Abstract
Echinococcus multilocularis transmission predominantly occurs in Europe between the red fox (Vulpes vulpes) and various species of rodent intermediate hosts. We infected 3 species of rodent, Myodes glareolus (n = 47), Mesocricetus auratus (n = 11) and outbred Mus musculus (CD-1 IGS) (n = 9) with an E. multilocularis egg suspension that contained 100 eggs with viable oncospheres and performed post mortem examination 6, 8 (M. glareolus) and 10 weeks post inoculation (wpi). C57BL/6j mice (n = 4) were used as positive controls as they have been shown to exhibit macroscopic liver lesions 4 wpi. To the best of our knowledge, this is the first study to experimentally assess susceptibility in the ostensibly competent host M. glareolus. Lesions were only detected in 2 of 47 M. glareolus (4.3%) at 8 and 10 wpi and although both contained protoscolices (1675 at 8 wpi and 88 at 12 wpi) the low percentage of infected animals brings into question their role as transmitters of the parasite. Significant differences were observed between inbred and outbred mice with E. multilocularis infection in the former demonstrating increased establishment (p ≤ 0.0001) and growth (p ≤ 0.0001). No lesions were found in all 11 M. auratus.
Keywords: Echinococcus multilocularis, Intermediate hosts, Metacestode
Graphical abstract
Highlights
-
•
Various rodent species perorally infected with Echinococcus multilocularis oncospheres.
-
•
Fundamental differences revealed between establishment, growth and fertility.
-
•
Myodes glareolus may not be significant to E. multilocularis transmission.
-
•
These data will advance knowledge of E. multilocularis epizootiology.
1. Introduction
Studies of the fox tapeworm, Echinococcus multilocularis have focused on naturally infected human and animal populations for disease mapping and risk assessment whilst experimental work is often conducted in mouse models intended for medical benefit (Dematteis et al., 2003; Eckert and Deplazes, 2004, Vuitton and Gottstein, 2010). Although experimental studies have identified profound differences in the susceptibility of the definitive carnivore hosts (Kapel et al., 2006) very little information exists on experimental infections of these tapeworms in their naturally occurring intermediate hosts, although such studies would clarify which host species play a key role in the transmission, why they are physiologically suited for parasite establishment and growth, and which minimum infectious doses would be required in natural settings across various relevant species. In addition to its ecological value, such data would constitute novel information for risk assessment and prevention.
In Europe, Arvicolidae species of rodents serve as intermediate hosts although the parasite is capable of more or less normal development in small mammal species from several families. Thus, the range of intermediate hosts that may be susceptible seems to be wider as compared to that of the definitive hosts. Even rodents not sympatric with Echinococcus multilocularis may establish metacestodes with protoscolices when experimentally inoculated (Thompson and Lymbery, 1995). That said, the growth and persistency of metacestodes varies between species and genus (Ohbayashi et al., 1971) and thus the geographical distribution of intermediate hosts species ought to affect transmission dynamics.
Experimental E. multilocularis infection in rodents can be achieved via various routes. Oral inoculation of E. multilocularis eggs is referred to as primary infection, whereas secondary inoculation involves the injection of metacestode homogenates or oncospheres intraperitoneally (IP), intrahepatically (IH), subcutaneously (SC) or intravenously (IV). Although secondary inoculation bypasses the early gastrointestinal exposure responsible for oncosphere activation and development, and thus provides a more narrow view of E. multilocularis infection dynamics, the majority of experimental studies apply this mode of administration (Matsumoto and Yagi, 2008). SC however does constitute a very sensitive method for testing oncosphere viability (Federer et al., 2015). Primary inoculation is thus more similar to the natural route of exposure, as the inoculated oncospheres have to pass through the gastrointestinal passage prior to liver establishment. However, due to the extensive safety measures required and difficulties in obtaining eggs few laboratories utilise this method (Romig and Bilger, 1999).
Early studies that utilised primary inoculation of rodents (e.g. Yamashita et al., 1956, Yamashita et al., 1958, Yamashita et al., 1963, Ohbayashi, 1960, Ohbayashi et al., 1971) provided information on metacestode development, but unfortunately inoculation dose varied between infections even in the same experiment. Thus, it is difficult to interpret varying establishment among these rodent species from these experiments.
