Abstract
Aging is the primary risk factor underlying hypertension and incident cardiovascular disease. With aging, the vasculature undergoes structural and functional changes characterized by endothelial dysfunction, wall thickening, reduced distensibility, and arterial stiffening. Vascular stiffness results from fibrosis and extracellular matrix (ECM) remodelling, processes that are associated with aging and are amplified by hypertension. Some recently characterized molecular mechanisms underlying these processes include increased expression and activation of matrix metalloproteinases, activation of transforming growth factor-β1/SMAD signalling, upregulation of galectin-3, and activation of proinflammatory and profibrotic signalling pathways. These events can be induced by vasoactive agents, such as angiotensin II, endothelin-1, and aldosterone, which are increased in the vasculature during aging and hypertension. Complex interplay between the “aging process” and prohypertensive factors results in accelerated vascular remodelling and fibrosis and increased arterial stiffness, which is typically observed in hypertension. Because the vascular phenotype in a young hypertensive individual resembles that of an elderly otherwise healthy individual, the notion of “early” or “premature” vascular aging is now often used to describe hypertension-associated vascular disease. We review the vascular phenotype in aging and hypertension, focusing on arterial stiffness and vascular remodelling. We also highlight the clinical implications of these processes and discuss some novel molecular mechanisms of fibrosis and ECM reorganization.
Résumé
Le vieillissement constitue le principal facteur de risque d’apparition de l’hypertension et de la maladie cardiovasculaire. En vieillissant, le système vasculaire subit des modifications structurelles et fonctionnelles caractérisées par une dysfonction endothéliale ainsi que l’épaississement, la rigidification et la perte d’élasticité des parois vasculaires. La rigidité vasculaire est causée par la fibrose et le remodelage de la matrice extracellulaire, des processus qui sont associés au vieillissement et qui sont amplifiés en présence d’hypertension. Parmi les mécanismes moléculaires sous-jacents du vieillissement récemment identifiés, on retrouve l’augmentation de l’expression et de l’activation des métalloprotéinases matricielles, l’activation des voies de signalisation du facteur de croissance transformant bêta 1 impliquant les protéines SMAD, la régulation positive de la galectine-3 et l’activation des voies de signalisation pro-inflammatoires et profibrotiques. Ces mécanismes peuvent être induits par divers agents vasoactifs comme l’angiotensine II, l’endothéline-1 et l’aldostérone dont la présence s’accroît au fil du processus de vieillissement et en présence d’hypertension. Cette interaction complexe entre le « processus de vieillissement » et les facteurs pro-hypertensifs entraîne un remodelage et une fibrose accélérée ainsi que la rigidification des artères qu’on observe habituellement avec l’hypertension. Puisque le phénotype vasculaire de l’hypertendu jeune ressemble à celui de la personne âgée par ailleurs en bonne santé, on fait désormais de plus en plus souvent appel au vocable de vieillissement vasculaire « précoce » ou « prématuré » pour désigner la maladie vasculaire liée à l’hypertension. Nous passons ici en revue le phénotype vasculaire du vieillissement et de l’hypertension en mettant l’accent sur la rigidité artérielle et le remodelage vasculaire. Nous traitons également de l’incidence clinique de ces processus, en plus d’aborder quelques-uns des mécanismes moléculaires de la fibrose et de la réorganisation de la matrice extracellulaire.
Hypertension is the largest contributor to the global burden of cardiovascular disease. The World Health Organization estimates that the number of adults with high blood pressure will increase from 1 billion to 1.5 billion worldwide by 2020.1 This increase is related in part to the fact that the population is aging. Of all the factors contributing to hypertension, such as genetics, obesity, dyslipidemia, sedentary lifestyle, and diabetes, advancing age is the most important risk factor. Both aging and hypertension are associated with structural, mechanical, and functional changes in the vasculature, characterized by increased arterial stiffness, reduced elasticity, impaired distensibility, endothelial dysfunction, and increased vascular tone. The prevalence of vascular stiffness and high blood pressure increases with age and as such, hypertension has been considered to be a condition of aging. Arterial stiffening precedes the development of hypertension, and both phenomena occur more frequently in the elderly. The relationship between aging, cardiovascular disease, and vascular stiffening is further exemplified in patients with progeria (premature aging), who exhibit accelerated vascular aging and often die of cardiovascular disease.2 Arterial stiffening is caused primarily by excessive fibrosis and reduced elasticity, with associated increased collagen deposition, increased elastin fiber fragmentation/degeneration, laminar medial necrosis, calcification, and cross-linking of collagen molecules by advanced glycation end-products.
Fibrosis as a dynamic process initially is an adaptive repair response that is reversible. However, the fibrogenic process is progressive, leading to further worsening of arterial stiffness and fibrosis that gradually extends into the neighbouring interstitial space. Fibrosis occurs in both large and small arteries. In large vessels, vascular stiffening leads to hemodynamic damage to peripheral tissues.3 Fibrosis and stiffening of the resistance circulation impair endothelial function, increase vasomotor tone, promote vascular rarefaction, and alter tissue perfusion. The combination of “aging” and prohypertensive elements, such as activation of the renin-angiotensin-aldosterone system, inflammation, oxidative stress, salt consumption, and genetic factors, results in excessive arterial fibrosis and extracellular matrix (ECM) deposition with amplification of aging-related vascular injury and stiffness. These processes lead to excessive fibrosis, which often extends from small arteries and replaces parenchymal tissue, thereby leading to tissue fibrosis, scarring, and hypertension-associated target organ damage of the heart, kidney, and brain.
At the molecular and cellular levels, arterial aging and hypertension-associated vascular changes are characterized by reduced nitric oxide production, increased generation of reactive oxygen species (ROS) (oxidative stress), activation of transcription factors, induction of “aging” genes, stimulation of proinflammatory and profibrotic signalling pathways, reduced collagen turnover, calcification, vascular smooth muscle cell proliferation, and ECM remodelling. These processes contribute to increased fibrosis, which is further promoted by prohypertensive vasoactive agents, such as angiotensin II (Ang II), endothelin-1 (ET-1), and aldosterone, which stimulate profibrotic signalling cascades, including p38 mitogen-activated protein kinases (p38 MAPK) and the transforming growth factor-β (TGF-β)/SMAD pathway. Activation of galectin-3 and dysregulation of MMPs and TIMPs are involved in ECM remodelling and further enhance vascular fibrosis. Many of these events are upregulated with advancing age and in human and experimental hypertension. We review the vascular phenotype in physiological aging and in hypertension, focusing particularly on arterial stiffness and fibrosis.
Aging-Associated Vascular Alterations
With aging, the vasculature undergoes functional, structural, and mechanical changes characterized by endothelial dysfunction, thickening (remodelling) of the vascular wall, and increased stiffening, respectively (Fig. 1). These changes result in a reduced capacity of arteries to adapt to tissue demands and accordingly may lead to ischemic injury. Preclinical and clinical studies have clearly demonstrated that with aging, there is impaired endothelium-dependent vasorelaxation with associated increased permeability and vascular inflammation.
