1% in those who took placebo (P < 0.001 compared to placebo). The percentages achieving sPGA 0 or 1 were 78.6% and 58.0% for brodalumab 210 mg and brodalumab 140 mg, respectively, compared to 4.0% in those who took placebo (P < 0.001 compared to placebo). In comparison to ustekinumab 45 mg or 90 mg, brodalumab 210 mg was shown to be statistically superior in terms of the proportion of patients achieving sPGA 0 or 1, PASI 90, and PASI 100 (P < 0.01 compared to ustekinumab). Both dosages of brodalumab were also statistically superior to placebo in terms of PASI 90, PASI 100, and PSI response (P < 0.001 compared to placebo) (Table 2). Data beyond 12 weeks revealed that 65% of patients in the brodalumab 210 mg Q2W group and 43% in the brodalumab 140 mg Q2W group achieved sPGA 0 or 1 at 52 weeks.
Adverse Events
At 12 weeks, a greater proportion of patients receiving brodalumab 210 mg and brodalumab 140 mg experienced any treatment-related adverse events (57.8% and 60.1%, respectively) compared to placebo (53.4%). The rates of or serious adverse events at 12 weeks were 1.0%, 2.1%, and 2.6% for patients taking brodalumab 210 mg, brodalumab 140 mg, or placebo, respectively. The most common adverse events were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia. Most adverse events brought on by treatment were mild or moderate in severity with most patients continuing treatment with brodalumab. Depression was observed in 0.3% of individuals on brodalumab 210 mg, 0.7% in those on brodalumab 140 mg, and 0.3% in those on placebo at 12 weeks, while there was one suicide attempt in the brodalumab 210 mg group. By 12 weeks, candida infections were more frequent in the brodalumab 210 mg groups (1.6%) and brodalumab 140 mg group (1.3%) compared to placebo (0.6%). Both the brodalumab 210 mg and brodalumab 140 mg groups had one case each of neutropenia by 12 weeks, while placebo had 0 cases. Through 52 weeks, there was one reported case of completed suicide in a patient who had started on placebo and switched to brodalumab 210 mg at 12 weeks, occurring 27 days after the last dose. There was an additional completed suicide beyond 52 weeks in a patient who had received brodalumab 210 mg every 2 weeks, occurring 19 days after the last dose. The patient on brodalumab 210 mg Q2W who had a suicide attempt prior to 12 weeks also had two further suicide attempts while on brodalumab 210 mg Q2W through week 52. It is important to note that comparisons in adverse events are not statistically significant, as the studies are powered to detect differences in efficacy rather than rates of adverse events.
AMAGINE-3
Study design
This study employed the same design method as AMAGINE-2, with a randomized, double-blind, placebo-controlled, multicenter trial that consisted of 1881 patients randomly distributed in a 2:2:1:1 ratio to receive either brodalumab 210 mg or brodalumab 140 mg (both given as a subcutaneous injection on day 1 and weeks 1, 2, 4, 6, 8, and 10), ustekinumab [given as subcutaneous injection of 45 mg for patients with body weight ≤100 kg and 90 mg for patients with body weight >100 kg, on day 1 and week 4 and every 12 weeks (Q12W) thereafter), or placebo given as a subcutaneous dummy injection on day 1 and weeks 1, 2, 4, 6, 8, and 10], respectively [21]. As in AMAGINE-2, at 12 weeks, patients in the brodalumab treatment arms were re-randomized in a 2:2:2:1 ratio to receive brodalumab at 210 mg every 2 weeks (Q2W), 140 mg Q2W, 140 mg every 4 weeks (Q4W), or 140 mg every 8 weeks (Q8W), respectively, through week 52. Patients originally taking placebo were switched to brodalumab 210 mg Q2W at 12 weeks through 52 weeks. Those taking ustekinumab continued to receive ustekinumab at the same dose Q12W. The study incorporated the same co-primary and secondary end points as AMAGINE-2.
