Abstract
At the single-channel level, oxidation of the cardiac ryanodine receptor (RyR2) is known to activate and inhibit the channel depending on the level of oxidation. However, the mechanisms through which these changes alter the activity of RyR2 in a cellular setting are poorly understood. In this study, we determined the effect of oxidation on a common form of RyR2 regulation; store overload-induced Ca2+ release (SOICR). We found that oxidation resulted in concentration and time-dependent changes in the activation threshold for SOICR. Low concentrations of the oxidant H2O2 resulted in a decrease in the threshold for SOICR, which led to an increase in SOICR events. However, higher concentrations of H2O2, or prolonged exposure, reversed these changes and led to an increase in the threshold for SOICR. This increase in the threshold for SOICR in most cells was to such an extent that it led to the complete inhibition of SOICR. Acute exposure to high concentrations of H2O2 led to an initial decrease and then increase in the threshold for SOICR. In the majority of cells the increased threshold could not be reversed by the application of the reducing agent dithiothreitol. Therefore, our data suggest that low levels of RyR2 oxidation increase the channel activity by decreasing the threshold for SOICR, whereas high levels of RyR2 oxidation irreversibly increase the threshold for SOICR leading to an inhibition of RyR2. Combined, this indicates that oxidation regulates RyR2 by the same mechanism as phosphorylation, methylxanthines, and mutations, via changes in the threshold for SOICR.
Introduction
During excitation-contraction coupling, Ca2+ is released via the cardiac ryanodine receptor (RyR2) from the sarcoplasmic reticulum (SR) and provides the major source of Ca2+ for contraction. However, uncontrolled Ca2+ release through RyR2 is pathogenic. A number of mechanisms by which RyR2 function becomes compromised have been identified, including mutations within RyR2, mutations in proteins associated with RyR2, phosphorylation of RyR2, and oxidation of RyR2 (1). A unifying theory by which these modifications lead to disease is via an increase in store overload-induced Ca2+ release (SOICR), otherwise known as Ca2+ leak or spontaneous Ca2+ release (2, 3, 4, 5, 6). We and others have shown that SOICR occurs due to an increase in the luminal Ca2+ sensitivity of RyR2 and concomitant decrease in the SR Ca2+ concentration required to trigger RyR2 opening (2, 3, 4).
RyR2 contains ∼21 free cysteine residues per monomer or ∼84 thiols per channel (7), deemed to be susceptible to reactive oxygen species (ROS) mediated oxidation. It has been shown that RyR2 is oxidized in a number of forms of cardiac disease and leads to enhanced Ca2+ leak (8, 9, 10, 11). Oxidation-induced activation of RyR2 has been observed at the single-channel level (7, 12) and in cardiac myocytes, where the application of H2O2 leads to a rapid increase in Ca2+ sparks (13). RyR2 oxidation can occur on both sides of the channel with either resulting in an increase in channel open probability. Others have also shown that the presence of a reducing agent (dithiothreitol, DTT) can rapidly reverse the oxidation-induced increase in RyR2 open probability (12, 14).
However, oxidation of RyR2 appears to have a more complex effect on RyR2 function than simple activation of the channel. In addition to the rapid increase in RyR2 activity following its oxidation, some studies suggest that prolonged exposure to thiol oxidizing compounds can elicit a biphasic effect on the channel, reflected by the suppression of RyR2 activity following the initial activation (13, 15, 16). These data suggest that there are multiple groups of thiol-containing residues that can become modified, with those most susceptible to modification leading to an increase in RyR2 channel activity and those that require prolonged exposure leading to a loss of RyR2 activity (12, 17). Although the effect of acute oxidation of RyR2 appears to be reversed by reducing agents (12, 14), the reversibility of chronic oxidation and other forms of prolonged thiol modification of RyR2 (and RyR1) is less clear (11, 12, 18, 19).