A meta-analysis of literature on E. multilocularis prevalence in both definitive and intermediate hosts (Takeuchi-Storm et al., 2015) demonstrated that genera was a significant factor for parasite prevalence with Ondatra having the highest estimate followed by Arvicola, then Microtus with Apodemus and Myodes having the lowest. Significant differences were observed in all accept Microtus and Arvicola and Apodemus and Myodes. Considering the consensus of various observations (Hanosset et al., 2008, Raoul et al., 2015) it is not surprising that Ondatra, Microtus and Arvicola had the highest odds ratios (OR). However, in a global perspective, it is worth noting that the genus Myodes which is widely distributed on all continents where the parasite occurs (Eckert, 1998) was found to be of similar low importance for parasite transmission as Apodemus, which are not considered suitable intermediate hosts (Tsukada et al., 2000) however the Japanese field mouse (Apodemus argenteus) has been found infected in Japan (Tsukada et al., 2002).
Considering the low OR for Myodes and the key role played in E. multilocularis transmission by Myodes rufocanus in Japan (Saitoh and Takahashi, 1998) it was deemed appropriate to experimentally assess the susceptibility of Myodes glareolus in an effort to elucidate their potential role as transmitters of the parasite in Europe. Although it was not possible to obtain Apodemus spp., outbred Mus musculus (CD-1 IGS) was inoculated as murid representative. This species has been found naturally infected (Leiby and Kritsky, 1972, Pétavy et al., 1990). The Syrian hamster (Mesocricetus auratus) was also included. This species is capable of harbouring the adult worm in its intestine after immunosuppression (Kamiya et al., 1991, Nonaka et al., 1996) however, post infection with approximately 40 eggs the Alaskan strain of the parasite, did not result in establishment (Yamashita et al., 1958). As this represents the only study found and due to the relatively low inoculum of eggs used it was deemed appropriate to attempt experimental infections with a European strain of the parasite. C57BL6/j mice were used as positive controls for egg viability as they have been shown to demonstrate macroscopic lesions after 4 wpi (Matsumoto et al., 2010).
2. Materials and methods
2.1. Experimental inoculation
The E. multilocularis eggs used for inoculation were isolated from worms in naturally infected foxes from the city of Zurich and the surrounding area, during the official Swiss hunting season. Eggs were tested for viability by the sodium hypochlorite (s-h) resistant test (Deplazes et al., 2005). In brief, the percentage viability of the eggs was determined to be the number of eggs with intact oncospheres after the s-h solution had been applied.
Animals were anesthetized with isoflurane and the egg suspension containing approximately 100 viable E. multilocularis eggs was administered via gavage. This was calculated as follows: the total number of eggs per ml divided by the percentage viability (via s-h resistant test) to determine the percentage of viable eggs per ml. The number of viable eggs per ml was then used to calculate the volume of egg suspension that would contain 100 viable eggs. Animals were inoculated on different days with the s-h test conducted prior to each inoculation round and the volume of egg suspension adjusted accordingly. During the period of inoculation eggs were stored at 4 °C to maximise viability (Veit et al., 1995) in 1% penicillin-streptomycin solution.
Animals were housed in a safety facility (Biosafety Level 2++ approved by the Danish Working Environment Authority, Journal no. 20120014119/21) at the Department of Plant and Environmental Sciences (University of Copenhagen, Denmark), under experimental license no. 2012-15-2934-00150. All animals were imported under permission from the Danish AgriFish Agency (CVR: 29979812, No. 1013624417).
Four species/strains of rodent were experimentally inoculated with 100 viable E. multilocularis eggs:
-
•
Myodes glareolus
Female (n = 23) and male (n = 24) M. glareolus were obtained from Institute of Environmental Sciences, Jagiellonian University Kraków, Poland. All animals were 56 days old at inoculation (DAI). Animals were euthanized at 6 wpi (Female n = 4, Male n = 4), 8 wpi (Female n = 15, Male n = 16) and 10 wpi (Female n = 4, Male n = 4). The animals euthanized at 8 wpi were a control group of a separate study investigating E. multilocularis infection in relation to basal metabolic rate (BMI). The conditions that these rodents were exposed to (housing, nutrition, and E. multilocularis infection) were precisely the same as the 6 and 10 wpi animals and were thus included. All animals were inoculated between 20/04/2015 and 29/04/2015.
-
•
Mus musculus (CD-1® IGS)
Male (n = 3) and female (n = 6) CD-1 animals were obtained from Charles River Germany. Animals were 56 DAI. All animals were euthanized 6 wpi. The original study design was for 12 animals to be inoculated but a shortage of eggs meant that it was not possible for 3 male animals to be inoculated. Animals were inoculated 1 month after the M. glareolus and C57BL/6j mice on 26/05/2015. As such it was intended to also inoculate an additional two C57BL/6j mice. The shortage of eggs precluded this but in the interest of the 3 R’s (Russell et al., 1959) it was decided to proceed with the inoculations.