Efficacy
At 12 weeks, the study demonstrated statistically significant superiority of brodalumab 210 mg and brodalumab 140 mg over placebo. The proportion of patients achieving PASI 75 were 85.1% and 69.2% for brodalumab 210 mg and brodalumab 140 mg, respectively, compared to 6.0% in those who took placebo (P < 0.001 compared to placebo). The percentage achieving sPGA 0 or 1 were 79.6% and 59.9% for brodalumab 210 mg and brodalumab 140 mg, respectively, compared to 4.1% in those who took placebo (P < 0.001 compared to placebo). In comparison to ustekinumab 45 mg or 90 mg, brodalumab 210 mg was shown to be statistically superior in terms of the proportion of patients achieving sPGA 0 or 1, PASI 75, PASI 90, and PASI 100 (P < 0.01 compared to ustekinumab). Both dosages of brodalumab were also statistically superior to placebo in terms of PASI 90, PASI 100, and PSI response (P < 0.001 compared to placebo) (Table 2). Data beyond 12 weeks revealed that 61% of patients in the brodalumab 210 mg Q2W group and 45% in the brodalumab 140 mg Q2W group achieved sPGA 0 or 1 at 52 weeks.
Adverse Events
At 12 weeks, a greater proportion of patients receiving brodalumab 210 mg and brodalumab 140 mg experienced any treatment-related adverse events (56.8% and 52.6%, respectively) or serious adverse events (1.4% and 1.6%, respectively) compared to placebo (adverse events: 48.6%, serious adverse events: 1.0%). The most common adverse events were nasopharyngitis, upper respiratory tract infection, headache, and arthralgia. Most adverse events brought on by treatment were mild or moderate in severity with most patients continuing treatment with brodalumab. Depression was observed in 0.3% of individuals on brodalumab 210 mg, 0.6% in those on brodalumab 140 mg, and 0.6% in those on placebo at 12 weeks, with no reported suicide attempts by this 12-week point. In this same time interval, candida infections were more frequent in the brodalumab 210 mg groups (1.3%) and brodalumab 140 mg group (0.5%) compared to placebo (0.3%). There were 12 reported cases of neutropenia in the brodalumab groups by 12 weeks (7 in the brodalumab 210 mg group and 5 in the brodalumab 140 mg group), while placebo had 0 cases. Through 52 weeks and beyond, there were no reported completed suicides or attempts of suicide. Important to note, comparisons in adverse events are not statistically significant, as the studies are powered to detect differences in efficacy rather than rates of adverse events.
Pooled Results
Efficacy
At 12 weeks, each of the studies demonstrated statistically significant superiority of brodalumab 210 mg Q2W and brodalumab 140 mg Q2W over placebo (P < 0.001 for all groups compared to placebo). Within these studies, the pooled proportion of patients reaching PASI 75 were 85.3% and 64.1% for brodalumab 210 mg and 140 mg, respectively, compared to 5.9% in those who took placebo. The pooled percentage of patients achieving sPGA 0 or 1 were 78.6% and 58.2% for brodalumab 210 mg and 140 mg, respectively, compared to 3.3% among those who took placebo. Brodalumab was similarly superior to placebo in terms of PASI 90 and PASI 100 (Table 2). The pooled data beyond 12 weeks for all three trials revealed a positive trend in results, with 64% of the overall patients in the brodalumab 210 mg Q2W pooled group and 45.9% in the brodalumab 140 mg Q2W pooled group achieving sPGA 0 or 1 at 52 weeks.
Adverse Events
The pooled proportion of patients who experienced adverse events at 12 weeks in all three studies was 57.6% among patients taking brodalumab 210 mg Q2W, 55.6% among patients taking brodalumab 140 mg Q2W, and 51.0% among patients on placebo. The most common adverse events among the studies were nasopharyngitis, headache, upper respiratory tract infection, and arthralgia. Throughout all three studies, 15 out of 2916 patients experienced neutropenia in the combined brodalumab dose groups at 12 weeks, while placebo groups experienced 0 cases of neutropenia. All cases were mild and transient, without associated infections. Through 52 weeks and beyond, two completed suicides were reported in the AMAGINE-2 study among patients taking brodalumab.
