Abstract
Simvastatin reduces the blood concentration of cholesterol by inhibiting hydroxymethylglutaryl-coenzyme A reductase, the rate-limiting enzyme in cholesterol synthesis, and thereby reduces the risk of cardiovascular disease. In addition, simvastatin treatment leads to a reduction in fluxes in mitochondrial respiratory complexes I and II and might thereby reduce the formation of reactive oxygen species, which have been implicated in the pathogenesis of arteriosclerosis. Therefore, we hypothesized that simvastatin may reduce oxidative stress in humans in vivo.
We conducted a randomized, double-blinded, placebo-controlled study in which subjects were treated with either 40 mg of simvastatin or placebo for 14 days. The endpoints were six biomarkers for oxidative stress, which represent intracellular oxidative stress to nucleic acids, lipid peroxidation and plasma antioxidants, that were measured in urine and plasma samples.
A total of 40 participants were included, of which 39 completed the trial. The observed differences between simvastatin and placebo groups in the primary outcomes, DNA and RNA oxidation, were small and nonsignificant (p=0.68), specifically, 3% in the simvastatin group compared to 7.1% in the placebo group for DNA oxidation and 7.3% in the simvastatin group compared to 3.4% in the placebo group. The differences in biomarkers related to plasma were not statistically significant between the treatments groups, with the exception of total vitamin E levels, which, as expected, were reduced in parallel with the reduction in plasma cholesterol.
In healthy young male volunteers, short-term simvastatin treatment, which considerably reduces cholesterol, does not lead to a clinically relevant reduction in a panel of measures of oxidative stress. Whether simvastatin has effects on oxidative stress in diseased populations, such as diabetes or hemochromatosis, where oxidative stress is prominent, is unknown but seems unlikely.
Keywords: Simvastatin, Randomized clinical trial, Oxidative stress, Reactive oxygen species, 8-oxodg, 8-oxoguo, DNA oxidation, RNA oxidation
Graphical abstract
Highlights
-
•
Simvastatin reduces plasma cholesterol but not oxidative stress.
-
•
Oxidative stress was measured in both intracellular and extracellular compartments.
-
•
Simvastatin does not influence nuclelic acid oxidation.
1. Introduction
The statin group of drugs is widely used to treat hypercholesterolemia and to reduce the risk of cardiovascular events in risk groups [1]. One common side effect of statins is muscle pain, and the mechanism by which this pain occurs is not fully understood but could include oxidative stress. Statins have different effects in skeletal and cardiac muscle, which may be why muscle problems are only observed in skeletal muscle [2] and not in other types of muscles. The primary site of ROS production is in mitochondria [3], [4]. However, there are many sources of ROS, both intracellular [5] and environmental [6]. Recently, a reduction in mitochondrial respiration in skeletal muscle was reported in patients treated with simvastatin. This reduction was mainly related to reduced respiration in mitochondrial respiratory complexes I and II mediated via a reduction in the content of the electron carrier Q10 [7]. Furthermore, atorvastatin and simvastatin reduced the oxidative stress induced by calcium [8].
Oxidative stress to RNA can be measured non-invasively based on the excretion of the ribonucleoside 8-oxoGuo (8-oxo-7,8-dihydroguanosine) in urine, and its clinical relevance has been demonstrated in type 2 diabetes, where high oxidative stress is predictive of death [9]. We have hypothesized that RNA oxidation is related to the production of hydrogen peroxide by mitochondria, which could contribute to mitochondrial respiration/dysfunction and excess ROS production in diabetes [10], [11]. A reduction in oxidative stress by statins would therefore represent a novel and interesting therapeutic mechanism.
We conducted a randomized, double blinded, controlled trial of simvastatin versus placebo in healthy male volunteers with DNA and RNA oxidation as the primary endpoints. The secondary endpoints were the plasma concentrations of malondialdehyde, vitamin C, vitamin E, and biopterin, which are markers of extracellular stress.
2. Materials and methods
2.1. Study design
The study was designed as a randomized, double-blinded, placebo-controlled intervention study. A total of 40 healthy male volunteers were recruited, and after informed consent was obtained from the participants, they were randomized to receive a 40 mg simvastatin tablet (N=20) or a placebo tablet (N=20) daily for 14 days. The inclusion criteria were as follows: healthy, Caucasian male between 18 and 50 years of age with a BMI between 18 kg/m2 and 30 kg/m2. The recruitment was conducted using online advertisement and posters at universities and dormitories. Potential participants received written information if they met the inclusion criteria. A flow chart of recruitment and randomization is given in Fig. 1.