-
•
Mesocricetus auratus
Female (n = 5) and male (n = 6) Mesocricetus auratus were obtained from Charles River France.
Animals were 56 DAI. These animals were euthanized 6 wpi (Female n = 3, Male n = 3) and 10 wpi (Female n = 2, Male n = 3). Animals were inoculated 07/04/2014. These animals were inoculated in the same period as the Woolsey et al., 2015b study that demonstrated heavy E. multilocularis infection in Microtus arvalis (which were inoculated with the same egg suspension spanning dates before and after 07/04/2014).
-
•
Mus musculus (C57BL/6j)
Female (n = 4) M. musculus were obtained from Charles River Germany in March 2015. Mice were 42 DAI. All animals were inoculated 20/04/2015.
2.2. Animal euthanasia
All animals were anesthetised with isoflurane and then euthanized with CO2 (gradual fill). Rodents were removed from the CO2 once they were observed to cease breathing. Cervical dislocation was performed subsequently as a precaution. Rodents were weighed and their livers were removed and lesions counted by eye and measured along their length (longest dimension). Protoscolex quantification was conducted as described by (Burlet et al., 2011). Internal liver lesions were investigated and counted and measured by palpating the liver between two clear plastic sheets. Although more accurate determination of internal lesions would have been obtained by slicing the organ, this would have reduced the accuracy of protoscolex enumeration, which was deemed more important to understanding the transmission potential than lesion number (as this parameter is a more robust determinant of the animal’s potential to transmit the parasite). All other organs (with the exception of the brain) were inspected for any metacestode growth.
2.3. Statistical analysis
The number of metacestodes, their size and species susceptibility (defined here as the number of all inoculated animals per species displaying E. multilocularis metacestodes in the liver), was run in a multiple linear regression analyses against species and sex at 6 wpi. Single rodents were found infected at 8 and 10 wpi respectively, negating statistical analysis at these time points. All analyses was conducted in R (R Core Team, 2014) and differences considered significant when (p < 0.05).
3. Results
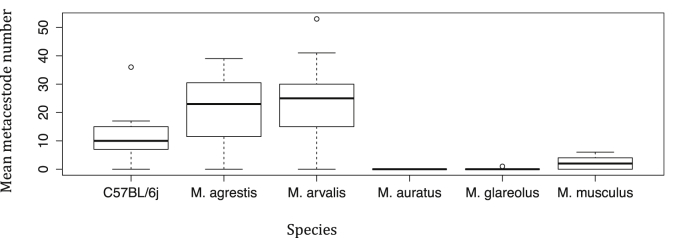
At 6 wpi 4/4 C57BL/6j and 5/9 CD-1 (3 female, 2 male) mice had developed visible E. multilocularis infections. No metacestodes were observed in M. auratus or M. glareolus at 6 wpi. Initial C57BL/6j susceptibility was significantly greater than M. auratus (p ≤ 0.0001), M. glareolus (p ≤ 0.0001) and CD-1 (p ≤ 0.0001). No rodents exhibited protoscolices at 6 wpi. Sex was not found to be significant (Fig 1).
Fig. 1.
Mean establishment of E. multilocularis oncospheres in the different rodent intermediate hosts after receiving 100 viable eggs at 6 wpi. (M. glareolus at 8 wpi).
No CD-1 outbred mice harboured metacestodes larger than 1 mm. Three C57BL/6j harboured metacestodes larger than this (>1 mm < 2 mm) and this was significant (p ≤ 0.0001).
Only 2/47 M. glareolus and no M. auratus developed metacestodes. At 8 wpi, one male M. glareolus developed an infection with a single mass of metacestode material (1.1 × 1.2 × 1 cm) located in the liver with 1675 protoscolices. At 10 wpi, one female M. glareolus was found infected with a single metacestode mass in the liver (2 × 0.9 × 0.6 cm) with 88 protoscolices.