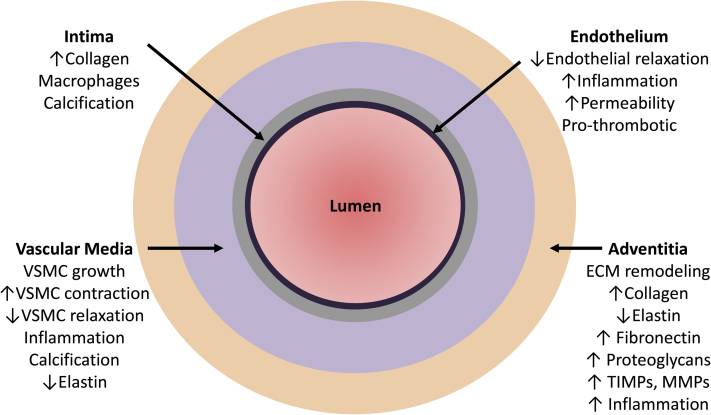
Figure 1.
The vascular phenotype in aging and hypertension. With aging and during the development of hypertension, the endothelium, vascular wall, and adventitia undergo functional and structural changes. Endothelial function is impaired and the vascular media is thickened. The adventitial extracellular matrix undergoes remodelling, with increased collagen deposition, reduced elastin content, and increased proinflammatory cells. These processes contribute to vascular fibrosis and stiffening. ECM, extracellular matrix; MMP, matrix metalloproteinases; TIMPs, tissue inhibitory metalloproteinases; VSMC, vascular smooth muscle cell.
Epidemiologic, cross-sectional, clinical, and postmortem studies in healthy individuals of variable ages have clearly demonstrated that intimal wall thickening and dilatation are noticeable structural changes that occur in conduit arteries with advanced age. Findings from noninvasive vascular phenotyping studies in healthy individuals have demonstrated that intima-media thickness increases 2- to 3-fold between 20 and 90 years of age.4 Studies in aging nonhuman primates also showed a relationship between intimal thickness in the thoracic aorta and aging.5 Exact factors causing progressive intimal thickening with aging in otherwise healthy individuals remain elusive, but a number of distinctive changes at the cellular and morphologic levels have been identified, including fracture of elastin fibres within the tunica media, increased collagen deposition, cellular senescence, and dysregulated cell proliferation. Associated with these events is remodelling of the ECM, which is an essential component of the connective tissue surrounding the vascular wall.
The ECM is composed of basic structural elements (collagen and elastin) and more specialized proteins including fibronectin and proteoglycans. The ECM is a dynamic structure and its components are continuously being turned over through highly regulated systems involving activation of MMPs and TIMPs. Dysregulation of these processes, together with alterations in profibrotic and proinflammatory signalling pathways, likely contribute to aging-associated vascular structural changes.
The Vascular Phenotype in Hypertension Resembles Aging-Associated Vascular Remodelling
The overall vascular phenotype of an individual at any 1 time depends not only on “aging” but also on a combination of multiple interacting factors, such as genetic factors, diet, smoking, diabetes, dyslipidemia, oxidative stress, and obesity.6, 7 Moreover, in the presence of prohypertensive factors, there is acceleration of aging-associated vascular changes that leads to exaggerated vascular injury and arterial stiffening. In susceptible individuals, the interplay between aging and hypertension leads to “early vascular aging” and arterial stiffness, in which the vascular phenotype in young hypertensive individuals resembles that of elderly otherwise healthy individuals (Fig. 1).
Arterial Stiffness
Normally, conduit arteries distend to accommodate large pressure ejections from the heart during systole to facilitate perfusion to tissues during diastole. This is determined in large part by the elasticity, distensibility, and compliance of the arterial system. Loss of elasticity and increased stiffness demand greater force to accommodate blood flow, leading to increased systolic blood pressure, increased cardiac work load, and consequent cardiac hypertrophy and risk of cardiovascular events. Aortic stiffness also affects the microcirculation and vice versa.7, 8 Aortic wall stiffening causes increased pulse wave velocity (PWV) and premature reflected waves with elevated central hemodynamic load leading to damage of peripheral small arteries.9 Remodelling of small arteries in turn leads to increased peripheral vascular and pulse wave reflection, which can further contribute to aortic stiffness.10 Arterial stiffness can be assessed by measuring PWV, pulse wave analysis, ambulatory arterial stiffness (using 24-hour ambulatory blood pressure monitoring) and evaluating endothelial function (flow-mediated dilation). PWV is the most commonly used approach and measures the speed of the pressure pulse from the heart as it is propagated through the arteries; it is calculated by dividing the distance travelled by the time taken to travel the defined distance. Stiffer arteries result in a more rapid travel time and hence a higher PWV. Various approaches can be used to measure PWV, including applanation tonometry, oscillometry, Doppler echocardiography, and magnetic resonance imaging. Although the measurement of PWV is considered to be the most simple, noninvasive, robust, and reproducible method to determine arterial stiffness,11 it is not yet used in routine clinical practice. Carotid-femoral PWV is a direct measure of aortic stiffness and is now considered the gold standard for its evaluation in clinical and epidemiologic studies.12
Arterial stiffness is a natural consequence of advancing age and is accelerated in hypertension. It is also an independent predictive risk factor for cardiovascular events and, as such, aortic PWV is now recognized as an important biomarker in the determination of cardiovascular risk. Arterial stiffness has a bidirectional causal relationship with blood pressure, because high blood pressure causes arterial wall injury, which promotes stiffening, whereas arterial stiffening itself is the major cause of increased systolic blood pressure, especially in the elderly,8, 13 Multiple interacting factors at the systemic (blood pressure, hemodynamics), vascular (vascular contraction/dilatation, ECM remodelling), cellular (cytoskeletal organization, inflammatory responses), and molecular (oxidative stress, intracellular signalling, mechanotransduction) levels contribute to arterial stiffness in aging and hypertension. Dysregulation of endothelial cells, vascular smooth muscle cells, and adaptive immune responses has also been implicated in arterial aging and vascular damage in hypertension. A detailed discussion of all these mechanisms is beyond the scope of this review and is addressed elsewhere this issue of the Canadian Journal of Cardiology.14 Here we focus on some molecular and cellular events that contribute to vascular fibrosis and ECM remodelling.
The ECM and Vascular Fibrosis in Aging and Hypertension
The ECM is an essential component of the connective tissue that surrounds cells. In addition to maintaining cellular and vascular integrity, it plays a fundamental role in cell signalling and regulation of cell-cell interactions. The ECM comprises multiple structural proteins, including collagens, elastin, fibronectin, and proteoglycans. Composition of the ECM varies from organ to organ, with collagen types I and III representing the predominant isoforms in the vascular ECM.15 The absolute and relative quantities of collagen and elastin determine biomechanical properties of vessels, in which an elastin deficiency/collagen excess leads to vascular fibrosis and increased stiffness.4, 15 In healthy individuals, collagen deposition and turnover are tightly regulated, and the ratio of collagen to elastin remains relatively constant. However, an imbalance in these processes leads to excessive ECM protein deposition, particularly collagen and fibronectin, contributing to vascular fibrosis and stiffening in aging and during the development of hypertension.15 Collagens are particularly important in these processes because they are the most abundant and stiffest of the ECM proteins. Increased collagen content and destruction of the elastin fiber network together with a proinflammatory microenvironment contribute to ECM remodelling and increased intima-media thickening and vascular stiffness in small and large arteries in human and experimental hypertension.