Fig. 1.
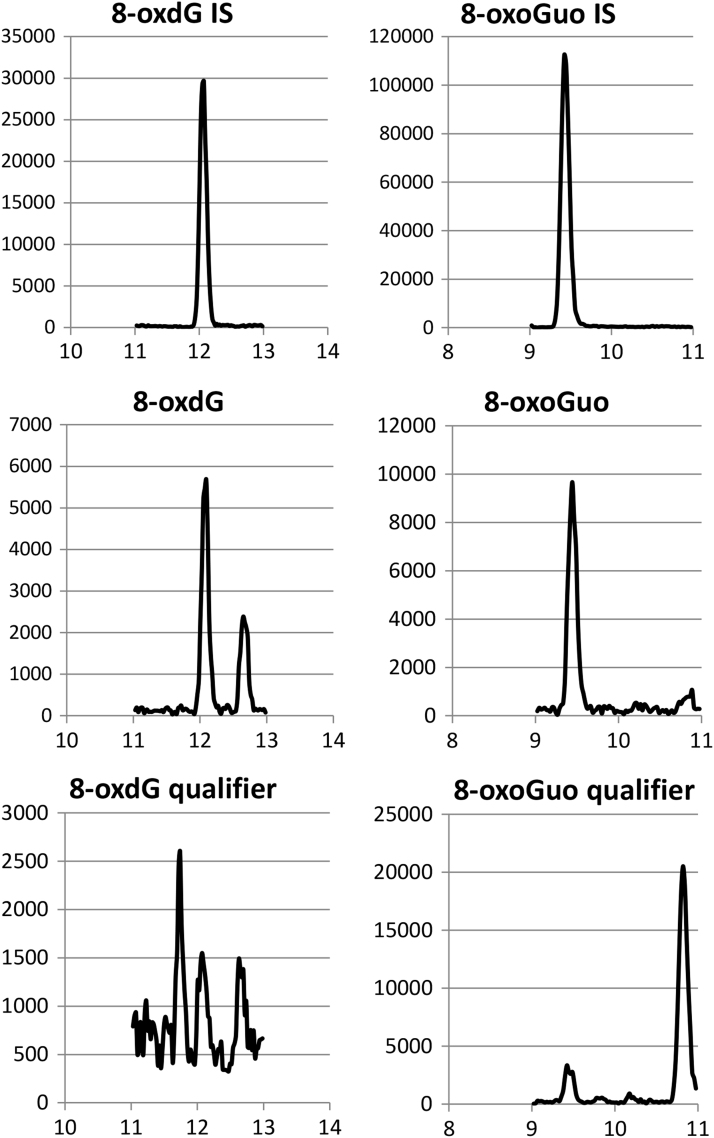
Chromatogram tracings from analysis of a urine sample showing the applied ion-traces for each analyte, the 15N5-labelled internal standard (IS), the quantifier ion, and the qualifier ion. The sample concentrations of 8-oxoGuo and 8-oxodG was 14.0 nM and 14.2 nM, respectively. The transitions are: m/z 303.186>212.99 (8-oxoGuo IS). m/z 298.186>207.99 (8-oxoGuo). m/z 298.186>165 (8-oxoGuo qualifier). m/z 287.186>196.939 (8-oxodG IS). m/z 282.186>191.939 (8-oxodG). m/z 282.186>150 (8-oxodG qualifier).
The trial was performed at the Laboratory of Clinical Pharmacology Q7642, Rigshospitalet from October 2014 to February 2015.
The production of trial medicines and treatment randomization were performed by Glostrup Apotek (Glostrup, Denmark) according to present GMP and ICH guidelines.
The study was approved by the Danish Health and Medicines Agency (2,014,031,149), the Regional Ethics Committee (H-4–2014-19) and the Danish Data Protection: (BBH-2014-026/I-suite 2882); the study is registered at the European Clinical Trials Database (2014-000959-92) and at clinicaltrials.gov (NCT02256254).
The study was conducted according to Danish law and current ICH-GCP guidelines. It was monitored by the GCP unit at the Copenhagen University Hospital (2014–647).
2.2. Study outcome
The primary endpoint was the individuals’ change in DNA and RNA oxidation, as determined by the 24 h urine excretion of 8-oxodG and 8-oxoGuo before and after simvastatin treatment compared to the changes observed in individuals in the placebo treatment group.
The secondary endpoints were changes in the blood plasma concentrations of the biomarkers, malondialdehyde [12] (MDA), vitamin C [13], vitamin E [14] and biopterin [15] between simvastatin treatment and placebo treatment groups.