We have recently shown that short-term exposure of cells expressing RyR2 to the oxidizing agent 2,2′-dithiodipyridine increases the activity of RyR2 by reducing the release threshold for SOICR (20). This suggests that the cellular mechanism by which oxidation leads to Ca2+ leak is a sensitization to luminal store Ca2+. However, whether the biphasic single-channel response of RyR2, to prolonged oxidation, results in similar biphasic changes in the release threshold for SOICR in intact cells remains unknown.
In this study, we determined the effect of prolonged oxidation of RyR2 by H2O2 on the release threshold for SOICR. We found that consistent with our previous data, initial oxidation increases Ca2+ leak by decreasing the release threshold for SOICR. After this initial decrease in the release threshold, we found that prolonged oxidation led to an increase in the release threshold for SOICR. The increase in the release threshold for SOICR was to such an extent that SOICR ceased. These data suggest that prolonged oxidation of RyR2 leads to a loss of RyR2 activity due to an increase in the release threshold for SOICR. We also assessed whether the change in SOICR could be restored to normal levels after the removal of the oxidative challenge or by the application of a reducing agent. We found that even short-term oxidation of RyR2 by high levels of H2O2 led to an initial decrease and then irreversible increase in the release threshold for SOICR in most cells. Our data reveal that the oxidation-mediated increase and subsequent decrease in RyR2 activity in intact cells is due to corresponding changes in the release threshold for SOICR.
Materials and Methods
Materials
All experimental materials were purchased from Sigma (Sigma Aldrich, St. Louis, MO) unless otherwise stated.
Methods
Generation of tetracycline-inducible human embryonic kidney cell lines: HEK293
Stable inducible HEK293 cells expressing RyR2 were generated as previously described (2).
Cytosolic Ca2+ measurements
Measurements of cytosolic Ca2+ were conducted in stable, inducible HEK293 cells expressing RyR2 as previously described (21). RyR2 expression was induced ∼18 h before measurements commenced via the addition of tetracycline (0.1 μg/ml). Cytosolic Ca2+ transients were measured after loading the cells with the acetoxymethyl ester (AM) form of the Ca2+ indicators, Fluo-4 (Thermo Fisher Scientific, North Shore City, New Zealand) dissolved in anhydrous dimethyl sulfoxide and 12% (w/vol) pluronic F127 (Thermo Fisher Scientific). Loading of Fluo-4 was carried out by incubating HEK293 cells in 0 Ca2+ Krebs-Ringer Hepes (KRH) buffer containing 125 mM NaCl, 5 mM KCl, 25 mM HEPES, 6 mM glucose, and 1.2 mM MgCl2, containing bovine saline albumin (1 mg/ml) adjusted to pH 7.4 with NaOH, and Fluo-4 AM dye (2 μM) for 8 min at room temperature. Cells were then continuously superfused with KRH solution containing various concentrations of CaCl2 (1 to 2 mM), H2O2 (0.01–1 mM), and DTT (10 mM) at room temperature. At the end of the experiments, 20 mM caffeine was applied to deplete the intracellular Ca2+ store. Fluo-4 AM dye was excited at 470 nm (40 nm bandwidth) every 2 s with an exposure time of 100 ms using a CoolLED system (Coherent Scientific Pty., Hilton, Australia). Fluorescence of Fluo-4 was detected through a longpass dichroic mirror (495 nm) and a longpass emission filter (>515 nm) by a CoolSNAP HQ2 charge-coupled device camera (Photometrics, Tucson, AZ). The cytosolic Ca2+ fluorescence is represented by F/F0, where F is the Ca2+ fluorescence intensity at any time and F0 is the average fluorescence intensity recorded in 0 Ca2+ KRH solution.