Contributing to the profibrotic process is transglutaminase (TG2), which is secreted into the ECM, where it catalyzes formation of ε-(γ-glutamyl)lysine isopeptide, in a Ca2+-dependent manner.16 TG2 acts as an extracellular scaffold protein as well as a cross-linking enzyme. Numerous ECM proteins are TG2 substrates, such as fibronectin, collagen, and laminin.16 Under physiological conditions, TG2 regulates fibroblast activity and ECM organization, with little protein cross-linking. However, in pathologic conditions, increased TG2/ECM protein crosslinking and altered TG2 activity cause increased rigidity and stiffening of the vascular wall, processes that may contribute to remodelling in aging and cardiovascular disease. Recent evidence indicates altered TG2 activity and functionality in large arteries of hypertensive rats.17 TG2 dysregulation has also been implicated in small-vessel changes and inward remodelling in hypertension.18 Fundamental to many of the processes underlying ECM reorganization and fibrosis in aging and hypertension is activation of MMPs and TIMPs.
MMPs and TIMPs
ECM proteins, including collagen and elastin, are regulated by MMPs, a family of endopeptidases, which are activated by many factors associated with aging and hypertension, such as proinflammatory signalling molecules (cytokines, interleukins), growth factors, vasoactive agents (Ang II, ET-1, aldosterone) and ROS. MMP activity is controlled at 3 levels: gene transcription, proenzyme activation, and activity inhibition.18 Signalling pathways involved in regulating MMP transcription include p38 MAPK, which can enhance or repress MMP expression in a cell type–dependent manner (Fig. 2). Commonly, MMPs are activated in the pericellular space by other MMPs, including membrane-type MMPs and MMP-3, or by serine proteases like plasmin and chymase. Activated MMPs degrade collagen, elastin, and other ECM proteins, resulting in a modified ECM, often associated with a proinflammatory microenvironment that triggers a shift of endothelial and vascular smooth muscle cells to a more secretory, migratory, proliferative, and senescent phenotype, which contributes to fibrosis, calcification, endothelial dysfunction, and increased intima-media thickness, further impacting on vascular remodelling and arterial stiffness.
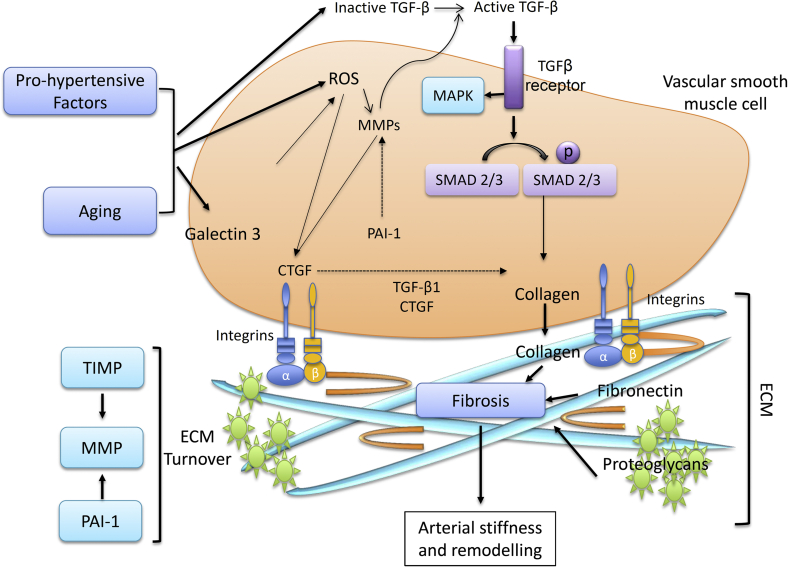
Figure 2.
Vascular signalling mediating extracellular matrix (ECM) remodelling, fibrosis, and arterial stiffening in aging and hypertension. Prohypertensive factors and physiological aging promote ECM remodelling through activation of transforming growth factor-β (TGF-β) and subsequently, mitogen-activated protein kinase (MAPK) and SMAD pathways, reactive oxygen species (ROS) production, leading to matrix metalloproteinase (MMP) and connective tissue growth factor (CTGF) activation and upregulation of galectin-3. Subsequently, collagen, fibronectin, and proteoglycan deposition is increased, leading to fibrosis and increased arterial stiffness. PAI, plasminogen activator inhibitor.
The effect that MMPs have on vascular fibrosis in hypertension is not completely elucidated, with both inhibitory and stimulatory modulation observed.19 This probably relates to activation of different MMP isoforms and downstream signalling pathways. For instance, MMP-1 overexpression attenuates fibrosis,20 whereas MMP-9 activation potentiates fibrosis and DNA damage.21 MMP2 activation leads to stimulation of TGF-β1 signalling; increased vascular smooth muscle cell production of collagens I, II, and III; and increased fibronectin secretion, processes that lead to collagen accumulation in the vascular wall. Although activation of vascular MMP2 and MMP9 in hypertension is associated with collagen accumulation, activation of MMP8 and MMP13 is associated with collagen degradation, processes especially important in arterial wall plaque and plaque rupture.22, 23 MMP2/MMP9 activation through TGF-β1/SMAD signalling also induces activation of myofibroblasts and increased infiltration of monocytes/macrophages, leading to oxidative stress, inflammation, and vascular wall injury. Vascular MMP2 and MMP9 are activated by numerous prohypertensive factors, including Ang II, ET-1, and salt, as well as mechanical and physical factors, such as shear stress and pressure. MMP2, MMP7, MMP9, and MMP14 are upregulated by aging. MMP2 activation is increased in aged rat aorta, leading to increased TGF-β1 and SMAD activation.24 Young rats infused with Ang II exhibit increased MMP2 activation with intima-media thickness and vascular fibrosis changes that are typical in old untreated rats.24 The importance of MMPs in vascular fibrosis in aging and hypertension is further evidenced by MMP inhibitors, such as PD166793, which blunted age-associated vascular fibrosis and remodelling in experimental models.25, 26
MMPs are normally inhibited by endogenous inhibitors called TIMPs, of which there are multiple isoforms. Alterations in the balance between ECM MMPs and TIMPs may contribute to the profibrotic phenotype in aging and hypertension.19, 24 The 4 TIMP isoforms—TIMP1, TIMP2, TIMP3, and TIMP4—are responsible for the inhibition of > 20 MMPs, and the relationship between MMPs and TIMPs changes with age. For instance, increased MMP2 expression and activity is observed in vessels of old rats and nonhuman primates compared with young counterparts.5, 27 Furthermore, TIMPs are downregulated in aged animals with heart failure but not in young animals.28
Molecular and Cellular Mechanisms of Vascular Fibrosis in Aging and Hypertension
TGF-β/SMAD signalling
