Highlights
-
•
A rare case of undifferentiated carcinomas with osteoclast-like giant cells of the ampullary region successfully treated with pancreaticoduodenectomy.
-
•
Carcinoma in situ was also observed, spreading from the tumor at the terminal segment of the common bile duct to the surgical margin of the common hepatic duct.
-
•
It is important to intraoperatively confirm negative bile duct margins by rapid pathologic diagnosis.
Keywords: Undifferentiated carcinoma with osteoclast-like giant cells, Ampullary region, Duodenal papilla, Case report
Abstract
Introduction
Undifferentiated carcinomas with osteoclast-like giant cells (UC-OGCs) of the ampullary region are very rare, with only a few cases reported to date. The clinicopathological features, treatment options, and prognosis of UC-OGCs are unclear. This report describes a patient with UC-OGCs of the ampullary region.
Presentation of case
A 78-year-old male patient was admitted for epigastric pain and fever. Contrast-enhanced computed tomography revealed a 2.6-cm mass at the duodenal papilla. Duodenoscopy revealed a smooth red protruding mass compressing the orifice of the papilla of Vater. Biopsy of the mass showed proliferation of osteoclast-like giant cells. A subtotal stomach-preserving pancreaticoduodenectomy was performed, and the tumor was histologically diagnosed as an UC-OGCs of the ampullary region. Carcinoma in situ was also observed, spreading from the tumor at the terminal segment of the common bile duct to the common hepatic duct, with carcinoma cells at the surgical margin of the common hepatic duct. One year after surgery, the patient is alive and without tumor recurrence.
Discussion
UC-OGCs of the ampullary region is very rare neoplasm containing osteoclast-like giant cells and mononuclear cells. Osteoclast-like giant cells may originate from reactive mesenchymal cells and carcinoma in situ may spread to the common hepatic duct. Surgery including pancreaticoduodenectomy may be a treatment option for resectable tumors, whereas gemcitabine may be a treatment option for unresectable tumors.
Conclusion
Carcinoma in situ may spread quite far (5 cm) to the common hepatic duct, making it desirable to intraoperatively confirm negative bile duct margins by rapid pathologic diagnosis.
1. Introduction
Undifferentiated carcinomas with osteoclast-like giant cells (UC-OGCs), similar to those present in long bones, have been observed in many organs but are generally rare. These tumors occur more frequently in the gallbladder and pancreas than in extrahepatic bile ducts and the ampullary region [1]. Because there have been few case reports of UC-OGCs of the ampullary region, their clinical features and treatment strategies are not well understood. This report describes a patient with UC-OGCs of the ampullary region who was successfully treated with surgery.
2. Presentation of case
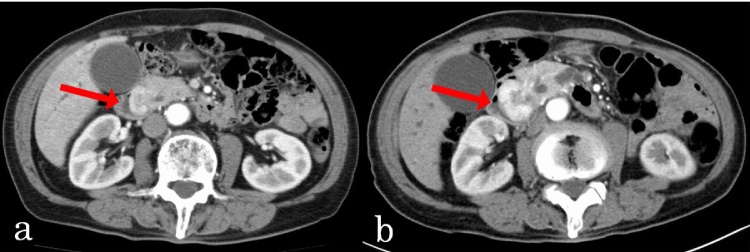
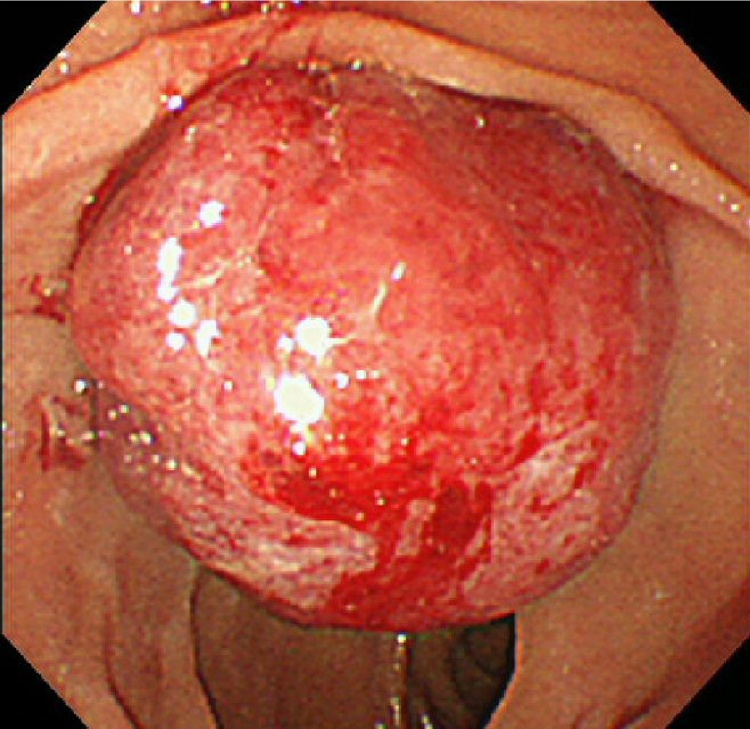