Luminal Ca2+ measurements
Stable, inducible HEK293 cells expressing RyR2 as described previously were used with the addition of transfection with D1ER cDNA. D1ER transfection took place 24 h before RyR2 induction. The cells were perfused continuously at room temperature with KRH containing various concentrations of Ca2+ (1 and 2 mM), H2O2 (0.01 to 1 mM), tetracaine (2 mM to block RyR2), and caffeine (20 mM to deplete the SR store). Fluorescence images of HEK293 cells were acquired every 2 s with an exposure time of 100 ms and excitation at 436 nm (20 nm bandwidth). The emissions of yellow fluorescent protein and cyan fluorescent protein were captured with the addition of a dual dichroic beamsplitter at 535 nm (40 nm bandwidth) and 480 nm (30 nm bandwidth), respectively. Two images of 170 × 256 pixels each were collected with a simultaneous dual-channel imaging system. The amount of fluorescence resonance energy transfer (FRET) was determined from the ratio of the emissions at 535 and 480 nm.
Statistical analysis
Results are presented as mean ±SE. For statistical analysis one-way or two-way analysis of variance was applied as appropriate. Differences were considered statistically significant if p < 0.05. All data analysis, curve fitting, and plotting were performed using GraphPad Prism 6 (GraphPad, La Jolla, CA).
Results
Oxidation has a biphasic effect on the frequency of SOICR
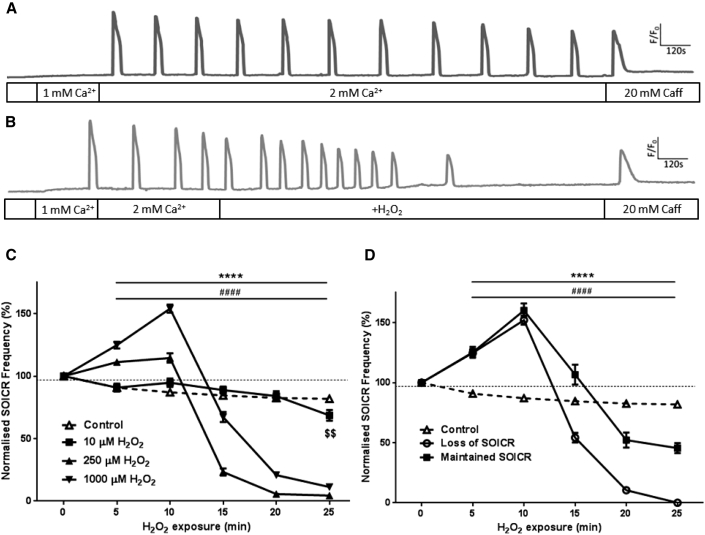
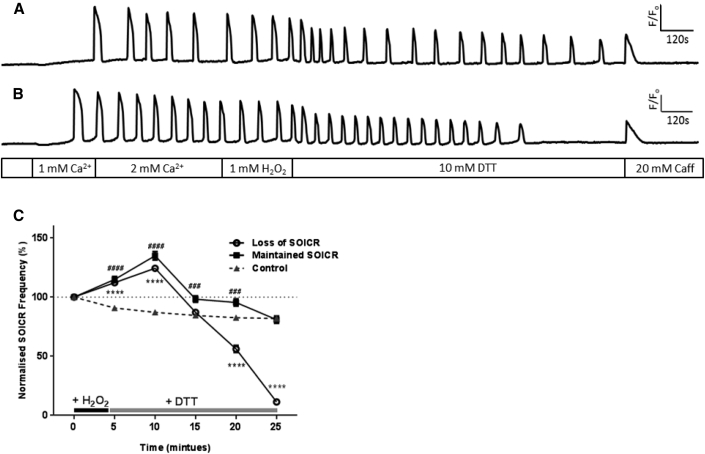
To assess the functional effect of RyR2 oxidation on SOICR, changes in SOICR frequency were observed in the presence of H2O2 (1 mM). H2O2 induced a biphasic effect on the activity of RyR2 (Fig. 1). Initially, within 10 min, a significant increase in the frequency of SOICR was observed, followed by a slight reduction in the frequency, and ultimately abrupt inhibition of SOICR after a prolonged time in H2O2 (Fig. 1 B). Within the first 5 min of H2O2 exposure the frequency of SOICR increased to 125 ± 2.7%, p < 0.0001 of control. By 10 min the frequency of SOICR reached 154 ± 3.2% of pre-H2O2 levels (p < 0.0001) (Fig. 1 C). However, further exposure to H2O2 led to a suppression of SOICR, which is clearly seen in Fig. 1 B by the abrupt loss of SOICR events. In the majority of cells there was only a small decrease in the frequency of SOICR (from 10 min onward) before it ceased (Fig. 1 B). The apparent gradual decline in SOICR frequency between the 15 to 25 min depicted in Fig. 1 C results from the complete loss of SOICR occurring at varying time points in different cells exposed to H2O2. SOICR ceased in 85.8% ± 6.89% (mean of inhibited cells per assay) of cells exposed to 1 mM H2O2 by 25 min. The mean time at which SOICR events ceased in these cells was 14.41 ± 0.74 min following initial H2O2 exposure. Because SOICR was not inhibited in all cells exposed to 1 mM H2O2, we performed separate frequency analysis on each of the two cell populations. As shown in Fig. 1 D both populations of cells showed the same initial