The TGF-β superfamily consists of > 40 members that share common sequence elements and structural motifs and includes TGF-β, bone morphogenetic protein, activin, inhibin, and growth differentiation factors.29, 30, 31, 32 Disruption of the TGF-β pathway has been implicated in arterial aging and vascular fibrosis.29, 30, 31, 32 Three isoforms (TGF-β1, TGF-β2, and TGF-β3) exist; TGF-β1 is most frequently upregulated in ECM remodelling and fibrosis and is consequently regarded as an important regulator of the ECM. In the vascular system, TGF-β1 is expressed in endothelial cells, vascular smooth muscle cells, myofibroblasts, and adventitial macrophages. Activation of vascular TGF-β1, and its downstream signalling effector SMAD, increases the synthesis of ECM proteins such as fibronectin, collagen, and plasminogen activator inhibitor-1 (PAI-1).33, 34 TGF-β reduces collagenase production and stimulates expression of TIMPS, resulting in excessive matrix accumulation, in part resulting from inhibition of ECM degradation.35 TGF-β signalling predominantly occurs through the cytoplasmic proteins, SMADs, which translocate to the nucleus and act as transcription factors. The SMAD family comprises receptor-activated SMADs (SMAD2, SMAD3, SMAD5, and SMAD8), inhibitory SMADs (SMAD6, SMAD 7) and common-partner SMADs (SMAD4). SMAD2 and SMAD3 are specific mediators of TGFβ/activin pathways, whereas SMAD7 inhibits both BMP and TGF-β/activin signalling. SMAD activation results in increased transcription of many genes involved in ECM formation, including fibronectin, procollagens, PAI-1, and connective tissue growth factor (CTGF).32 In vascular smooth muscle cells, overexpression of SMAD7 inhibits TGF-β–induced fibronectin, collagen, and CTGF production.36 Important non-SMAD pathways implicated in TGF-β profibrotic signalling include extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), p38 MAPK, and phosphoinositide 3-kinase/Akt.37 SMAD translocation to the nucleus can be modulated by Ras-activated ERK1/2. ERK inhibition reduces TGF-β–stimulated SMAD phosphorylation as well as collagen production, suggesting that ERK activation is necessary for an optimal response to TGF-β1.36
Activation of TGF-β1 and receptor-mediated signalling are increased in the aortic wall with aging and during development of hypertension.24 Important in the context of these conditions, Ang II,38, 39 mechanical stress,34, 40 ET-1,36and ROS41 are all elevated and are known to mediate TGF-β activation, with resultant vascular fibrosis. Additionally, MMPs (particularly MMP2 and MMP9) enhance release of TGF-β1, whereas TGF-β1 stimulates TIMP, resulting in inhibition of ECM degradation, which further induces ECM accumulation and vascular remodelling and fibrosis. Ang II can activate the SMAD pathway independent of TGF-β1, with implications for fibrosis.36, 42
Plasminogen activator inhibitor-1
Plasminogen activator inhibitor-1 (PAI-1) is a member of the serine protease inhibitor (serpin) gene family and functions as an inhibitor of the serine proteases, urokinase-type plasminogen activator (uPA), and tissue-type plasminogen activator (tPA). PAI-1 inhibits fibrinolysis and hence regulates dissolution of fibrin and inhibits degradation of the ECM by reducing plasmin generation. PAI-1 normally maintains tissue homeostasis through regulating the activities of uPA, tPA, plasmin, and MMPs. In pathophysiological conditions, PAI-1 upregulation contributes to accumulation of ECM proteins and tissue fibrosis by preventing tissue proteolytic activity and reducing collagen degradation. Together with increased TGF-β1 activity, PAI-1 activity and expression are increased in experimental models of aging and in aged individuals.43, 44 PAI-1 is upregulated in aging-associated pathologic conditions, including hypertension.45 Increased PAI-1 is also recognized as a biomarker of cellular senescence in aging and hypertension.46
Connective tissue growth factor
CTGF is a 38-kDa cysteine-rich secreted potent profibrotic factor implicated in fibroblast proliferation, cellular adhesion, and ECM synthesis. CTGF expression in the vasculature is enhanced by several stimuli, including TGF-β1, tumor necrosis factor-α, and mechanical stress.47 Ang II–induced vascular fibrosis is mediated by CTGF, and vascular smooth muscle cells treated with CTGF antisense oligonucleotides are protected against agonist-induced ECM protein expression.36, 48 CTGF may play an important role in arterial aging and vascular fibrosis; a number of experimental models have demonstrated increased levels of CTGF and associated vascular fibrosis with increasing age.49, 50
Galectin-3
Galectin-3 (LGALS3) is a 29- to 35-kDa carbohydrate-binding lectin expressed on the cell surface of many cell types, including fibroblasts and endothelial and inflammatory cells. It is secreted mainly by activated macrophages, and it is ligand activated by oligosaccharides. Galectin-3 is also activated by other ligands, including glycosylated matrix proteins such as laminin, collagen, elastin, fibronectin, and integrin. The cellular actions of galectin-3 lead to cell proliferation, adhesion, and fibrosis. Galectin-3 has been shown to play an important role in fibrosis and tissue remodelling. In heart failure, plasma galectin-3 levels are increased.51 In the recent Prevention of Renal and Vascular End-Stage Disease (PREVEND) study in which plasma galectin-3 levels were measured in 7968 individuals, plasma levels correlated positively with increasing age and cardiovascular risk factors, including hypertension.52 Because of its role in fibrosis, galectin-3 is now considered by many to be an important biomarker of cardiovascular fibrosis. The precise mechanisms through which galectin-3 influences ECM remodelling and fibrosis are still unclear, although activation of the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) and protein kinase C (PKC) pathways,53, 54 as well as oxidative stress and inflammation, have been suggested. In addition, galectin-3 may directly increase production of ECM proteins. In rat vascular smooth muscle cells, overexpression of galectin-3 enhanced aldosterone-induced collagen 1 synthesis, whereas spironolactone or modified citrus pectin (galectin-3 inhibitor) reversed these effects.55 Galectin-3 inhibition also attenuated cardiovascular fibrosis and left ventricular dysfunction in a mouse model of heart failure.56
The Role of Prohypertensive Vasoactive Factors in Vascular Aging and Fibrosis
Many vasoactive factors activate profibrotic pathways, including Ang II, ET-1, and aldosterone (Figs. 2 and 3). Downstream signalling involves activation of redox-sensitive genes and transcription factors, early growth response factor-1, and activation of TGF-β1, MMPs, galectin-3, and MAP kinases.57, 58, 59, 60, 61 The aging vasculature is characterized by increased levels of Ang II,5 angiotensin-converting enzyme,17, 31, 61 mineralocorticoid receptors,62 and endothelin-converting enzyme-1.63, 64 As such, increased levels of these factors, their receptors, and downstream targets could represent an important event during aging that leads to vascular stiffness.
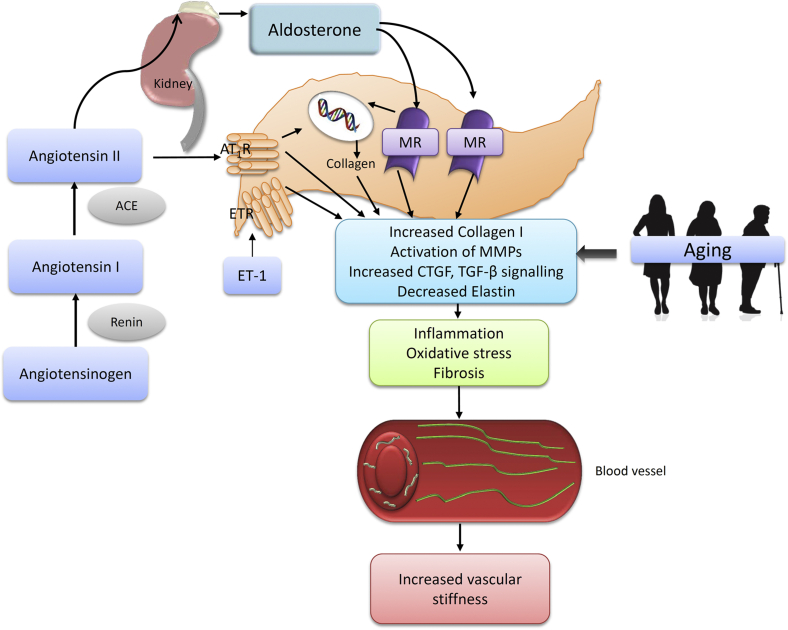
Figure 3.