Compliance levels of at least 65% trial medicine administration and 75% urine collection were accepted; levels were corrected from recorded void losses.
2.3. Statistical considerations
An a priory power calculation assuming a type 1 error risk of 5%, a type 2 error risk of 20% and a relevant difference of 20% was performed and resulted in 20 participants in each group.
The statistical analysis was carried out with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA), and p-values<0.05 were considered statistically significant.
Variables were tested for normal distribution (Kolmogorov-Smirnov). The mean changes in each endpoint between the simvastatin and the placebo groups were analyzed using a two-sided, unpaired t-test.
2.4. Analytical procedures
2.4.1. Chemicals
For this study, 8-oxo-7,8-dihydroguanosine (8-oxoGuo) was purchased from BioLog (Bremen, Germany) and 8-oxo-7,8-dihydrodeoxyguanosine (8-oxodG) from Berry & Associates (Dexter, MI). The internal standards, 15N5-8-oxodG and 15N5-8-oxoGuo, were produced in the lab by electrochemical oxidation [16] of 15N5-dG and 15N5-Guanosine, which were purchased from Cambridge Isotope Laboratories (Tewksbury, MA).
Acetonitrile (isocratic grade) was purchased from Merck KgaA (Darmstadt, Germany). Methanol (HPLC-grade), lithium acetate dihydrate, acetic acid, and aqueous ammonia (25%) were all from Sigma-Aldrich (Steinheim, Germany). LC-MS Ultra Chromasolv water from Sigma-Aldrich was used for preparation of the Lithium acetate buffer and mobile phase. QC samples were prepared from a pool of urine samples and stored at –20 °C.
2.4.2. Sample preparation
Urine samples were stored at −20 °C prior to analysis. The frozen urine was thawed, mixed, and heated to 37 °C for 5 min to re-dissolve possible precipitates and trapped analytes [17]. The heated samples were then centrifuged at 10,000×g for 5 min. Calibration standards in the range of 1.0–60.0 nM and an internal standard solution were prepared in 0,1 M Lithium acetate, pH 6.4. The final sample preparation in 96-well plates was carried out in a fully automated process, using a Biomek 3000 (Beckman Coulter, CA, USA) that mixed 1) 90 µL of 100 mM lithium acetate buffer, 2) 110 µL of urine/calibration standard/QC sample, and 3) 90 µL of internal standard solution.
2.4.3. Chromatography
The chromatographic separation was performed on an Acquity UPLC I-class system (Waters, Milford, MA, USA). The column was an Acquity UPLC BEH Shield RP18 column (1.7 µm, 2.1×100 mm) protected with a VanGuard precolumn (BEH Shield RP18, 1.7 µm, 2.1×5 mm), and both were from Waters. The column was operated at 4 °C to focus the analytes at the front end during the injection of 50 µL of sample volume. The mobile phase and gradient are shown in Table 1.
Table 1.
UPLC gradient program. Eluent A: 5.0 mM ammonium acetate, pH 5. Eluent B: acetonitrile.
| Time (min.) | Flow (µL/min) | %A | %B |
|---|---|---|---|
| 0.0 | 200 | 100 | 0 |
| 0.5 | 200 | 100 | 0 |
| 12.0 | 200 | 95 | 5 |
| 14.6 | 200 | 10 | 90 |
| 15.0 | 300 | 10 | 90 |
| 16.0 | 300 | 10 | 90 |
| 17.0 | 200 | 100 | 0 |
| 20.0 | 200 | 100 | 0 |
2.4.4. Mass spectrometry
The MS detection was performed by a Xevo TQ-S triple quadrupole mass spectrometer (Waters) that was working in the negative ionization electrospray mode. The MS-settings were optimized using the Intellistart function (MassLynx 4.1). A desolvation gas flow of 1000 L/h was applied and heated to 500 °C to ensure a stable spray of the UPLC mobile phase containing less than 5% organic solvent. To reduce contamination of the ion source, a switching valve diverted the mobile phase flow to waste with the exception of the fraction at the time for elution of the target peaks (9–14 min).
Detections were performed in the multi-reaction monitoring mode (MRM). The MS/MS transitions and collision energies are reported in Table 2. Quantification was based on the signal peak area from the transitions, 298/208 (8-oxoGuo) and 282/192 (8-oxodG) relative to the signal peak area of the respective internal standards. The transitions, 298/165 (8-oxoGuo) and 282/149 (8-oxodG), were applied as qualifier ions to confirm the presence of the analyte and the absence of false contributions from the co-elution of similar components in the urine sample. The chromatogram traces are shown in Fig. 2.