A previously healthy 78-year-old male patient was admitted to our hospital because of epigastric pain and fever. Over the previous month, he had unintentionally lost 4.0 kg in weight. Physical examination showed no evidence of abdominal tenderness or a palpable mass. Laboratory examination showed anemia (hemoglobin, 10.3 g/dl) and elevated serum concentrations of alkaline phosphatase (ALP) was 1876 IU/l, gamma glutamyl transpeptidase (γ-GTP; 373 IU/l), lipase (87 IU/l), aspartate aminotransferase (AST; 77 IU/l), and alanine aminotransferase (ALT; 117 IU/l). His total bilirubin concentration was 0.7 mg/dl, his carbohydrate antigen 19-9 (CA19-9) concentration was 5.3 U/ml (normal <37.0 U/ml) and his carcinoembryonic antigen (CEA) concentration was 1.5 ng/ml (normal < 5.0 ng/ml). Contrast-enhanced computed tomography (CECT) showed an approximately 2.6 × 1.5 cm mass at the duodenal papilla, enhanced in the arterial phase but washed-out in the venous phase (Fig. 1a). There was no evidence of lymph node metastasis or invasion of the common bile duct or pancreatic duct. Duodenoscopy revealed a smooth red protruding mass compressing the orifice of the papilla of Vater, making endoscopic retrograde cholangiopancreatography difficult (Fig. 2). Endoscopic ultrasonography showed dilation of the pancreatic duct and common bile duct. A biopsy of the mass showed proliferation of osteoclast-like giant cells. Follow-up CECT 1 month later showed enlargement of the tumor, to 3.8 × 2.7 mm (Fig. 1b). Therefore, we performed subtotal stomach-preserving pancreaticoduodenectomy (SSPPD) with regional lymphadenectomy.
Fig. 1.
(a) Abdominal CT scan showing an approximately 2.6 × 1.5 cm mass enhanced in an arterial phase. (b) Follow up CECT 1 month after admission, showing rapid enlargement of the tumor, to 3.8 × 2.7 mm.
Fig. 2.
Duodenoscopy results, showing a smooth red protruding mass compressing the orifice of the papilla of Vater.
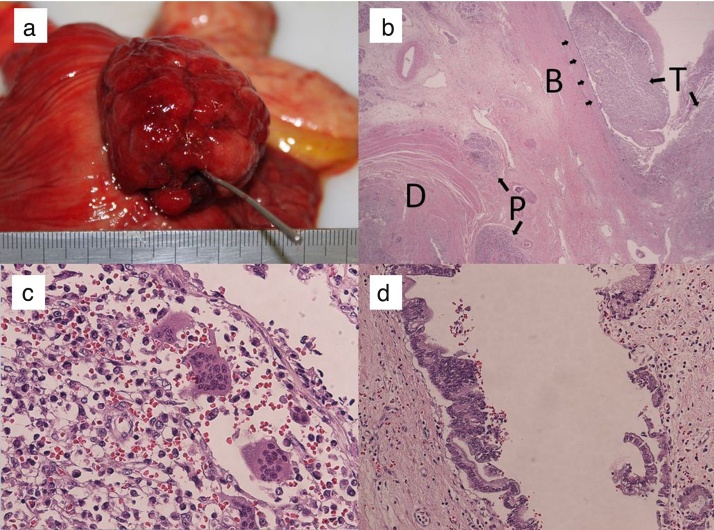
Macroscopic examination of the duodenum showed a protruding mass, measuring 50 × 40 × 35 mm, located in the periampulla of the duodenum (Fig. 3a). Microscopically, the tumor was found to originate from the terminal segment of the common bile duct in the ampullary region (Fig. 3b). The area of the tumor protruding into the duodenum consisted of sheets of anaplastic round and ovoid giant cells or fascicles of spindle cells mixed with multinucleated osteoclast-like giant cells. The former cells were negative for epithelial membrane markers like AE1/AE3, EMA and negative for CD68 while the later cells were negative for AE1/AE3, EMA and positive for CD68 (Fig. 3c). Carcinoma in situ (BilIN 3) was also observed, spreading from the terminal segment of the common bile duct to the common hepatic duct and the cystic duct. The surgical margin of the common hepatic duct was positive (Fig. 3d). There was no evidence of lymph node metastasis, lymphatic or vascular invasion, or invasion to the pancreas, pancreatic duct, or duodenum. The final diagnosis was UC-OGCs of the ampullary region, with the tumor staged as T1, N0, M0, stage IA according to the TNM staging system.