4. Discussion
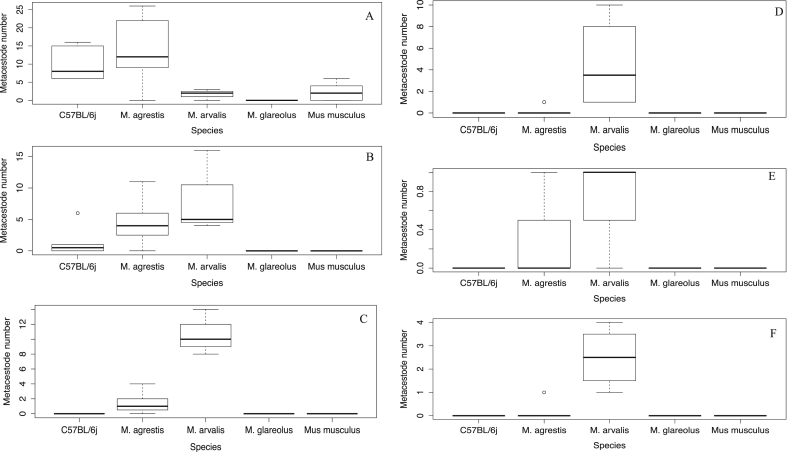
The infection rate of 4.3% in M. glareolus is of special interest considering that under very similar experimental conditions, M. arvalis and Microtus agrestis both exhibited much greater susceptibility to the parasite (95.2% and 88.9% of animals developing infection respectively) and many more metacestodes per rodent (27.5 ± 6.63 S.D. and 23.9 ± 15.3 S.D. respectively) at 6 wpi (Woolsey et al., 2015a, Woolsey et al., 2015b). Comparisons of these data with the current study can be seen in Fig. 2, Fig. 3. Two M. glareolus were infected and both harboured protoscolices, so the species is clearly capable of transmitting the parasite and in the literature there are numerous references to this species being infected with protoscolices in Europe e.g. (Eckert, 1998, Osterman Lind et al., 2011, Liccioli et al., 2013). Prevalence of the parasite in M. glareolus has been observed as high, 10.3% (6/58) (Reperant et al., 2009) but is generally reported as low with prevalence values of 4.3% (1/23) (Hanosset et al., 2008) and 2.4% (2/83, but with 108 000 protoscolices in one animal) (Stieger et al., 2002) in central Europe. It would be prudent however to consider this limited susceptibility in relation to Microtus spp. in assessing the competence of this species in parasite transmission. Limited susceptibility in this species is verified by the 100% infection rate in the C57BL/6j mice used as positive controls. The lack of susceptibility in this species is of even greater interest considering that in Japan, the transmission of E. multilocularis is largely based upon the grey-sided vole (Myodes rufocanus) (Saitoh and Takahashi, 1998) suggesting that susceptibility to this parasite is perhaps not determined by genus. It would have been desirable to see data on lesion size in M. glareolus at 6 wpi in order to determine some idea regarding the metacestode rate of growth but unfortunately no animals had lesions at this endpoint. To ensure infections at all time points when working with this species much larger cohort sizes will be needed.
Fig. 2.
Mean establishment of E. multilocularis oncospheres in the different rodent intermediate hosts after receiving 100 viable eggs at 6 wpi (M. glareolus at 8 wpi). Data from current study and (Woolsey et al., 2015a, Woolsey et al., 2015b).
Fig. 3.
Number of metacestodes of varying sizes in individual species at 6 wpi (M. glareolus at 8 wpi) after receiving 100 viable E. multilocularis eggs. A <1 mm, B 1 - ≤2 mm, C > 2 - ≤3 mm, D > 3 - ≤4 mm, E 4 - ≤5 mm, F > 5 mm. Data from current study and (Woolsey et al., 2015a, Woolsey et al., 2015b).
Lending support to the limited role played by this species in parasite transmission is the finding that variations in E. multilocularis prevalence in foxes was not associated with M. glareolus but was with Microtus spp. in the Swiss canton of Grisons, even though M. glareolus constituted the second highest prey item in the study (Tanner et al., 2006). Considering the role of intermediate host populations being highly important to variations in definitive host prevalence (Raoul et al., 2015), this is of great interest.
The C57BL/6j mice were significantly more susceptible to the parasite than the CD-1 outbred mice. C57BL/6j mice were not found to harbour protoscolices at 16 wpi after oral infection with 200 eggs (Matsumoto et al., 2010) and thus, considering the reduced susceptibility of CD-1 to the inbred strain it is unlikely that the outbred mice would have produced protoscolices either. Although there are a limited number of examples of this species harbouring the infection in the wild e.g. (Leiby et al., 1970, Leiby and Kritsky, 1972, Pétavy et al., 1990), these data are highly indicative of M. musculus not playing any significant role in E. multilocularis transmission. Although outbred, these CD-1 mice obtained from Charles River originate from a very small number of animals (2 males and 7 females), which represents a significant bottleneck, and thus caution should be exercised when interpreting these results as representative of wild type M. musculus. Furthermore, only 4 C57BL/6j mice were used in this study but their inbred nature should mitigate the small sample size. In previous studies using the same methodology similar infection dynamics were observed in this strain (Woolsey et al., 2015a, Woolsey et al., 2015b).