Table 2.
Analytical parameters and mass transitions of the MS/MS detection.
| Analyte | Rt. (min.) | Ionization | MRM mass transitions (Collision energy) | Fragment ratio Qualifier/quantifier | ||
|---|---|---|---|---|---|---|
| Quantifier | Qualifier | Internal standard | ||||
| 8-oxoGuo | 9.27 | ESI(-) | 298→208 (20 V) | 298→165 (24 V) | 303→213 (20 V) | 0.28 |
| 8-oxodG | 11.87 | ESI(-) | 282→192 (14 V) | 282→149 (32 V) | 287→197 (14 V) | 0.22 |
Fig. 2.
Participant flow for SIMOX. In total, 45 participants were assessed for eligibility, and 40 were included. Following treatment, one participant was excluded, and finally, 39 participants were analyzed.
The signal ratio of the two fragment-ions (qualifier/quantifier) was calculated for each sample. According to the tolerance guidelines [18], results were rejected if the fragment ratio in the urine samples deviated by more than ±25% from the mean ratio of the actual standards.
2.4.5. Validation procedures
The analytical method was validated in accordance with the FDA guidelines [19], including selectivity, accuracy, precision, linearity, ion suppression and LLOQ. Validation with respect to the stability of samples and standards was performed in a previous study [20].
Validation of method selectivity and accuracy was challenged by the fact that no human urine without detectable endogenous levels of the studied adducts could be found, and no accredited reference material was available. The determination of selectivity was therefore only possible with respect to the internal standards but not for the analytes, and the analytical accuracy could be determined only from their recovery in urine samples to which different levels of 8oxoGuo and 8oxodG standard solution had been added.
2.4.6. Limit of quantification
The lower limit of quantification (LLOQ) was 1.0 nM for both 8-oxoGuo and 8-oxodG based on the quality requirements of CV <20%.
2.4.7. Linearity, precision, and accuracy
For both analytes, linear relationships were obtained in the concentration range of 1.0–200 nM using a weighting factor of 1/x2. However, as the urine concentration of 8-oxoGuo and 8-oxodG rarely exceeds 60 nM, the applied calibration range was limited to 1–60 nM.
The within-day and between-day variations were estimated from three series of five human urine samples, all prepared in triplicate and ranging in concentration from 3.5 to 48.5 nM. Variation at repeated analyses of the same sample was, on average, 1.4% for 8oxoGuo and 2.7 for 8oxodG (n=4).
The average within-day precision was 2.9% for 8-oxoGuo and 3.7% for 8-oxodG, and the between-day precision was 1.5% and 3.4%, respectively. As a measure of accuracy, the average recovery in the human urine samples to which 8-oxoGuo and 8-oxodG had been added was 98.7% for 8oxoGuo and 95.7% for 8oxodG.
2.4.8. Ion suppression
Ion suppression due to the matrix effects was estimated from the ratio of the average peak area of the internal standard in the urine samples to the aqueous calibration standards because identical amounts of ISTD has been added to the urine samples and standards. Considerable ion suppression at 42–45% was observed and was calculated as [1–(ISTD peak areaurine)/(ISTDpeak areabuffer)]×100.
2.4.9. Selectivity
As no blank urine samples were available with respect to the natural occurring analytes, selectivity or specificity was evaluated based on the second fragment ion measured. The selectivity requirements were defined as follows: 1) Peaks from the quantifier ion, as well as the qualifier ion, should both be present in the chromatogram, and the retention time of the two peaks should not differ by more than 0.05 min 2) The peak area ratio of the two peaks should not deviate from the average ratio in the standards by more than ±25%. These requirements were fulfilled in all of the urine samples involved in the validation.
No interfering peaks at the relevant retention times were observed in the MS/MS transition trace of the two internal standards in the five urine samples when measured without the addition of the internal standard.
2.4.10. Drift
Repeated analyses of five urine samples proved that batches of at least 220 samples could be analyzed without drift during the analysis time (ca. 75 h).
3. Results
3.1. Study population and flow
The baseline characteristics of the included participants are presented in Table 3. The physical and biochemical data were collected at the screening visit as part of their eligibility criteria. There were no significant differences in any of the characteristics between the two groups.
Table 3.