Discussion
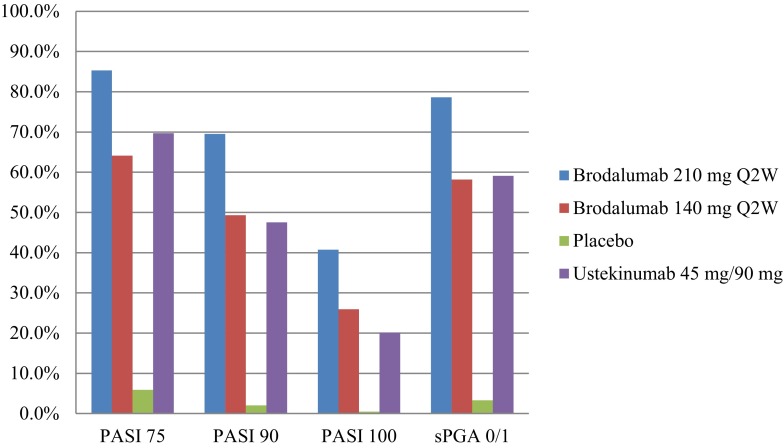
Results from the examined phase III clinical trials demonstrated that brodalumab is a very potent agent in the treatment of plaque-type psoriasis. At week 12, the proportions of patients achieving PASI 75 or sPGA scores of 0 or 1 were higher in the brodalumab 210 mg arm compared to the brodalumab 140 mg arm, and both were far superior to the portion of patients that received placebo (Table 2; Fig. 1). Brodalumab 210 mg was also found to be statistically superior to ustekinumab at 12 weeks with respect to PASI 90, PASI 100, and sPGA 0 or 1. While current long-term results seem encouraging, further long-term trials are needed. The advantage in brodalumab is most distinct in regards to the percentage of patients who attain PASI 90 and PASI 100 (Table 2). Based on evidence that patients who reach PASI 90 or PASI 100 experience greater improvement in quality of life than PASI 75 responders [22], we can estimate that these efficacy achievements are more significant than PASI 75 from a patient perspective. In comparison to secukinumab and ixekizumab, brodalumab achieves similar treatment results at 12 weeks. However, brodalumab appears to deliver greater PASI 100 rates at 12 weeks and better overall maintenance at 52 weeks, although no head-to-head studies have been conducted and research is still in its early states [23]. In any case, the development of this unique, highly effective class of biologic therapy provides optimism for patients whose psoriasis remains refractory to older biologic agents.
Fig. 1.
Percentage of patients achieving PASI 75, PASI 90, PASI 100, and sPGA 0 or 1 at the most efficacious phase III dosage for each drug, at week 12, among the three AMAGINE trials.
Disclaimer: Data were tabulated from independent studies that were not conducted in a head-to-head manner. PASI Psoriasis Area and Severity Index, Q2W Every 2 weeks, sPGA Static Physician Global Assessment
Among the phase III trials of brodalumab, the most common adverse events included nasopharyngitis, headache, upper respiratory tract infection, and arthralgia. The majority of adverse events were mild or moderate in severity. Less than 2% of patients experienced either neutropenia or candida infection while on brodalumab 210 mg or brodalumab 140 mg through the first 12 weeks. Neutropenia was transient and without associated infections, and candida infections were mild to moderate in intensity and resolved without discontinuation of treatment. Anti-IL-17 therapeutics, including brodalumab, have the theoretical risk of increasing the incidence of mucocutaneous candidiasis infection, based on genetic studies of patients lacking IL-17 immunity [24, 25]. Candida infections were more common among patients taking brodalumab than in those taking placebo among the studies we examined. In comparison to secukinumab and ixekizumab, no substantial differences were noted in the safety profile, with nasopharyngitis, upper respiratory infection, and headache comprising the most common adverse events [23]. Both neutropenia and candida infections generally occurred more frequently, although not significantly, in patients on the three therapeutics. Of utmost significance, the AMAGINE-2 trial included two patients who completed suicide (one within 52 weeks and another one beyond 52 weeks) while on brodalumab. Although concerning, these events do not necessarily constitute a causative relationship between brodalumab and suicidal ideation, especially given that patients with psoriasis are already at higher risk for depression, suicidal ideation, suicide attempt, and completed suicide [26, 27]. Regardless, Amgen, the company involved in the devlopment of brodalumab, has decided to withdraw from co-development of the drug because of worries of a potential black box warning about suicide, citing “events of suicidal ideation and behavior in the brodalumab program” [19]. Valeant Pharmaceuticals has since assumed a lead role in the further development of brodalumab [28].