Fig. 3.
(a) Macroscopic findings of the duodenum, showing a fungiform exposed protruding mass, 50 × 40 × 35 mm, located in the periampulla of the duodenum. (b) Microscopically, the tumor was located mainly in the ampullary region, originating from the terminal segment of the common bile duct in the ampullary region. (c) Histologic examination showed that the tumor consisted of sheets of anaplastic round, ovoid or spindle giant cells admixed with multinucleated osteoclast-like giant cells. (d) Carcinoma in situ was observed, continuously spreading from the tumor at the terminal segment of the common bile duct to the common hepatic duct and cystic duct.
Twelve days after surgery, the patient was discharged from hospital without any complications. One year after surgery, the patient remains alive, with follow-up abdominal CT scan showing no evidence of tumor recurrence.
3. Discussion
Although generally rare, UC-OGCs similar to those in the long bones have been detected in many organs, including soft tissue of the extremities [2], as well as the mediastinum [3], larynx [4] and skin [5]. In visceral organs, this neoplasm is more common in the gallbladder and pancreas than in the extrahepatic bile ducts and ampullary region [1]. The ampullary region contains the sphincter of Oddi, which acts as a channel leading from the point at which the common bile duct enters the wall of the duodenum into the major duodenal papilla [6]. In our patient, the tumor was originated from the terminal segment of the common bile duct and had not spread into the pancreatic duct or duodenum, resulting in a diagnosis of a UC-OGCs of the ampullary region.
Histologically, UC-OGCs are composed of two types of cells: osteoclast-like giant cells and mononuclear cells. Proposed origins of the osteoclast-like giant cells have included neoplastic acinar cells, neoplastic ductal cells, neoplastic epithelial cells, neoplastic mesenchymal cells, and reactive mesenchymal cells [1]. In our patient, osteoclast-like giant cells were lack of atypia, and positive for CD68 while negative for cytokeratin. These results may support the hypothesis that the osteoclast-like giant cells are originated from reactive mesenchymal cells. Preoperative biopsy of the mass in our patient showed proliferation of osteoclast-like giant cells without carcinoma cells. However, the presence of osteoclast-like giant cells raises the possibility of the presence of the neoplastic mononuclear cells. Because lymph node metastasis was reported in UC-OGCs of pancreas [7], [8], we chose to perform pancreaticoduodenectomy with regional lymphadenectomy. UC-OGCs of pancreas accompanying PanIN lesions has reported [9]. In our patient, BilIN 3 was found in the terminal segment of the common bile duct, close to the anaplastic tumor; however, we could not detect areas of transition between BilIN 3 lesions and anaplastic tumor components. BilIN 3 may spread more than 40 mm from the macroscopic tumor margins of bile duct carcinoma [10]. In our patient, BilIN 3 had spread more than 50 mm. Negative bile duct margins of UC-OGCs of the ampullary region should be confirmed intraoperatively by rapid pathologic diagnosis.