Baseline characteristics of the included participants. The physical and biochemical data were collected at the screening visit. Data for compliance were collected at their first and final visits. The data are depicted as the means (standard deviation) and p values.
| Placebo | Simvastatin | ||
|---|---|---|---|
| N | 19 | 20 | |
| Physical data | Mean (SD) | Mean (SD) | p |
| Age (yrs.) | 25.5 (5.49) | 25.2 (6.30) | 0.86 |
| Weight (kg) | 80.4 (10.85) | 79.8 (11.55) | 0.99 |
| Height (m) | 1.84 (0.10) | 1.83 (0.06) | 0.86 |
| Body Mass Index (kg/m2) | 23.8 (2.47) | 23.7 (2.95) | 0.95 |
| Biochemical data | Mean (SD) | Mean (SD) | |
| Ferritin (μg/L) | 154.37 (80.44) | 135.95 (93.43) | 0.51 |
| Hemoglobin (mmol/L) | 9.42 (0.44) | 9.17 (0.63) | 0.16 |
| Iron (μmol/L) | 21.95 (5.20) | 23.55 (7.07) | 0.43 |
| Creatinine (μmol/L) | 91.74 (10.19) | 89.75 (11.31) | 0.57 |
| Total cholesterol (mmol/L) | 4.11 (0.75) | 4.10 (0.77) | 0.97 |
| HDL cholesterol (mmol/L) | 1.40 (0.17) | 1.46 (0.29) | 0.47 |
| LDL cholesterol (mmol/L) | 2.41 (0.69) | 2.32 (0.76) | 0.70 |
| Triglycerides (mmol/L) | 0.83 (0.52) | 0.85 (0.50) | 0.88 |
| Compliance | Mean (SD) | Mean (SD) | |
| Trial medicine administration (%) | 100 (0) | 98.9 (3.49) | 0.19 |
| Urine collection 1, before (%) | 99.7 (1.26) | 99.5 (1.51) | 0.66 |
| Urine collection 2, after (%) | 99.1 (3.16) | 99.2 (3.62) | 0.94 |
3.2. Compliance
Data for compliance was collected at the participants’ first and final visit. The overall urine collection compliance was 92%, and the overall trial medicine.
administration compliance was 99%.
3.3. Adverse events
The 39 participants who completed the study reported a total of 57 events. In total, 15 events were assessed as adverse reactions, but none were serious. No adverse events caused withdrawal or code breach. The most common adverse events or reactions were changes in a safety measurement, symptoms of the flu, gastrointestinal problems and headaches.
3.4. Primary endpoints
The data for the primary endpoints of the participants who completed the study are presented in Table 4 and Fig. 3.
Table 4.
Urinary excretion/24 h of the primary endpoints, 8-oxodG and 8-oxoGuo, for the placebo and simvastatin groups. The p values were calculated using an unpaired t-test.
| Before | After | Delta | p | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| 8-oxodG(nM/24 h) | Placebo (N=19) | 24.38 (9.65) | 26.11 (10.10) | 1.73 (5.90) | |
| Simvastatin (N=20) | 20.62 (7.08) | 21.24 (8.17) | 0.62 (3.90) | 0.49 | |
| 8-oxoGuo (nM/24 h) | Placebo (N=19) | 31.10 (9.05) | 32.16 (9.67) | 1.06 (7.51) | |
| Simvastatin (N=20) | 26.19 (5.68) | 28.11 (6.42) | 1.92 (5.57) | 0.68 | |
Fig. 3.
Delta values of urinary excretion/24 h of the primary endpoints. 8-oxodG (circle) and 8-oxoGuo (square) for the placebo (open) and simvastatin (full) groups are shown. The data are denoted as the mean±SD for the placebo (N=19) and simvastatin (N=20) groups.
There was no statistically significant difference in the mean change in RNA oxidation (8-oxoGuo/24 h) after simvastatin treatment compared to the difference before and after placebo treatment (p=0.68). The mean difference in RNA oxidation in the simvastatin group was +7.3% (95% CL: −9.3–16.6%), and +3.4% (95% CL: −10.8–14.2%) in the placebo group.
There was no statistically significant difference in the mean DNA oxidation (8-oxodG/24 h) following simvastatin treatment compared to placebo treatment (p=0.49), and the difference in the simvastatin group was 3% (95% CL: −8.3–11.3%) and 7.1% (95% CL: −10.9–18.0%) in the placebo group.
3.5. Secondary endpoints
The data for the secondary endpoints of the participants who completed the study are presented in Table 5 and Fig. 4.
Table 5.