increase in the frequency of SOICR in response to H2O2, suggesting that the initial increase in SOICR is independent of whether it is inhibited. The increase in frequency peaked (160 ± 5.8% and 152 ± 3.9% in cells where SOICR was maintained or lost, respectively) at 10 min post-H2O2 application. The reduction in the amplitude of SOICR depicted in Fig. 1 B is likely due to the loss and bleaching of the Fluo-4 dye rather than changes in the magnitude of Ca2+ release because the same trend can be seen in the nontreated cell (Fig. 1 A). However, it is also possible that prolonged treatment with H2O2 leads to the gradual depletion of the Ca2+ store and subsequently Ca2+ release. Of importance, loss of SOICR in cells exposed to H2O2 was not due to the complete depletion of the Ca2+ store as the application of 20 mM caffeine was able to elicit a release of store Ca2+ to a level comparable to the untreated cells (Fig. 1, A and B).
Figure 1.
H2O2 increases and then decreases the occurrence of SOICR. HEK293 cells stably expressing RyR2 were loaded with Fluo-4 in KRH buffer. Cells were superfused with KRH containing 0, 1, or 2 mM Ca2+, with or without various concentrations of H2O2. At the end of each experiment cells were perfused with 20 mM caffeine. Representative traces from cells treated without (A) or with 1 mM H2O2 (B). (C) The frequency of SOICR events, per 5 min bin, normalized to the frequency of events before the application of H2O2. (D) Separate frequency analysis of cells where SOICR was maintained or inhibited in cells exposed to 1 mM H2O2. Control n = 158, 10 μM n = 71, 250 μM n = 93, 1000 μM n = 162. Data shown are mean ± SE. $$ 10 μM p < 0.01; ∗∗∗∗ 250 μM, p < 0.0001; ##### 1000 μM, p < 0.0001; all compared to control (no H2O2).
Oxidation initially decreases the release threshold for SOICR in a concentration-dependent manner
To investigate the mechanism by which oxidation of RyR2 alters the frequency for SOICR, we monitored luminal Ca2+ directly using the luminally expressed Ca2+ indicator D1ER. We have previously used this technique to monitor how point mutations within RyR2 and proarrhythmic drugs such as caffeine alter the thresholds for SOICR (3, 4, 22). Those experimental data have demonstrated that the occurrence and magnitude of Ca2+ release was determined by both the release threshold for SOICR (the intracellular Ca2+ store level, expressed as a percentage of the total store, at which SOICR events occur (FSOICR), governed by the sensitivity of RyR2 to SR luminal Ca2+ (3)), and the termination threshold (the intracellular Ca2+ store level, expressed as a percentage of the total store, at which SOICR events terminate (FTermi), controlled by the inactivation of RyR2 due to partial depletion of SR Ca2+ (22, 23) or luminal Ca2+-dependent deactivation of RyR2 (24)). To establish a concentration-dependent effect of H2O2 on the release and termination threshold for SOICR, cells were exposed to a range of H2O2 concentrations (10 μM–1 mM) for 15 min.