The CD-1 mice were included in this study as a representative of the family Muridae. Based on trapping studies aiming to determine E. multilocularis prevalence, it is clear that Apodemus spp. are caught a great deal more frequently than M. musculus (Takeuchi-Storm et al., 2015). This is due to habitat overlap between this species and species widely regarded to be key drivers of E. multilocularis transmission in Europe whereas M. musculus are found in villages, away from these species (Giraudoux et al., 2003). Due to this, Apodemus spp. would constitute a much better representative of the Muridae family. That said, if protoscolices had been found in mice in this study it would have made this species potentially important considering the urbanisation of foxes (Deplazes et al., 2004).
It should be noted that the CD-1 mice were inoculated approximately 1 month after the M. glareolus and C57BL/6j mice and although the s-h resistance test was conducted prior to inoculation of the outbred mice, recent studies have demonstrated that this primarily tests the maturity of the eggs, not necessarily their infectivity (Federer et al., 2015) and this may have declined in the time between inoculations. As such it would have been ideal to have an additional group of C57BL/6j mice inoculated at the same time for a more robust comparison of susceptibility between these two strains and results need to be considered in this context. It does seem however that even in highly susceptible species, inoculated with 100–1000 eggs, maximum establishment in the liver is < 35 (Woolsey et al., 2015a, Woolsey et al., 2015b) and thus the egg dose used in this study was much higher than any possible establishment. It therefore seems likely that the CD-1 mice received enough infective eggs for a robust comparison as eggs have been demonstrated to survive for 78 days in summer conditions (Veit et al., 1995) far less favourable than the ideal storage conditions of the eggs in this study.
The infections observed in M. glareolus and both strains of mouse are indicative of two distinct mechanisms pertaining to E. multilocularis infection dynamics in the host; i) establishment of the oncosphere and ii) its persistence and subsequent growth. Clearly, the establishment of the oncosphere in the mouse is much more successful than in M. glareolus however, growth of the metacestode if it does establish in this latter species is far more prolific, with protoscolices appearing 8 wpi. This may permit the distinction of two types of host in relation to E. multilocularis infection, those that have the physiological/morphological/immunological profile to permit establishment of the oncosphere but not its proliferation in the liver and those that are refractory to oncosphere invasion but permit rapid growth if it does occur. M. arvalis and M. agrestis, which along with high metacestode establishment and growth (Woolsey et al., 2015a, Woolsey et al., 2015b) would clearly represent a third type which posses both of these ‘attributes’. Of course, there may be a range of issues that result in the lack of establishment of the oncosphere in the liver such as the physio/chemical conditions in the stomach or the morphology of the small intestine, however the two positive M. glareolus in this study would indicate that such aspects are not relevant in this species.
The mechanisms behind the increased resistance of the outbred strain to E. multilocularis infection are impossible to establish with the data obtained in this study. In order to elucidate such mechanisms, assessment of various immune parameters, such as varying cytokine profiles, would have been useful. Investigating such aspects in ecologically relevant Cricetidae species in the manner that has been achieved with various mouse strains (Vuitton and Gottstein, 2010) is a clear avenue for prospective study. If the cause does lie in differing immune mechanisms it would not be surprising that outbred mice demonstrate greater resistance to infection considering that inbreeding is thought to reduce host immunity as a result of decreased heterozygosity and inbreeding increases homozygosity (Carrington et al., 1999). Indeed, it was found in song sparrows (Melospiza melodia) that a greater inbreeding coefficient decreased the effectiveness of cell mediated immune response (Reid et al., 2003), precisely the type associated with resistance to E. multilocularis (Emery et al., 1996, Emery et al., 1997, Vuitton and Gottstein, 2010). It should be noted however that in the context of oncosphere establishment and metacestode growth, it is the latter that has been demonstrated to be inhibited by cell mediated immune responses and the aspects governing establishment in the liver are far less investigated (not least due to the lack of studies utilising primary infection).
Mesocricetus auratus appears not to be of relevance in transmission as they were not susceptible to E. multilocularis, but it is not possible to determine whether this is a consequence of the oncosphere failing to establish in the liver or another mechanism by which the parasite was prevented from reaching the organ. Furthermore, it should be considered that with a greater number of animals an infection might have been observed. Considering M. glareolus in this study this is particularly relevant with only 2 infections resulting from 47 inoculations.