Plasma concentrations of the secondary endpoints, Malondialdehyde (MDA), Biopterin, Vitamin C and Vitamin E, for the placebo and simvastatin groups. The p values were calculated using an unpaired t-test.
| Before | After | Delta | p | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| MDA (nmol/mL) | Placebo (N=19) | 0.14 (0.045) | 0.15 (0.053) | 0.0046 (0.046) | |
| Simvastatin (N=20) | 0.16 (0.042) | 0.16 (0.041) | 0.0018 (0.027) | 0.60 | |
| Biopterin (nmol/mL) | Placebo (N=19) | 0.34 (0.13) | 0.33 (0.091) | 0.0083 (0.13) | |
| Simvastatin (N=20) | 0.36 (0.10) | 0.35 (0.10) | 0.0095 (0.10) | 0.98 | |
| Vitamin C (μmol/L) | Placebo (N=19) | 55.01 (20.34) | 50.63 (21.36) | 4.39 (16.2) | |
| Simvastatin (N=20) | 53.26 (18.77) | 50.11 (19.27) | 3.15 (10.3) | 0.78 | |
| Vitamin E (μmol/L) | Placebo (N=19) | 22.29 (6.46) | 21.91 (5.99) | 0.39 (2.75) | |
| Simvastatin (N=20) | 22.12 (3.97) | 18.89 (4.75) | 3.23 (2.88) | 0.0003 | |
Fig. 4.
Delta values of plasma concentrations of the secondary endpoints. A) Malondialdehyde (MDA) (rhomb), B) Biopterin, ratio of BH2/BH4 (upward triangle), C) Vitamin C (hexagon) and D) Vitamin E (downward triangle) for the placebo (open) and simvastatin (full) groups are shown. The data are denoted as the mean±SD for the placebo (N=19) and simvastatin (N=20) groups.
The differences between the two treatment groups for MDA (Fig. 4A), biopterin (Fig. 4B), and Vitamin C (Fig. 4C) were small and nonsignificant, with p=0.60, p=0.98 and p=0.78, respectively.
There was a statistically significant difference between the treatment groups for total vitamin E (p=0.0003) (Fig. 4D), which was expected due to the corresponding reduction in LDL observed in the simvastatin treated group. There were 3.4% and 26.7% reductions in total cholesterol after placebo and simvastatin treatment, respectively, and the LDL cholesterol level was correspondingly reduced by 4.1% and 46.6%. The total reduction of vitamin E was 1.7% after placebo treatment compared to 14% after simvastatin treatment.
4. Discussion
We found that in healthy male volunteers, simvastatin did not influence markers of oxidative stress that relate to intracellular oxidative stress (DNA and RNA oxidation), lipid peroxidation or plasma concentrations of antioxidant vitamins. These data show that simvastatin does not reduce oxidative stress in vivo.
The efficacy of simvastatin in the treatment of hypercholesterolemia and its use in patients, such as those with type II diabetes, is well documented. Additionally, ex vivo, the inhibition of mitochondrial respiration was demonstrated [7] after treatment for one year. However, in vivo treatment with simvastatin does not lead to a reduction of oxidative stress.
Our study was designed as a short-term study lasting 2 weeks. In cells, simvastatin exposure leads to immediate dose-related effects [27], and in our 2-week study, we observed clear effects with a 25% and 50% reduction in plasma and LDL cholesterol concentrations, respectively. In the study by Larsen et al. [7], participants took simvastatin for one year. However, that study showed that simvastatin had a rather small effect on mitochondrial respiration, reducing it from 64 to 54 pmol/mg/s, which may not be clinically relevant and impossible to find in vivo, where a larger number of factors influence mitochondrial respiration. Larsen et al. also found a reduced concentration of Q10, just as we found a reduction in vitamin E, which corresponded to the reduction in cholesterol. In long-term treatment with simvastatin, the changes induced in lipid metabolism and lipid soluble antioxidants may take time to manifest. Additionally, these changes might not be specific for simvastatin and may be indirect effects rather than a direct effect of simvastatin on mitochondria.
The previously observed difference between smokers and non-smokers in DNA oxidation was approximately 50% [20]. In patients with hemochromatosis [21], RNA oxidation is increased 2–3-fold. Furthermore, in patients with type 2 diabetes, the highest quartile versus the lowest quartile differ approximately 2-fold in RNA oxidation levels [9], and high values of RNA oxidation are associated with a considerable risk of premature death in the high quartile [9]. In the present study, the small, nonsignificant 7% difference with an upper 19% confidence limit ensures that the possibility of overlooking a true difference due to type 2 error is low and without clinical relevance.