Brodalumab is a human IgG2 monoclonal antibody that may better achieve full clearance (PASI 100) relative to the other IL-17A cytokine specific agents, secukinumab and ixekizumab, because it is the only treatment in development that inhibits the IL-17 receptor (IL-17RA). Although IL-17A is recognized as the most significant IL-17 isotype in the pathogenesis of psoriasis [5], IL-17F and IL-25 also interact with the IL-17 receptor to provoke inflammatory signaling [4, 20]. The ability of brodalumab to block the effects of all cytokines that interact with IL-17RA is likely to contribute to the higher efficacy observed with brodalumab compared to ixekizumab and secukinumab, whose antagonistic effects are limited to IL-17A [29]. Both secukinumab and ixekizumab have passed phase III trials and are FDA approved, with ixekizumab obtaining approval most recently on 22 March 2016, while brodalumab remains in development. Both brodalumab and secukinumab are fully human monoclonal antibodies, whereas ixekizumab is a humanized antibody. A humanized antibody contains nonhuman regions, but still behaves very similarly to a fully human antibodies. In regards to injection frequency, brodalumab provides a more advantageous option with only four syringes injected by the end of the first month for the 210-mg regimen compared to secukinumab, which necessitates the injection of ten syringes by the end of the first month for the 300-mg regimen (both dosing regimens are the highest for the respective drug). Thus far, there have been no head-to-head comparisons of brodalumab against secukinumab and ixekizumab.
In addition to treating psoriasis, brodalumab has the potential to offer further systemic benefits. With regards to psoriatic arthritis, which affects between 5% and 30% of those with psoriasis, anti-IL-17 agents may have a critical role, as higher levels of IL-17 and IL-17RA have been observed within the synoviocytes and synovial fluid of psoriatic arthritis patients [30–33]. Consequently, a phase III clinical trial has shown that brodalumab was statistically superior to placebo in the treatment of patients with active psoriatic arthritis at 12 weeks, as measured by the proportion of patients achieving an American College of Rheumatology 20% improvement response (ACR20) and 50% improvement response (ACR50) [34]. Brodalumab is also being tested as a treatment for asthma and axial spondyloarthritis [35, 36]. Additionally, treatment of the inflammatory state underlying psoriasis has the ability to reduce the incidence of cardiovascular disease [37]. Increased IL-17 has been detected in atherosclerotic plaques [38] and is believed to pair with other inflammatory signals in forming plaques [39]. These observations together convey the possibility of using IL-17 pathway antagonists to treat not only psoriasis, but also psoriatic arthritis and to reduce the risk of cardiovascular disease in those with psoriasis [40].
Conclusion
Brodalumab has generated encouraging data for efficacy and safety in the treatment of chronic plaque psoriasis. The phase III clinical trials results suggest that brodalumab 210 mg and brodalumab 140 mg dosed every 2 weeks are far superior statistically to placebo in all categories at 12 weeks and better able to achieve complete clearance compared to the contemporary biologic agent ustekinumab. Further results from open-label extension studies are required to validate the promising long-term efficacy and safety profile of this unique therapeutic.
Acknowledgments
No funding or sponsorship was received for publication of this article. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
Dr. John Koo is a speaker for AbbVie, Leo, and Celgene. Dr. Koo conducts research for Amgen, Janssen, Novartis, Photomedex, Galderma, Pfizer, and Merck. Dr. Tina Bhutani is an advisor for Cutanea. Dr. Bhutani conducts research for Abbvie, Janssen, and Merck. Dr. Koo and Dr. Bhutani have no stocks, employment, or board memberships with any pharmaceutical company. Mr. Benjamin Farahnik, Mr. Kourosh Beroukhim, Dr. Mio Nakamura, Mr. Michael Abrouk, Mr. Henry Zhu, Ms. Rasnik Singh, and Ms. Kristina Lee have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D0D4F0601FA6776C.