Influence of prohypertensive factors and aging in the development of vascular fibrosis and arterial stiffening. The renin-angiotensin-aldosterone system, acting through angiotensin receptor type 1 (AT1R) and mineralocorticoid receptor (MR), and endothelin-1 (ET-1) acting through endothelin receptor (ETR) activate matrix metalloproteinase (MMPs), connective tissue growth factor (CTGF), and transforming growth factor-β (TGF-β) signalling, resulting in inflammation, oxidative stress, and fibrosis, leading to increased arterial stiffness. This process is also induced by ET-1 signalling through ETR, aldosterone signalling through MR, and aging. ACE, angiotensin converting enzyme.
Ang II signalling and vascular fibrosis
The renin-angiotensin-aldosterone system plays a central role in structural and mechanical changes in the vasculature. Ang II acts through activation of 2 receptors—AT1 and AT2—in which AT1 plays a major role in the production of ECM proteins.65, 66, 67, 68 This is highlighted by studies demonstrating that antagonism of Ang II receptors results in decreased fibrosis.69, 70 The precise signalling events involved in Ang II-induced vascular fibrosis are incompletely determined; however, in mesangial cells, TGF-β1 activity is increased by Ang II, an effect not observed when activator protein 1 binding sites or PKC- and p38 MAPK–dependent pathways are inhibited.65 In addition, galectin-3 seems to be associated with Ang II–induced fibrosis, and its expression is related to the severity of renal dysfunction in aging; mice subjected to Ang II infusion develop cardiac fibrosis,71 an effect not observed in galectin-3 knockout animals. Furthermore, cultured fibroblasts exposed to galectin-3 have reduced collagen production and deposition.60 Ang II–induced activation of p38 MAPK is also associated with the development and progression of fibrosis, commonly observed in aging and hypertension.72, 73, 74 It has been suggested that Ang II induces activity of MMPs and TIMPs and upregulation of CTGF during aging.75, 76, 77, 78, 79, 80
Aldosterone and vascular fibrosis
Accumulating evidence implicates aldosterone as an important pathophysiological mediator in cardiovascular remodelling by promoting vascular hypertrophy, fibrosis, inflammation, and oxidative stress.81, 82, 83 Evidence from animal models and clinical trials of heart failure and hypertension demonstrate that chronic blockade of mineralocorticoid receptors, through which aldosterone signals, reduces cardiovascular fibrosis. In rats, aldosterone infusion increases aortic media cross-sectional area associated with elevated collagen levels, particularly increased collagen I synthesis.84, 85
In the context of aging, aldosterone levels have been shown to decline in older age.86, 87 This is associated with increased expression of mineralocorticoid receptors in intact vessels, as well as in cultured vascular smooth muscle cells, and has been shown to correlate with markers of vascular fibrosis.62 Whether increased signalling through mineralocorticoid receptors plays a role in vascular fibrosis associated with aging has yet to be confirmed.
ET-1 and vascular fibrosis
ET-1 is a secreted peptide produced primarily in endothelial cells after conversion of preproendothelin to proendothelin and subsequently to mature endothelin, which has potent vasoconstrictor activity. The vascular actions of ET-1 are mediated by 2 distinct endothelin receptor subtypes: the ETA and ETB receptors located on both vascular smooth muscle and endothelial cells. In addition to well-established hypertrophic and mitogenic properties, ET-1 can modulate ECM remodelling by stimulating fibroblast-induced collagen synthesis. ET-1 stimulates synthesis of collagen through both ETA and ETB receptor subtypes.88, 89 Reduced cardiac and renal MMP activity and expression has been reported after administration of ETA receptor antagonists.90, 91, 92 Similarly, treatment with an endothelin antagonist normalizes expression of the collagen I gene and leads to the regression of renal vascular fibrosis and improved survival.93
Numerous findings have reported elevated ET-1 levels in healthy older adult humans.94, 95 In cultured aortic endothelial cells, ET-1 synthesis is greater in cells obtained from older donors vs young adult donors.96 In Wistar-Kyoto (WKY) rats, aging is associated with a 3.6-fold elevation in kidney ET-1 protein expression in the kidney. In rodent models, dual ETA/ETB receptor antagonism had no effect on the age-associated increase in aortic MMP-2 activity in WKY rats but markedly reduced pro and active MMP-2 activity in aged hypertensive rats, demonstrating that ET-1 may represent an important mediator of vascular stiffness in aging in the presence of other vascular diseases.63
Conclusions
With aging, the vasculature undergoes structural and functional changes characterized by arterial remodelling, vascular fibrosis, and stiffening, which are processes that are evident in aging and hypertension. Arterial stiffening is common, occurring in > 60% of individuals older than 70 years and is a major independent predictor for serious cardiovascular events. Accordingly, there is a need to understand the fundamental processes that cause vascular stiffness so that mechanism-based therapeutic strategies can be developed to ameliorate or prevent processes of “vascular aging” in hypertension and associated cardiovascular diseases. Arterial stiffening is caused primarily by excessive fibrosis from excessive accumulation of vascular collagen and degradation of elastin. It is a dynamic phenomenon, which initially is an adaptive repair response that is reversible. However, the fibrogenic process is progressive, leading to further worsening of arterial stiffness and fibrosis that gradually extends into the neighbouring interstitial space, causing tissue and organ damage. A number of noninvasive methods are currently available to evaluate large-artery stiffness in the clinical setting, including carotid-femoral PWV. Increased PWV in aging and hypertension reflects increased arterial stiffness and is emerging as a biomarker for cardiovascular risk stratification. Perhaps over the next decade, PWV assessment may become a routine investigation in the clinical tool kit to better predict hypertension and cardiovascular disease.
Funding Sources
This work was supported by grants from the British Heart Foundation (BHF) (RG/13/7/30099). R.M.T. is supported through a BHF Chair (CH/12/4/29762) and R.A.L. is supported by a PhD scholarship from FAPESP-Brazil (2012/12178-6).
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
See page 666 for disclosure information.