For patients with coronary heart disease, statin therapy resulted in a decrease in MDA [22] as measured by the thiobarbituric acid reaction (TBA). However, this method is much less specific for oxidative stress in vivo than the MDA measurement we used. In patients with hypercholesterolemia [23], changes in total antioxidant capacity were found. However, we regard this assay as being easy to perform but unspecific and difficult to interpret as oxidative stress. In vivo studies on hypertensive hypercholesterolemia mice and humans [24] have also used the unspecific TBA reaction for testing the effects of fluvastatin treatment. Similarly, the TBA reaction has also been used to study effects of fluvastatin, alone and in combination with the antihypertensive agent, Losartan, in humans and rats [25]. In summary, as these studies have used unspecific methodology [26], their results are very difficult to interpret. Our study is the first to study the anti-oxidative effects of simvastatin in healthy young males using specific and validated methodologies, and based on our findings, we can reject the hypothesis that simvastatin has antioxidant effects. It is possible that simvastatin or other statins could have different effects in various patient populations, such as those mentioned above. Although it is unknown, there are no indications that the effect on the markers we have measured for oxidative stress should differ between such diseased populations and the normal controls investigated in the present study.
Estimating DNA and RNA oxidation from urine as a biomarker has limitations, particularly as it reflects an average of the intracellular oxidative stress in all cells in the body. Consequently, if simvastatin has differential effects in other organs, for example, such as opposite effects in heart and skeletal muscle (5), potentially positive and negative effects could cancel each other out, thereby obscuring the detection of positive effects in major organs. Likewise, if the positive effect is confined to a minor organ, such as the endothelium cells, such an effect, positive or negative, could be undetectable due to the large background noise from the remainder of the body.
In conclusion, this study demonstrated that in healthy young male volunteers, simvastatin treatment per se does not reduce specific biomarkers for intracellular and extracellular oxidative stress.
Acknowledgement
Supported by a Grant from Region Hovedstaden, Denmark and the Toyota foundation, Denmark.
References
- 1.Grundy S.M. HMG-CoA reductase inhibitors for treatment of hypercholesterolemia. N. Engl. J. Med. 1988;319:24–33. doi: 10.1056/NEJM198807073190105. [DOI] [PubMed] [Google Scholar]
- 2.Bouitbir J., Charles A.L., Echaniz-Laguna A., Kindo M., Daussin F., Auwerx J. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: a'mitohormesis’ mechanism involving reactive oxygen species and PGC-1. Eur. Heart J. 2012;33:1397–1407. doi: 10.1093/eurheartj/ehr224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller F.L., Liu Y., Van R.H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 4.Van H.B., Woshner V., Santos J.H. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair. 2006;5:145–152. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Holmstrom K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 6.Vallyathan V., Shi X. The role of oxygen free radicals in occupational and environmental lung diseases. Env. Health Perspect. 1997;105(1):165–177. doi: 10.1289/ehp.97105s1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen S., Stride N., Hey-Mogensen M., Hansen C.N., Bang L.E., Bundgaard H. Simvastatin effects on skeletal muscle: relation to decreased mitochondrial function and glucose intolerance. J. Am. Coll. Cardiol. 2013;61:44–53. doi: 10.1016/j.jacc.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 8.Parihar A., Parihar M.S., Zenebe W.J., Ghafourifar P. Statins lower calcium-induced oxidative stress in isolated mitochondria. Hum. Exp. Toxicol. 2012;31:355–363. doi: 10.1177/0960327111429141. [DOI] [PubMed] [Google Scholar]