In conclusion, clear differences between rodent susceptibility to E. multilocularis transmission have been demonstrated. This methodology that has the potential to shed light on parasite transmission dynamics that cannot be obtained through trapping studies or secondary infections in non-ecologically relevant species. In Europe, there ought to be a clear emphasis on conducting such studies on Arvicola and Apodemus spp. and assessment of cytokine and endocrine profiles in all species aiming to determine why these species manifest such differences in response to the parasite. Furthermore, early stages of parasite-host interaction are still unclear and this present model could be used to compare e.g. intestinal dynamics in susceptible/refractory species.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank the Institute of Environmental Sciences, Jagiellonian University, Kraków, Poland and LUKE (Natural Resources Institute), Finland for providing the voles for this study. Charlotte Fischer is thanked for her assistance in animal experimentation and expertise in rodent handling. This study was funded and conducted under the framework project “Echinococcus multilocularis In Rodents (EMIRO)” funded by EMIDA, Era-Net under the EU-FP7.
References
- Burlet P., Deplazes P., Hegglin D. Age, season and spatio-temporal factors affecting the prevalence of Echinococcus multilocularis and Taenia taeniaeformiss in Arvicola terrestris. Parasit. Vectors. 2011;4:6. doi: 10.1186/1756-3305-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington M., Nelson G.W., Martin M.P., Kissner T., Vlahov D., Goedert J.J., Kaslow R., Buchbinder S., Hoots K., O’Brien S.J. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- Dematteis S., Rottenberg M., Baz A. Cytokine response and outcome of infection depends on the infective dose of parasites in experimental infection by Echinococcus granulosus. Parasite Immunol. 2003;25:189–197. doi: 10.1046/j.1365-3024.2003.00620.x. [DOI] [PubMed] [Google Scholar]
- Deplazes P., Hegglin D., Gloor S., Romig T. Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends Parasitol. 2004;20:77–84. doi: 10.1016/j.pt.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Deplazes P., Grimm F., Sydler T., Tanner I., Kapel C.M. Experimental alveolar echinococcosis in pigs, lesion development and serological follow up. Vet. Parasitol. 2005;130:213–222. doi: 10.1016/j.vetpar.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Eckert J. Alveolar Echinococcosis (Echinococcus multilocularis) and other forms of Echinococcosis (Echinococcus vogeli and Echinococcus oligarthus) In: Palmer S.R., Soulsby L., Simpson D.I.H., editors. Zoonoses: Biology, Clinical Practice, and Public Health Control. Oxford University Press; Oxford: 1998. [Google Scholar]
- Eckert J., Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin. Microbiol. Rev. 2004;17:107–135. doi: 10.1128/CMR.17.1.107-135.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery I., Liance M., Deriaud E., Vuitton D.A., Houin R., Leclerc C. Characterization of T-cell immune responses of €chinococcus rnultilocularis-infected C57BL/6J mice. Parasite Immunol. 1996;18:463–472. doi: 10.1111/j.1365-3024.1996.tb01030.x. [DOI] [PubMed] [Google Scholar]
- Emery I., Liance M., Leclerc C. Secondary Echinococcus multilocularis infection in A/J mice: delayed metacestode development is associated with Th1 cytokine production. Parasite Immunol. 1997;19:493–503. doi: 10.1046/j.1365-3024.1997.d01-162.x. [DOI] [PubMed] [Google Scholar]
- Federer K., Armua-Fernandez M.T., Hoby S., Wenker C., Deplazes P. In vivo viability of Echinococcus multilocularis eggs in a rodent model after different thermo-treatments. Exp. Parasitol. 2015;154:14–19. doi: 10.1016/j.exppara.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Giraudoux P., Craig P.S., Delattre P., Bao G., Bartholomot B., Harraga S., Qur J.P., Raoul F., Wang Y., Shi D. Interactions between landscape changes and host communities can regulate Echinococcus multilocularis transmission. Parasitology. 2003;127:S119–S129. [PubMed] [Google Scholar]