References
- 1.World Health Organization, International Society of Hypertension Writing Group 2003 World Health Organization (WHO)/International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21:1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Baker P.B., Baba N., Boesel C.P. Cardiovascular abnormalities in progeria. Case report and review of the literature. Arch Pathol Lab Med. 1981;105:384–386. [PubMed] [Google Scholar]
- 3.Huveneers S., Daemen M.J., Hordijk P.L. Between Rho(k) and a hard place: the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res. 2015;116:895–908. doi: 10.1161/CIRCRESAHA.116.305720. [DOI] [PubMed] [Google Scholar]
- 4.Lakatta E.G., Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 5.Stout L.C., Whorton E.B., Jr., Vaghela M. Pathogenesis of diffuse intimal thickening (DIT) in non-human primate thoracic aortas. Atherosclerosis. 1983;47:1–6. doi: 10.1016/0021-9150(83)90065-5. [DOI] [PubMed] [Google Scholar]
- 6.Lopes R.A., Neves K.B., Tostes R.C., Montezano A.C., Touyz R.M. Downregulation of nuclear factor erythroid 2-related factor and associated antioxidant genes contributes to redox-sensitive vascular dysfunction in hypertension. Hypertension. 2015;66:1240–1250. doi: 10.1161/HYPERTENSIONAHA.115.06163. [DOI] [PubMed] [Google Scholar]
- 7.AlGhatrif M., Strait J.B., Morrell C.H. Longitudinal trajectories of arterial stiffness and the role of blood pressure: the Baltimore Longitudinal Study of Aging. Hypertension. 2013;62:934–941. doi: 10.1161/HYPERTENSIONAHA.113.01445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AlGhatrif M., Lakatta E.G. The conundrum of arterial stiffness, elevated blood pressure, and aging. Curr Hypertens Rep. 2015;17:1–9. doi: 10.1007/s11906-014-0523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilsson P.M., Boutouyrie P., Cunha P. Early vascular ageing in translation: from laboratory investigations to clinical applications in cardiovascular prevention. J Hypertens. 2013;31:1517–1526. doi: 10.1097/HJH.0b013e328361e4bd. [DOI] [PubMed] [Google Scholar]
- 10.Laurent S., Boutouyrie P. The structural factor of hypertension: large and small artery alterations. Circ Res. 2015;116:1007–1021. doi: 10.1161/CIRCRESAHA.116.303596. [DOI] [PubMed] [Google Scholar]
- 11.Laurent S., Cockcroft J., Van Bortel L., European Network for Noninvasive Investigation of Large Arteries Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 12.Van Bortel L.M., Laurent S., Boutouyrie P., Artery Society. European Society of Hypertension Working Group on Vascular Structure and Function. European Network for Noninvasive Investigation of Large Arteries Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–448. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 13.Kotsis V., Stabouli S., Karafillis I., Nilsson P. Early vascular aging and the role of central blood pressure. J Hypertens. 2011;29:1847–1853. doi: 10.1097/HJH.0b013e32834a4d9f. [DOI] [PubMed] [Google Scholar]
- 14.Kida Y., Goligorsky M.S. Sirtuins, cell senescence, and vascular aging. Can J Cardiol. 2016;32:634–641. doi: 10.1016/j.cjca.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakatta E.G. The reality of aging viewed from the arterial wall. Artery Res. 2013;7:73–80. doi: 10.1016/j.artres.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z., Griffin M. TG2, a novel extracellular protein with multiple functions. Amino Acids. 2012;42:939–949. doi: 10.1007/s00726-011-1008-x. [DOI] [PubMed] [Google Scholar]
- 17.Petersen-Jones H.G., Johnson K.B., Hitomi K. Transglutaminase activity is decreased in large arteries from hypertensive rats compared with normotensive controls. Am J Physiol Heart Circ Physiol. 2015;308:H592–H602. doi: 10.1152/ajpheart.00402.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chakraborti S., Mandal M., Das S., Mandal A., Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 19.Giannandrea M., Parks W.C. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iimuro Y., Nishio T., Morimoto T. Delivery of matrix metalloproteinase-1 attenuates established liver fibrosis in the rat. Gastroenterology. 2003;124:445–458. doi: 10.1053/gast.2003.50063. [DOI] [PubMed] [Google Scholar]
- 21.Prakobwong S., Yongvanit P., Hiraku Y. Involvement of MMP-9 in peribiliary fibrosis and cholangiocarcinogenesis via Rac1-dependent DNA damage in a hamster model. Int J Cancer. 2010;127:2576–2587. doi: 10.1002/ijc.25266. [DOI] [PubMed] [Google Scholar]
- 22.Newby A.C. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- 23.Wang M., Kim S.H., Monticone R.E., Lakatta E.G. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension. 2015;65:698–703. doi: 10.1161/HYPERTENSIONAHA.114.03618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang M., Zhao D., Spinetti G. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signalling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26:1503–1509. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 25.Wang M., Zhang J., Telljohann R. Chronic matrix metalloproteinase inhibition retards age-associated arterial proinflammation and increase in blood pressure. Hypertension. 2012;60:459–466. doi: 10.1161/HYPERTENSIONAHA.112.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zavaczki E., Jeney V., Agarwal A. Hydrogen sulfide inhibits the calcification and osteoblastic differentiation of vascular smooth muscle cells. Kidney Int. 2011;80:731–739. doi: 10.1038/ki.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z., Froehlich J., Galis Z.S., Lakatta E.G. Increased expression of matrix metalloproteinase-2 in the thickened intima of aged rats. Hypertension. 1999;33:116–123. doi: 10.1161/01.hyp.33.1.116. [DOI] [PubMed] [Google Scholar]
- 28.Horn M.A., Graham H.K., Richards M.A. Age-related divergent remodeling of the cardiac extracellular matrix in heart failure: collagen accumulation in the young and loss in the aged. J Mol Cell Cardiol. 2012;53:82–90. doi: 10.1016/j.yjmcc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Bonnema D.D., Webb C.S., Pennington W.R. Effects of age on plasma matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) J Card Fail. 2007;13:530–540. doi: 10.1016/j.cardfail.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M., Takagi G., Asai K. Aging increases aortic MMP-2 activity and angiotensin II in nonhuman primates. Hypertension. 2003;41:1308–1316. doi: 10.1161/01.HYP.0000073843.56046.45. [DOI] [PubMed] [Google Scholar]
- 31.Wang M., Zhang J., Jiang L.Q. Proinflammatory profile within the grossly normal aged human aortic wall. Hypertension. 2007;50:219–227. doi: 10.1161/HYPERTENSIONAHA.107.089409. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Ortega M., Rodriguez-Vita J., Sanchez-Lopez E., Carvajal G., Egido J. TGF-beta signalling in vascular fibrosis. Cardiovasc Res. 2007;74:196–206. doi: 10.1016/j.cardiores.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Douillet C.D., Velarde V., Christopher J.T. Mechanisms by which bradykinin promotes fibrosis in vascular smooth muscle cells: role of TGF-beta and MAPK. Am J Physiol Heart Circ Physiol. 2000;279:H2829–H2837. doi: 10.1152/ajpheart.2000.279.6.H2829. [DOI] [PubMed] [Google Scholar]
- 34.O'Callaghan C.J., Williams B. Mechanical strain-induced extracellular matrix production by human vascular smooth muscle cells: role of TGF-beta(1) Hypertension. 2000;36:319–324. doi: 10.1161/01.hyp.36.3.319. [DOI] [PubMed] [Google Scholar]