References
- 1.Rachakonda T, Schupp C, Armstrong A. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014 doi: 10.1016/j.jaad.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 2.Feldman SR, Malakouti M, Koo JY. Social impact of the burden of psoriasis: effects on patients and practice. Dermatol Online J. 2014;20(8):1–8. [PubMed] [Google Scholar]
- 3.Lebwohl MG, Bachelez H, Barker J, et al. Patient perspectives in the management of psoriasis: results from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis Survey. J Am Acad Dermatol. 2014;70(5):871–81.e1–30. doi:10.1016/j.jaad.2013.12.018. [DOI] [PubMed]
- 4.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 5.Krueger JG, Fretzin S, Suárez-Fariñas M, et al. IL-17A is essential for cell activation and inflammatory gene circuits in subjects with psoriasis. J Allergy Clin Immunol. 2012;130(1):145–54.e9. doi:10.1016/j.jaci.2012.04.024. [DOI] [PMC free article] [PubMed]
- 6.Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17(5):435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 7.Chan JR, Blumenschein W, Murphy E, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J Exp Med. 2006;203(12):2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lowes MA, Kikuchi T, Fuentes-Duculan J, et al. Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol. 2008;128(5):1207–1211. doi: 10.1038/sj.jid.5701213. [DOI] [PubMed] [Google Scholar]
- 9.Pappu R, Ramirez-Carrozzi V, Sambandam A. The interleukin-17 cytokine family: critical players in host defence and inflammatory diseases. Immunology. 2011;134(1):8–16. doi: 10.1111/j.1365-2567.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang C, Chen S, Qian H, Huang W. Interleukin-23: as a drug target for autoimmune inflammatory diseases. Immunology. 2012;135(2):112–124. doi: 10.1111/j.1365-2567.2011.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritchlin C, Rahman P, Kavanaugh A, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990–999. doi: 10.1136/annrheumdis-2013-204655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laan M, Cui ZH, Hoshino H, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162(4):2347–2352. [PubMed] [Google Scholar]
- 13.Kao C-YY, Chenv Y, Thai P, et al. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J Immunol. 2004;173(5):3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 14.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 15.Li J, Li D, Tan Z. The expression of interleukin-17, interferon-gamma, and macrophage inflammatory protein-3 alpha mRNA in patients with psoriasis vulgaris. J Huazhong Univ Sci Technol Med Sci. 2004;24(3):294–296. doi: 10.1007/BF02832000. [DOI] [PubMed] [Google Scholar]
- 16.Ariza M-EE, Williams MV, Wong HK. Targeting IL-17 in psoriasis: from cutaneous immunobiology to clinical application. Clin Immunol. 2013;146(2):131–139. doi: 10.1016/j.clim.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Yilmaz SB, Cicek N, Coskun M, Yegin O, Alpsoy E. Serum and tissue levels of IL-17 in different clinical subtypes of psoriasis. Arch Dermatol Res. 2012;304(6):465–469. doi: 10.1007/s00403-012-1229-1. [DOI] [PubMed] [Google Scholar]
- 18.Brown G, Malakouti M, Wang E, Koo J, Levin E. Anti-IL-17 phase II data for psoriasis: a review. J Dermatol Treat. 2014;26(1):32–36. doi: 10.3109/09546634.2013.878448. [DOI] [PubMed] [Google Scholar]
- 19.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130(5):1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papp K, Reich K, Leonardi C, et al. Efficacy and safety of brodalumab in patients with moderate to severe plaque psoriasis: results of AMAGINE-1, a phase 3, randomized, double-blind, placebo controlled study through week 12. In: Presented at the 73rd annual meeting of the American academy of dermatology; 2015 March 20–24; San Francisco, CA.