Because of the rarity of UC-OGCs of the ampullary region, their clinicopathological features remain unclear. A search of the literature found only two previous reports of UC-OGCs of the ampullary region (Table 1). All patients were elderly(>70 years) and presented with unspecific symptoms like abdominal pain or without symptoms. The prognosis of patients with carcinomas containing osteoclast-like giant cells is unclear. For example, the prognosis of patients with UC-OGCs of the breast is similar to that of patients with invasive breast cancer. The prognosis of undifferentiated carcinoma of the pancreas is also poor, with a median survival period of less than 1 year [11]. Older age, males, small tumors, positive metastases and a concomitant component of ductal adenocarcinoma are suggested as poor prognostic factors of UC-OGCs of pancreas [12]. Owing to their asymptomatic nature, UC-OGCs of the pancreas are usually not detected until they are quite large and have reached advanced stages with widespread metastases. UC-OGCs of the ampullary region may also be difficult to detect early, because tumor in ampullary region often lack symptoms in early stage [13]. Almost all patients with UC-OGCs of the pancreas who have survived for a long time have undergone surgery. The reported patient of UC-OGCs in the ampullary region who survived more than 2 years was performed pancreaticoduodenectomy [14] while the other reported patient of UC-OGCs in the ampullary region who died within 6 month was performed only local resection [1]. These results may suggest that pancreaticoduodenectomy may be the treatment of choice for resectable UC-OGCs in the ampullary region. Our patient was performed pancreaticoduodenectomy, which may lead to a short-term survive even though the surgical margin of the common hepatic duct was positive [15]. Although there is no standard chemotherapy for UC-OGCs of the ampullary region and related metastases, gemcitabine induced a complete response in a patient with recurrent liver and para-aortic lymph node lesions following surgical resection of a UC-OGCs of the ampullary region [14]. In addition, gemcitabine was found effective in a patient with UC-OGC of the pancreas [16], suggesting that gemcitabine would be feasible in patients with unresectable or recurrent UC-OGCs of the ampullary region. Larger case series and longer term follow-up are necessary to clarify the clinicopathological features, surgical outcome and effects of chemotherapy in patients with UC-OGCs of the ampullary region.
Table 1.
Clinical and pathological features of patients with UC-OGC in the ampullary region.
| case | author | age/sex | Clinical Presentation | location | size (cm) | surgery | other therapy | follow up |
|---|---|---|---|---|---|---|---|---|
| 1 | Molberg [1] | 85/F | NA | ampullary | 3.5 | local resection | none | DOD 6 months |
| 2 | Matsuzawa [14] | 71/F | abdominal pain | ampullary | 6 | Pancreaticoduodenectomy | gemcitabine | Alive 24 months |
| 3 | present case | 78/M | epigastric pain, mild fever | ampullary | 5 | Pancreaticoduodenectomy | none | Alive 12 months |
NA; not available, DOD; dead of disease.
4. Conclusion
Surgery, including pancreaticoduodenectomy, appears to be a fair treatment option for patients with resectable UC-OGCs of the ampullary region. Carcinoma in situ may spread quite far (5 cm) to the common hepatic duct, making it important to intraoperatively confirm negative bile duct margins by rapid pathologic diagnosis.
Conflict of interest
The authors have no conflicts of interest.
Funding
None.
Ethical approval
Ethical approval not required.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Author contributions
Yusuke Kawamoto, Yusuke Ome, Kazuki Hashida performed the operation. Kazuhiro Terada made pathological diagnosis. Yusuke Kawamoto wrote the manuscript, while Yusuke Ome, Kazuyuki Kawamoto and Tadashi Ito critically revised it.
Guarantor
Yusuke Kawamoto.
Contributor Information
Yusuke Kawamoto, Email: yk13105@kchnet.or.jp.
Yusuke Ome, Email: yo14408@kchnet.or.jp.
Kazuhiro Terada, Email: kt13958@kchnet.or.jp.
Kazuki Hashida, Email: kh14813@kchnet.or.jp.
Kazuyuki Kawamoto, Email: kk7159@kchnet.or.jp.
Tadashi Ito, Email: ti4820@kchnet.or.jp.