- 35.Duncan M.R., Frazier K.S., Abramson S. Connective tissue growth factor mediates transforming growth factor beta-induced collagen synthesis: down-regulation by cAMP. FASEB J. 1999;13:1774–1786. [PubMed] [Google Scholar]
- 36.Rodriguez-Vita J., Sanchez-Lopez E., Esteban V. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-beta-independent mechanism. Circulation. 2005;111:2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- 37.Li J.H., Huang X.R., Zhu H.J. Advanced glycation end products activate Smad signalling via TGF-beta-dependent and independent mechanisms: implications for diabetic renal and vascular disease. FASEB J. 2004;18:176–178. doi: 10.1096/fj.02-1117fje. [DOI] [PubMed] [Google Scholar]
- 38.Gibbons G.H., Pratt R.E., Dzau V.J. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest. 1992;90:456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itoh H., Mukoyama M., Pratt R.E., Gibbons G.H., Dzau V.J. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J Clin Invest. 1993;91:2268–2274. doi: 10.1172/JCI116454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sucosky P., Balachandran K., Elhammali A., Jo H., Yoganathan A.P. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009;29:254–260. doi: 10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhyu D.Y., Yang Y., Ha H. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- 42.Russo I., Frangogiannis N.G. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84–93. doi: 10.1016/j.yjmcc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeshita K., Yamamoto K., Ito M. Increased expression of plasminogen activator inhibitor-1 with fibrin deposition in a murine model of aging, “Klotho” mouse. Semin Thromb Hemost. 2002;28:545–554. doi: 10.1055/s-2002-36699. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto Y., Kobayashi A., Yamazaki N. Relationship between age and plasma t-PA, PA-inhibitor, and PA activity. Thromb Res. 1987;46:625–633. doi: 10.1016/0049-3848(87)90264-7. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto K., Takeshita K., Saito H. Plasminogen activator inhibitor-1 in aging. Semin Thromb Hemost. 2014;40:652–659. doi: 10.1055/s-0034-1384635. [DOI] [PubMed] [Google Scholar]
- 46.Vlachopoulos C., Xaplanteris P., Aboyans V. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241:507–532. doi: 10.1016/j.atherosclerosis.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 47.Oemar B.S., Luscher T.F. Connective tissue growth factor. Friend or foe? Arterioscler Thromb Vasc Biol. 1997;17:1483–1489. doi: 10.1161/01.atv.17.8.1483. [DOI] [PubMed] [Google Scholar]
- 48.Ruperez M., Lorenzo O., Blanco-Colio L.M. Connective tissue growth factor is a mediator of angiotensin II-induced fibrosis. Circulation. 2003;108:1499–1505. doi: 10.1161/01.CIR.0000089129.51288.BA. [DOI] [PubMed] [Google Scholar]
- 49.van Almen G.C., Verhesen W., van Leeuwen R.E. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell. 2011;10:769–779. doi: 10.1111/j.1474-9726.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bigot A., Jacquemin V., Debacq-Chainiaux F. Replicative aging down-regulates the myogenic regulatory factors in human myoblasts. Biol Cell. 2008;100:189–199. doi: 10.1042/BC20070085. [DOI] [PubMed] [Google Scholar]
- 51.Van Kimmenade R.R., Januzzi J.L., Ellinor P.T. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 52.De Boer R., van Veldhuisen D., Gansevoort R. The fibrosis marker galectin-3 and outcome in the general population. J Intern Med. 2012;272:55–64. doi: 10.1111/j.1365-2796.2011.02476.x. [DOI] [PubMed] [Google Scholar]
- 53.Koopmans S.M., Bot F.J., Schouten H.C., Janssen J., van Marion A. The involvement of Galectins in the modulation of the JAK/STAT pathway in myeloproliferative neoplasia. Am J Blood Res. 2012;2:119–127. [PMC free article] [PubMed] [Google Scholar]
- 54.Song X., Qian X., Shen M. Protein kinase C promotes cardiac fibrosis and heart failure by modulating galectin-3 expression. Biochim Biophys Acta. 2015;1853:513–521. doi: 10.1016/j.bbamcr.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 55.Calvier L., Miana M., Reboul P. Galectin-3 mediates aldosterone-induced vascular fibrosis. Arterioscler Thromb Vasc Biol. 2013;33:67–75. doi: 10.1161/ATVBAHA.112.300569. [DOI] [PubMed] [Google Scholar]
- 56.Vergaro G., Prud'homme M., Fazal L. Inhibition of galectin-3 pathway prevents isoproterenol-induced left ventricular dysfunction and fibrosis in mice. Hypertension. 2016;67:606–612. doi: 10.1161/HYPERTENSIONAHA.115.06161. [DOI] [PubMed] [Google Scholar]
- 57.Mendoza-Torres E., Oyarzun A., Mondaca-Ruff D. ACE2 and vasoactive peptides: novel players in cardiovascular/renal remodeling and hypertension. Ther Adv Cardiovasc Dis. 2015;9:217–237. doi: 10.1177/1753944715597623. [DOI] [PubMed] [Google Scholar]
- 58.Martinez-Martinez E., Calvier L., Fernandez-Celis A. Galectin-3 blockade inhibits cardiac inflammation and fibrosis in experimental hyperaldosteronism and hypertension. Hypertension. 2015;66:767–775. doi: 10.1161/HYPERTENSIONAHA.115.05876. [DOI] [PubMed] [Google Scholar]
- 59.Messaoudi S., He Y., Gutsol A. Endothelial Gata5 transcription factor regulates blood pressure. Nat Commun. 2015;6:8835. doi: 10.1038/ncomms9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu L., Ruifrok W.P., Meissner M. Genetic and pharmacological inhibition of galectin-3 prevents cardiac remodeling by interfering with myocardial fibrogenesis. Circ Heart Fail. 2013;6:107–117. doi: 10.1161/CIRCHEARTFAILURE.112.971168. [DOI] [PubMed] [Google Scholar]
- 61.Neves K., Nguyen Dinh Cat A., Lopes R.A. Chemerin regulates crosstalk between adipocytes and vascular cells through Nox. Hypertension. 2015;66:657–666. doi: 10.1161/HYPERTENSIONAHA.115.05616. [DOI] [PubMed] [Google Scholar]
- 62.Krug A.W., Allenhofer L., Monticone R. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 2010;55:1476–1483. doi: 10.1161/HYPERTENSIONAHA.109.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spiers J.P., Kelso E.J., Siah W.F. Alterations in vascular matrix metalloproteinase due to ageing and chronic hypertension: effects of endothelin receptor blockade. J Hypertens. 2005;23:1717–1724. doi: 10.1097/01.hjh.0000176787.04753.ee. [DOI] [PubMed] [Google Scholar]
- 64.Park J.B., Schiffrin E.L. Cardiac and vascular fibrosis and hypertrophy in aldosterone-infused rats: role of endothelin-1. Am J Hypertens. 2002;15:164–169. doi: 10.1016/s0895-7061(01)02291-9. [DOI] [PubMed] [Google Scholar]
- 65.Weigert C., Brodbeck K., Klopfer K., Häring H., Schleicher E. Angiotensin II induces human TGF-β1 promoter activation: similarity to hyperglycaemia. Diabetologia. 2002;45:890–898. doi: 10.1007/s00125-002-0843-4. [DOI] [PubMed] [Google Scholar]
- 66.Montezano A.C., Paravicini T.M., Chignalia A.Z. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (nox5) regulation by angiotensin ii and endothelin-1 is mediated via calcium/calmodulin-dependent pathways in human endothelial cells. Circ Res. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Qi G., Jia L., Li Y. Angiotensin II infusion–induced inflammation, monocytic fibroblast precursor infiltration, and cardiac fibrosis are pressure dependent. Cardiovasc Toxicol. 2011;11:157–167. doi: 10.1007/s12012-011-9109-z. [DOI] [PubMed] [Google Scholar]
- 68.Carver K.A., Smith T.L., Gallagher P.E., Tallant E. Angiotensin-(1-7) prevents angiotensin II-induced fibrosis in cremaster microvessels. Microcirculation. 2015;22:19–27. doi: 10.1111/micc.12159. [DOI] [PubMed] [Google Scholar]