- 21.Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015 doi: 10.1056/NEJMoa1503824. [DOI] [PubMed] [Google Scholar]
- 22.Revicki DA, Willian MK, Menter A, Saurat J-HH, Harnam N, Kaul M. Relationship between clinical response to therapy and health-related quality of life outcomes in patients with moderate to severe plaque psoriasis. Dermatology (Basel). 2008;216(3):260–270. doi: 10.1159/000113150. [DOI] [PubMed] [Google Scholar]
- 23.Farahnik B, Beroukhim K, Nakamura M, et al. Anti-IL-17 agents for psoriasis: a review of phase III data. J Drugs Dermatol. 2016;15(3):311–316. [PubMed] [Google Scholar]
- 24.Puel A, Cypowyj S, Bustamante J, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332(6025):65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boisson B, Wang C, Pedergnana V, et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39(4):676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta MA, Gupta AK. Depression and suicidal ideation in dermatology patients with acne, alopecia areata, atopic dermatitis and psoriasis. Br J Dermatol. 1998;139(5):846–850. doi: 10.1046/j.1365-2133.1998.02511.x. [DOI] [PubMed] [Google Scholar]
- 27.Kurd SK, Troxel AB, Crits-Christoph P, Gelfand JM. The risk of depression, anxiety, and suicidality in patients with psoriasis: a population-based cohort study. Arch Dermatol. 2010;146(8):891–895. doi: 10.1001/archdermatol.2010.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu JJ. Letter regarding phase 3 studies comparing brodalumab with ustekinumab in psoriasis. J Psoriasis Psoriatic Arthritis. 2016;1:61. [Google Scholar]
- 29.Garber K. Anti-IL-17 mAbs herald new options in psoriasis. Nat Biotechnol. 2012 doi: 10.1038/nbt0612-475. [DOI] [PubMed] [Google Scholar]
- 30.Langenbruch A, Radtke MA, Krensel M, Jacobi A, Reich K, Augustin M. Nail involvement as a predictor of concomitant psoriatic arthritis in patients with psoriasis. Br J Dermatol. 2014 doi: 10.1111/bjd.13272. [DOI] [PubMed] [Google Scholar]
- 31.Mease PJ, McInnes IB, Kirkham B, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373(14):1329–1339. doi: 10.1056/NEJMoa1412679. [DOI] [PubMed] [Google Scholar]
- 32.Raychaudhuri SP. Role of IL-17 in psoriasis and psoriatic arthritis. Clin Rev Allergy Immunol. 2013;44(2):183–193. doi: 10.1007/s12016-012-8307-1. [DOI] [PubMed] [Google Scholar]
- 33.Raychaudhuri SP, Raychaudhuri SK, Genovese MC. IL-17 receptor and its functional significance in psoriatic arthritis. Mol Cell Biochem. 2012;359(1–2):419–429. doi: 10.1007/s11010-011-1036-6. [DOI] [PubMed] [Google Scholar]
- 34.Mease PJ, Genovese MC, Greenwald MW, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–2306. doi: 10.1056/NEJMoa1315231. [DOI] [PubMed] [Google Scholar]
- 35.McIvor A. Emerging therapeutic options for the treatment of patients with symptomatic asthma. Ann Allergy Asthma Immunol. 2015 doi: 10.1016/j.anai.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Yeremenko N, Paramarta JE, Baeten D. The interleukin-23/interleukin-17 immune axis as a promising new target in the treatment of spondyloarthritis. Curr Opin Rheumatol. 2014;26(4):361–370. doi: 10.1097/BOR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 37.Wu JJ, Poon K-YTY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148(11):1244–1250. doi: 10.1001/archdermatol.2012.2502. [DOI] [PubMed] [Google Scholar]
- 38.De Boer OJ, van der Meer JJ, Teeling P, et al. Differential expression of interleukin-17 family cytokines in intact and complicated human atherosclerotic plaques. J Pathol. 2010;220(4):499–508. doi: 10.1002/path.2667. [DOI] [PubMed] [Google Scholar]
- 39.Csiszar A, Ungvari Z. Synergistic effects of vascular IL-17 and TNFalpha may promote coronary artery disease. Med Hypotheses. 2004;63(4):696–698. doi: 10.1016/j.mehy.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Gan EY, Chong W-SS, Tey HL. Therapeutic strategies in psoriasis patients with psoriatic arthritis: focus on new agents. BioDrugs. 2013;27(4):359–373. doi: 10.1007/s40259-013-0025-6. [DOI] [PubMed] [Google Scholar]