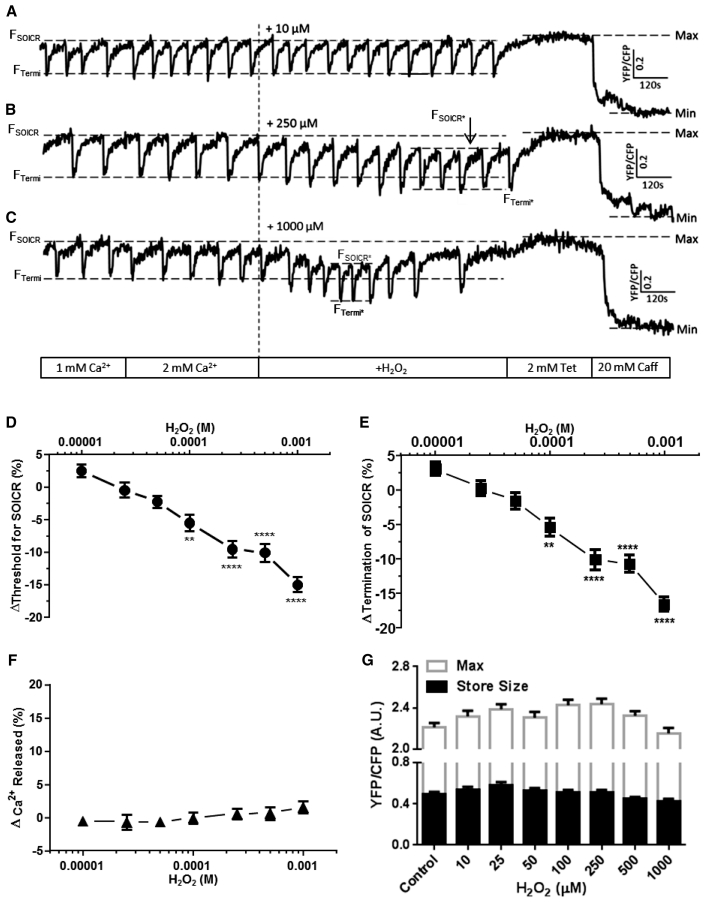
Control cells (in the absence of H2O2) exhibited a negligible change in the release or termination thresholds throughout the duration of an experiment (data not shown). Lower concentrations of H2O2 (10, 25, and 50 μM) also did not significantly affect the release or termination thresholds for SOICR (Fig. 2 A). The percentage change in threshold is characterized as the difference between the baseline release threshold (before H2O2 application for each condition, FSOICR) and the maximum change in release threshold following H2O2 addition (FSOICR∗). As the concentration of H2O2 surpassed 100 μM, both the release (FSOICR vs. FSOICR∗) and termination (FTermi vs. FTermi∗) thresholds for SOICR displayed significant decreases (100 μM H2O2; release threshold: −5.5 ± 1.25%, termination threshold: −5.4 ± 1.28%; both p < 0.01 vs. control) (Fig. 2, D and E). Because the thresholds for release and termination fell to the same extent under each condition, there was no change in the amount of Ca2+ released per SOICR event (Fig. 2 F). The largest decrease in the SOICR thresholds was observed when the cells were exposed to 1 mM H2O2 (release threshold −15.0 ± 1.14%, termination threshold: −16.5 ± 1.05%; both p < 0.0001 vs. control) (Fig. 2, C, D, and E). Of importance, the maximum store content (Max) and store size (Max-Min) were unaltered by H2O2 suggesting that the ability of SERCA to completely fill the store was not changed by H2O2 (Fig. 2 G). The lack of change in the magnitude of release and maximum store size confirms that the apparent reduction in magnitude of release when using Fluo-4 (Fig. 1, A and B), is due to photobleaching or loss of the dye rather than changes in the magnitude of Ca2+ release. During cytosolic Ca2+ imaging, an increased SOICR frequency was observed by 5 min, with the maximum increase occurring at ∼10 min (Fig. 1). These time-dependent changes in SOICR frequency coincide with the time at which H2O2 led to the maximum decrease in the release threshold for SOICR during luminal Ca2+ imaging. On average the release threshold was maximally decreased at 6.19 ± 0.218 min following 1 mM H2O2 addition. Interestingly, exposure to 1 mM H2O2 induced an