- 69.Ishidoya S., Morrissey J., McCracken R., Reyes A., Klahr S. Angiotensin II receptor antagonist ameliorates renal tubulointerstitial fibrosis caused by unilateral ureteral obstruction. Kidney Int. 1995;47:1285–1294. doi: 10.1038/ki.1995.183. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz-Ortega M., Gonzalez S., Seron D. ACE inhibition reduces proteinuria, glomerular lesions and extracellular matrix production in a normotensive rat model of immune complex nephritis. Kidney Int. 1995;48:1778–1791. doi: 10.1038/ki.1995.476. [DOI] [PubMed] [Google Scholar]
- 71.AbouEzzeddine O.F., Haines P., Stevens S. Galectin-3 in heart failure with preserved ejection fraction: a RELAX trial substudy (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure) JACC Heart Fail. 2015;3:245–252. doi: 10.1016/j.jchf.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Z., Li J., Bu X. Age-induced augmentation of p38 MAPK phosphorylation in mouse lung. Exp Gerontol. 2011;46:694–702. doi: 10.1016/j.exger.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 73.Wu Z., Yu Y., Liu C. Role of p38 mitogen-activated protein kinase in vascular endothelial aging: interaction with arginase-II and S6K1 signalling pathway. Aging (Albany NY) 2015;7:70–81. doi: 10.18632/aging.100722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsieh C., Papaconstantinou J. The effect of aging on p38 signalling pathway activity in the mouse liver and in response to ROS generated by 3-nitropropionic acid. Mech Ageing Dev. 2002;123:1423–1435. doi: 10.1016/s0047-6374(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 75.Pons M., Cousins S.W., Alcazar O., Striker G.E., Marin-Castaño M.E. Angiotensin II–induced MMP-2 activity and MMP-14 and basigin protein expression are mediated via the angiotensin II receptor type 1–mitogen-activated protein kinase 1 pathway in retinal pigment epithelium: implications for age-related macular degeneration. Am J Pathol. 2011;178:2665–2681. doi: 10.1016/j.ajpath.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakai K., Kawato T., Morita T. Angiotensin II induces the production of MMP-3 and MMP-13 through the MAPK signalling pathways via the AT 1 receptor in osteoblasts. Biochimie. 2013;95:922–933. doi: 10.1016/j.biochi.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 77.Yaghooti H., Firoozrai M., Fallah S., Khorramizadeh M. Angiotensin II induces NF-κB, JNK and p38 MAPK activation in monocytic cells and increases matrix metalloproteinase-9 expression in a PKC-and Rho kinase-dependent manner. Braz J Med Biol Res. 2011;44:193–199. doi: 10.1590/s0100-879x2011007500008. [DOI] [PubMed] [Google Scholar]
- 78.Oelusarz A., Nichols L.A., Grunz-Borgmann E.A. Overexpression of MMP-7 increases collagen 1A2 in the aging kidney. Physiol Rep. 2013;1:e00090. doi: 10.1002/phy2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sangaralingham S.J., Wang B.H., Huang L. Cardiorenal fibrosis and dysfunction in aging: imbalance in mediators and regulators of collagen. Peptides. 2016;76:108–114. doi: 10.1016/j.peptides.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Odenbach J., Wang X., Cooper S. MMP-2 mediates angiotensin II-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension. 2011;57:123–130. doi: 10.1161/HYPERTENSIONAHA.110.159525. [DOI] [PubMed] [Google Scholar]
- 81.Sakurabayashi-Kitade S., Aoka Y., Nagashima H. Aldosterone blockade by spironolactone improves the hypertensive vascular hypertrophy and remodeling in angiotensin II overproducing transgenic mice. Atherosclerosis. 2009;206:54–60. doi: 10.1016/j.atherosclerosis.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 82.Sontia B., Montezano A.C.I., Touyz R.M. Downregulation of renal TRPM7 and increased cardiovascular and renal inflammation and fibrosis in aldosterone-infused mice—effects of magnesium supplementation. Hypertension. 2008;51:915–921. doi: 10.1161/HYPERTENSIONAHA.107.100339. [DOI] [PubMed] [Google Scholar]
- 83.Callera G.E., Yogi A., Briones A.M. Vascular proinflammatory responses by aldosterone are mediated via c-Src trafficking to cholesterol-rich microdomains: role of PDGFR. Cardiovasc Res. 2011;91:720–731. doi: 10.1093/cvr/cvr131. [DOI] [PubMed] [Google Scholar]
- 84.Savoia C., Touyz R.M., Schiffrin E.L. Selective mineralocorticoid receptor blocker eplerenone reduces resistance artery stiffness in hypertensive patients. Hypertension. 2008;51:432–439. doi: 10.1161/HYPERTENSIONAHA.107.103267. [DOI] [PubMed] [Google Scholar]
- 85.Briones A.M., Nguyen Dinh Cat A., Callera G.E. Adipocytes produce aldosterone through calcineurin-dependent signalling pathways: implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension. 2012;59:1069–1078. doi: 10.1161/HYPERTENSIONAHA.111.190223. [DOI] [PubMed] [Google Scholar]
- 86.Weidmann P., Beretta-Piccoli C., Ziegler W.H. Age versus urinary sodium for judging renin, aldosterone, and catecholamine levels: studies in normal subjects and patients with essential hypertension. Kidney Int. 1978;14:619–628. doi: 10.1038/ki.1978.171. [DOI] [PubMed] [Google Scholar]
- 87.Hegstad R., Brown R.D., Jiang N. Aging and aldosterone. Am J Med. 1983;74:442–448. doi: 10.1016/0002-9343(83)90971-3. [DOI] [PubMed] [Google Scholar]
- 88.Horstmeyer A., Licht C., Scherr G., Eckes B., Krieg T. Signalling and regulation of collagen I synthesis by ET-1 and TGF-β1. FEBS J. 2005;272:6297–6309. doi: 10.1111/j.1742-4658.2005.05016.x. [DOI] [PubMed] [Google Scholar]
- 89.Hafizi S., Wharton J., Chester A.H., Yacoub M.H. Profibrotic effects of endothelin-1 via the ETA receptor in cultured human cardiac fibroblasts. Cell Physiol Biochem. 2004;14:285–292. doi: 10.1159/000080338. [DOI] [PubMed] [Google Scholar]
- 90.Park J.B., Schiffrin E.L. ET(A) receptor antagonist prevents blood pressure elevation and vascular remodeling in aldosterone-infused rats. Hypertension. 2001;37:1444–1449. doi: 10.1161/01.hyp.37.6.1444. [DOI] [PubMed] [Google Scholar]
- 91.Ammarguellat F.Z., Gannon P.O., Amiri F., Schiffrin E.L. Fibrosis, matrix metalloproteinases, and inflammation in the heart of DOCA-salt hypertensive rats: role of ET(A) receptors. Hypertension. 2002;39:679–684. doi: 10.1161/hy0202.103481. [DOI] [PubMed] [Google Scholar]
- 92.Ebihara I., Nakamura T., Tomino Y., Koide H. Effect of a specific endothelin receptor A antagonist and an angiotensin-converting enzyme inhibitor on glomerular mRNA levels for extracellular matrix components, metalloproteinases (MMP) and a tissue inhibitor of MMP in aminonucleoside nephrosis. Nephrol Dial Transplant. 1997;12:1001–1006. doi: 10.1093/ndt/12.5.1001. [DOI] [PubMed] [Google Scholar]
- 93.Boffa J.J., Tharaux P.L., Dussaule J.C., Chatziantoniou C. Regression of renal vascular fibrosis by endothelin receptor antagonism. Hypertension. 2001;37:490–496. doi: 10.1161/01.hyp.37.2.490. [DOI] [PubMed] [Google Scholar]
- 94.Komatsumoto S., Nara M. Changes in the level of endothelin-1 with aging. Nihon Ronen Igakkai Zasshi. 1995;32:664–669. doi: 10.3143/geriatrics.32.664. [DOI] [PubMed] [Google Scholar]
- 95.Donato A.J., Gano L.B., Eskurza I. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–H432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tokunaga O., Fan J., Watanabe T. Endothelin. Immunohistologic localization in aorta and biosynthesis by cultured human aortic endothelial cells. Lab Invest. 1992;67:210–217. [PubMed] [Google Scholar]