- Hanosset R., Saegerman C., Adant S., Massart L., Losson B. Echinococcus multilocularis in Belgium: prevalence in red foxes (Vulpes vulpes) and in different species of potential intermediate hosts. Vet. Parasitol. 2008;151:212–217. doi: 10.1016/j.vetpar.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Kamiya M., Sato H., Kitaoka M., Ishiwata K., Oku Y., Ito M., Gathura P. Laboratory rodent models for the tapeworm-stage of Taenia saginata and other related Taeniid species. Southeast Asian J. Trop. Med. Public Health. 1991;22:262–267. [PubMed] [Google Scholar]
- Kapel C.M., Torgerson P.R., Thompson R.C., Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. Int. J. Parasitol. 2006;36:79–86. doi: 10.1016/j.ijpara.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Leiby P.D., Kritsky D.C. Echinococcus multilocularis: a possible domestic life cycle in Central North America and its public health implications. J. Parasitol. 1972;58:1213–1215. [PubMed] [Google Scholar]
- Leiby P.D., Patrick Carney W., Woods C.E. Studies on sylvatic echinococcosis. III. Host occurrence and geographic distribution of Echinococcus multilocularis in the north Central United States. J. Parasitol. 1970;56:1141–1150. [PubMed] [Google Scholar]
- Liccioli S., Duignan P.J., Lejeune M., Deunk J., Majid S., Massolo A. A new intermediate host for Echinococcus multilocularis: the southern red-backed vole (Myodes gapperi) in urban landscape in Calgary, Canada. Parasitol. Int. 2013;62:355–357. doi: 10.1016/j.parint.2013.03.007. [DOI] [PubMed] [Google Scholar]
- Matsumoto J., Kouguchi H., Oku Y., Yagi K. Primary alveolar echinococcosis: course of larval development and antibody responses in intermediate host rodents with different genetic backgrounds after oral infection with eggs of Echinococcus multilocularis. Parasitol. Int. 2010;59:435–444. doi: 10.1016/j.parint.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Matsumoto J., Yagi K. Experimental studies on Echinococcus multilocularis in Japan, focusing on biohazardous stages of the parasite. Exp. Parasitol. 2008;119:534–541. doi: 10.1016/j.exppara.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Nonaka N., Iida M., Yagi K., Ito A., Ooi H.K., Oku Y., Kamiya H. Time course of coproantigen excretion in Echinococcus multilocularis infections in foxes and an alternative definitive host. Gold. Hamsters. Int. J. Parasitol. 1996;26:1271–1278. doi: 10.1016/s0020-7519(96)00117-8. [DOI] [PubMed] [Google Scholar]
- Ohbayashi M. Studies on echinococcosis x: histological observations on experimental cases of multilocular echinococcosis. Jpn. J. Veterinary Res. 1960;8:134–160. [Google Scholar]
- Ohbayashi M., Rausch R.L., Fay F.H. On the ecology and distribution of Echinococcus spp. (Cestoda:Taenidae), and characteristics of their development in the intermediate host: II. Comparative studies on the development of larval E. Multilocularis Leukart, 1863, in the intermediate host. Jpn. J. Veterinary Res. 1971;19:1–53. [PubMed] [Google Scholar]
- Osterman Lind E., Juremalm M., Christensson D., Widgren S., Hallgren G., Agren E.O., Uhlhorn H., Lindberg A., Cedersmyg M., Wahlström H. First detection of Echinococcus multilocularis in Sweden. Euro Surveill. 2011;16:1–3. February to March 2011. [PubMed] [Google Scholar]
- Pétavy A.F., Deblock S., Walbaum S. The house mouse: a potential intermediate host for Echinococcus multilocularis in France. Trans. R. Soc. Trop. Med. Hyg. 1990;84:571–572. doi: 10.1016/0035-9203(90)90044-f. [DOI] [PubMed] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna, Austria: 2014. R: a Language and Environment for Statistical Computing.http://www.R-project.org/ [Google Scholar]
- Raoul F., Hegglin D., Giraudoux P. Trophic ecology, behaviour and host population dynamics in Echinococcus multilocularis transmission. Vet. Parasitol. 2015;213:162–171. doi: 10.1016/j.vetpar.2015.07.034. [DOI] [PubMed] [Google Scholar]
- Reid J.M., Arcese P., Keller L.F. Inbreeding depresses immune response in song sparrows (Melospiza melodia): direct and inter-generational effects. Proc. Biol. Sci. 2003;270:2151–2157. doi: 10.1098/rspb.2003.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reperant L.A., Hegglin D., Tanner I., Fischer C., Deplazes P. Rodents as shared indicators for zoonotic parasites of carnivores in urban environments. Parasitology. 2009;136:329–337. doi: 10.1017/S0031182008005428. [DOI] [PubMed] [Google Scholar]