- 9.Broedbaek K., Siersma V., Henriksen T., Weimann A., Petersen M., Andersen J.T. Association between urinary markers of nucleic acid oxidation and mortality in type 2 diabetes: a population-based cohort study. Diabetes Care. 2013;36:669–676. doi: 10.2337/dc12-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poulsen H.E., Specht E., Broedbaek K., Henriksen T., Ellervik C., Mandrup-Poulsen T. RNA modifications by oxidation: a novel disease mechanism? Free Radic. Biol. Med. 2012;52:1353–1361. doi: 10.1016/j.freeradbiomed.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Poulsen H.E., Nadal L.L., Broedbaek K., Nielsen P.E., Weimann A. Detection and interpretation of 8-oxodG and 8-oxoGua in urine, plasma and cerebrospinal fluid. Biochim. Biophys. Acta. 2014;1840:801–808. doi: 10.1016/j.bbagen.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Lykkesfeldt J. Determination of malondialdehyde as dithiobarbituric acid adduct in biological samples by HPLC with fluorescence detection: comparison with ultraviolet-visible spectrophotometry. Clin. Chem. 2001;47:1725–1727. [PubMed] [Google Scholar]
- 13.Lykkesfeldt J. Ascorbate and dehydroascorbic acid as biomarkers of oxidative stress: validity of clinical data depends on vacutainer system used. Nutr. Res. 2012;32:66–69. doi: 10.1016/j.nutres.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Sattler W., Mohr D., Stocker R. Rapid isolation of lipoproteins and assessment of their peroxidation by high-performance liquid chromatography postcolumn chemiluminescence. Methods Enzymol. 1994;233:469–489. doi: 10.1016/s0076-6879(94)33053-0. [DOI] [PubMed] [Google Scholar]
- 15.Mortensen A., Hasselholt S., Tveden-Nyborg P., Lykkesfeldt J. Guinea pig ascorbate status predicts tetrahydrobiopterin plasma concentration and oxidation ratio in vivo. Nutr. Res. 2013;33:859–867. doi: 10.1016/j.nutres.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Weimann A., Belling D., Poulsen H.E. Quantification of 8-oxo-guanine and guanine as the nucleobase, nucleoside and deoxynucleoside forms in human urine by high-performance liquid chromatography-electrospray tandem mass spectrometry. Nucleic Acids Res. 2002;30:E7. doi: 10.1093/nar/30.2.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogdanov M.B., Beal M.F., McCabe D.R., Griffin R.M., Matson W.R. A carbon column-based liquid chromatography electrochemical approach to routine 8-hydroxy-2′-deoxyguanosine measurements in urine and other biologic matrices: a one-year evaluation of methods. Free Radic. Biol. Med. 1999;27:647–666. doi: 10.1016/s0891-5849(99)00113-6. [DOI] [PubMed] [Google Scholar]
- 18.2002/657/EC: Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (Text with EEA relevance) (notified under document number C(2002) 3044). Official Journal L 221, 8–20; 2002.
- 19.US Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. 〈www.fda.gocv/downloads/Drugs/Guidances/ucm070107.pdf〉. 2001
- 20.Loft S., Vistisen K., Ewertz M., Tjonneland A., Overvad K., Poulsen H.E. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–2247. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- 21.Broedbaek K., Poulsen H.E., Weimann A., Kom G.D., Schwedhelm E., Nielsen P. Urinary excretion of biomarkers of oxidatively damaged DNA and RNA in hereditary hemochromatosis. Free Radic. Biol. Med. 2009;47:1230–1233. doi: 10.1016/j.freeradbiomed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Li J., Sun Y.M., Wang L.F., Li Z.Q., Pan W., Cao H.Y. Comparison of effects of simvastatin versus atorvastatin on oxidative stress in patients with coronary heart disease. Clin. Cardiol. 2010;33:222–227. doi: 10.1002/clc.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz M.I., Baykal Y., Kilic M., Sonmez A., Bulucu F., Aydin A. Effects of statins on oxidative stress. Biol. Trace Elem. Res. 2004;98:119–127. doi: 10.1385/BTER:98:2:119. [DOI] [PubMed] [Google Scholar]
- 24.Dursun S., Cuhadar S., Koseoglu M., Atay A., Aktas S.G. The anti-inflammatory and antioxidant effects of pravastatin and nebivolol in rat aorta. Anadolu Kardiyol. Derg. 2014;14:229–233. doi: 10.5152/akd.2013.4708. [DOI] [PubMed] [Google Scholar]
- 25.Abdel-Zaher A.O., Elkoussi A.E., Abudahab L.H., Elbakry M.H., Elsayed E.A. Effect of simvastatin on the antihypertensive activity of losartan in hypertensive hypercholesterolemic animals and patients: role of nitric oxide, oxidative stress, and high-sensitivity C-reactive protein. Fundam. Clin. Pharmacol. 2014;28:237–248. doi: 10.1111/fcp.12020. [DOI] [PubMed] [Google Scholar]
- 26.Moselhy H.F., Reid R.G., Yousef S., Boyle S.P. A specific, accurate, and sensitive measure of total plasma malondialdehyde by HPLC. J. Lipid Res. 2013;54:852–858. doi: 10.1194/jlr.D032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong J.H., Yum K.S., Chang J.Y., Kim M., Ahn J.Y., Kim S. Dose-specific effect of simvastatin on hypoxia-induced HIF-1alpha and BACE expression in Alzheimer’s disease cybrid cells. BMC Neurol. 2015;15:127. doi: 10.1186/s12883-015-0390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]