References
- 1.Molberg K.H., Heffess C., Delgado R., Albores-Saavedra J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer. 1998;82:1279–1287. doi: 10.1002/(sici)1097-0142(19980401)82:7<1279::aid-cncr10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 2.Angervall L., Hagmar B., Kindblom L.G. Malignant giant cell tumor of soft tissues: a clinicopathologic, cytologic, ultrastructural, angiographic, and microangiographic study. Cancer. 1981;47:736–747. doi: 10.1002/1097-0142(19810215)47:4<736::aid-cncr2820470419>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Fu K., Moran C.A., Suster S. Primary mediastinal giant cell tumors: a clinicopathologic and immunohistochemical study of two cases. Ann. Diagn. Pathol. 2002;6:100–105. doi: 10.1053/adpa.2002.32376. [DOI] [PubMed] [Google Scholar]
- 4.Wieneke J.A., Gannon F.H., Heffner D.K. Giant cell tumor of the larynx: a clinicopathologic series of eight cases and a review of the literature. Mod. Pathol. 2001;14:1209–1215. doi: 10.1038/modpathol.3880462. [DOI] [PubMed] [Google Scholar]
- 5.Maheswaran P., Addis B.J. Osteoclastoma-like giant cell tumour of the skin. Histopathology. 1990;16:604–607. doi: 10.1111/j.1365-2559.1990.tb01168.x. [DOI] [PubMed] [Google Scholar]
- 6.Japanese Society of Biliary Surgery . second english edition. Kanehara; Tokyo: 2004. Classification of Biliary Tract Carcinoma. [Google Scholar]
- 7.Newbould M.J., Benbow E.W., Sene A. Adenocarcinoma of the pancreas with osteoclast-like giant cells: a case report with immunocytochemistry. Pancreas. 1992;7:611–615. doi: 10.1097/00006676-199209000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Jo Sungho. Huge undifferentiated carcinoma of the pnacreas with steoclast-like giant cells. World J. Gastroenterol. 2014;20:2725–2730. doi: 10.3748/wjg.v20.i10.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmann F., Esposito I., Michalski C.W. Early undifferentiated pancreatic carcinoma with osteoclastlike giant cells: direct evidence for ductal evolution. Am. J. Surg. Pathol. 2007;31:1919–1925. doi: 10.1097/PAS.0b013e318067bca8. [DOI] [PubMed] [Google Scholar]
- 10.Ebata T., Watanabe H., Ajioka Y., Oda K., Nimura Y. Pathological appraisal of lines of resection for bile duct carcinoma. Br. J. Surg. 2002;89:1260–1267. doi: 10.1046/j.1365-2168.2002.02211.x. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi S., Nakano H., Ooike N. Long-term survivor of a resected undifferentiated pancreatic carcinoma with osteoclast-like giant cells who underwent a second curative resection: a case report and review of the literature. Oncol. Lett. 2014;8:1499–1504. doi: 10.3892/ol.2014.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holland R., van Haelst U.J. Mammary carcinoma with osteoclastlike giant cells: additional observations of six cases. Cancer. 1984;53:1963–1973. doi: 10.1002/1097-0142(19840501)53:9<1963::aid-cncr2820530927>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Winter J.M., Cameron J.L., Olino K. Clinicopathologic analysis of ampullary neoplasms in 450 patients: implications for surgical strategy and long-term prognosis. J. Gastrointest. Surg. 2010;14:379–387. doi: 10.1007/s11605-009-1080-7. [DOI] [PubMed] [Google Scholar]
- 14.Matsuzawa G., Shirabe K., Gion T. Surgecally resected undifferentiated carcinoma with osteoclast-like giant cells of the periampullary region involving the orifice of the papilla of vater: report of a case. Surg. Today. 2010;40:376–379. doi: 10.1007/s00595-009-4078-6. [DOI] [PubMed] [Google Scholar]
- 15.Nakanishi Y., Kondo S., Zen Y. Impact of residual in situ carcinoma on postoperative survival in 125 patients with extrahepatic bile duct carcinoma. J. Hepatobiliary Pancreat. Sci. 2010;17:166–173. doi: 10.1007/s00534-009-0127-1. [DOI] [PubMed] [Google Scholar]
- 16.Yoshioka M., Uchinami H., Watanabe G. Effective use of gemcitabine in the treatement of undifferentiated carinoma with osteoclast-like giant cells of the pancreas with portal vein tumor thorombus. Intern. Med. 2012;51:2145–2150. doi: 10.2169/internalmedicine.51.7670. [DOI] [PubMed] [Google Scholar]