- Romig T., Bilger B. Animal models of echinococcosis. In: Zak O., Sande M.A., editors. Animal Models of Infection. Academic Press; San Diego, London, Boston, New York, Sydney, Tokyo, Toronto: 1999. pp. 877–884. [Google Scholar]
- Russell W.M.W., Burch R.L., Hume C.W. Methuen; United Kingdom: 1959. The Principles of Humane Experimental Technique. [Google Scholar]
- Saitoh T., Takahashi K. The role of vole populations in the prevalence of the parasite (Echinococcus multilocularis) in foxes. Res. Popul. Ecol. 1998;40:97–105. [Google Scholar]
- Stieger C., Hegglin D., Schwarzenbach G., Mathis A., Deplazes P. Spatial and temporal aspects of urban transmission of Echinococcus multilocularis. Parasitology. 2002;124:631–640. doi: 10.1017/s0031182002001749. [DOI] [PubMed] [Google Scholar]
- Takeuchi-Storm N., Woolsey I.D., Jensen P.M., Fredensborg B.L., Pipper C.B., Kapel C.M. Predictors of Echinococcus multilocularis prevalence in definitive and intermediate hosts: a meta-analysis approach. J. Parasitol. 2015 doi: 10.1645/14-645.1. [DOI] [PubMed] [Google Scholar]
- Tanner F., Hegglin D., Thoma R., Brosi G., Deplazes P. Echinococcus multilocularis in Grisons: distribution in foxes and presence of potential intermediate hosts. Schweiz. Arch. Tierheilkd. 2006;148:501–510. doi: 10.1024/0036-7281.148.9.501. [DOI] [PubMed] [Google Scholar]
- Thompson R.A., Lymbery A.J. Echinococcus and hydatid disease. Cab. Int. 1995 [Google Scholar]
- Tsukada H., Hamazaki K., Ganzorig S., Iwaki T., Konno K., Lagapa J.T., Matsuo K., Ono A., Shimizu M., Sakai H. Potential remedy against Echincoccus multilocularis in wild red foxes using baits with anthelmintic distributed around fox breeding dens in Hokkaido, Japan. Parasitology. 2002;125:119–129. doi: 10.1017/s0031182002001968. [DOI] [PubMed] [Google Scholar]
- Tsukada H., Morishima Y., Nonaka N., Kamiya M. Preliminary study of the role of red foxes in Echinococcus multilocularis transmission in the urban area of Sapporo, Japan. Parasitology. 2000;120:423–428. doi: 10.1017/s0031182099005582. [DOI] [PubMed] [Google Scholar]
- Veit P., Bilger B., Schad V., Schäfer J., Frank W., Lucius R. Influence of environmental factors on the infectivity of Echinococcus multilocularis eggs. Parasitology. 1995;110:79–86. doi: 10.1017/s0031182000081075. [DOI] [PubMed] [Google Scholar]
- Vuitton D.A., Gottstein B. Echinococcus multilocularis and its intermediate host: a model of parasite-host interplay. J. Biomed. Biotechnol. 2010;2010:923193. doi: 10.1155/2010/923193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolsey I.D., Bune N.E., Jensen P.M., Deplazes P., Kapel C.M. Echinococcus multilocularis infection in the field vole (Microtus agrestis): an ecological model for studies on transmission dynamics. Parasitol. Res. 2015;114:1703–1709. doi: 10.1007/s00436-015-4355-9. [DOI] [PubMed] [Google Scholar]
- Woolsey I.D., Jensen P.M., Deplazes P., Kapel C.M. Establishment and development of Echinococcus multilocularis metacestodes in the common vole (Microtus arvalis) after oral inoculation with parasite eggs. Parasitol. Int. 2015;64:571–575. doi: 10.1016/j.parint.2015.08.006. [DOI] [PubMed] [Google Scholar]
- Yamashita J., Ohbayashi M., Kitamura Y., Suzuki K., Okugi M. Studies on echinococcosis VIII: experimental Echinococcosis multilocularis in various rodents: especially on the difference of susceptibility among uniform strains of the mouse. Jpn. J. Vet. Res. 1958;6:135–155. [Google Scholar]
- Yamashita J., Ohbayashi M., Konno S. Studies on Echinococcosis IV. Experimental infection of the white mouse. Jpn. J. Veterinary Res. 1956;4:123–128. [Google Scholar]
- Yamashita J., Ohbayashi M., Sakamoto T., Orihara M., Suzuki K., Okugi M. Studies on Echinococcosis XIV: further observations on the difference pf susceptibility to Echinococcus multilocularis among uniform strains of the mouse. Jpn. J. Veterinary Res. 1963;11:50–54. [Google Scholar]