additional change in the release threshold for SOICR from 10 min onward (Fig. 2 C), where both the release and termination thresholds began to increase. This effect, associated with prolonged exposure to high concentrations of H2O2, was further investigated below. The secondary change in SOICR seen during luminal Ca2+ imaging coincides with loss of SOICR seen during cytosolic Ca2+ imaging (Fig. 1). This suggests that the loss of SOICR mediated by H2O2 is also linked with modification of the release threshold for SOICR.
Figure 2.
H2O2 initially decreases the thresholds for SOICR. HEK293 cells stably expressing RyR2 were transfected with the luminally expressed FRET-based Ca2+ indicator; D1ER. The cells were superfused with KRH containing 1 or 2 mM Ca2+ and varying concentrations of H2O2 (10–1000 μM). At the end of each experiment cells were perfused with 2 mM tetracaine and 20 mM caffeine to determine the maximum and minimum store capacities, respectively. (A–C) Representative traces from cells exposed to 10, 250, and 1000 μM H2O2; dashed lines represent the release (before: FSOICR and after: FSOICR∗ H2O2) and termination (before: FTermi and after: FTermi∗ H2O2) SOICR thresholds or maximum (Max) and minimum (Min) store levels. (D–G) H2O2-mediated changes in the SOICR threshold for release, SOICR termination threshold, magnitude of Ca2+ release, and max store level and store size, respectively. Control n = 39, 10 μM n = 24, 25 μM n = 28, 50 μM n = 32, 100 μM n = 36, 250 μM n = 40, 500 μM n = 34, and 1000 μM n = 33. Data shown are mean ± SE. ∗∗p < 0.01; ∗∗∗∗p < 0.0001; compared to control (no H2O2).
Prolonged RyR2 oxidation increases the release threshold for SOICR
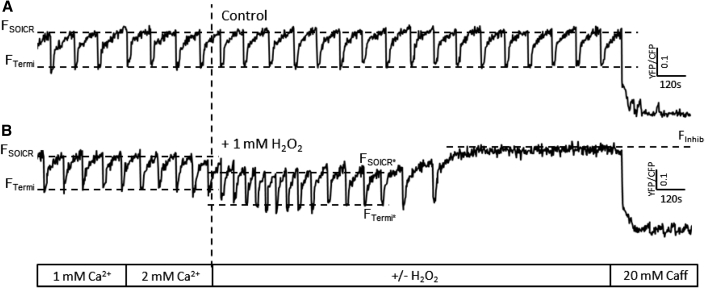
Cytosolic Ca2+ imaging showed that prolonged oxidation (15 min onward) was associated with the complete loss of SOICR activity in most cells. Given that we observed an increase in the release threshold for release during the luminal Ca2+ imaging, we hypothesized that loss of SOICR was due to an increase in the release threshold of SOICR. To investigate this, luminal Ca2+ imaging was performed over an extended time frame (25 min). Following the initial decrease in the release threshold (FSOICR∗) for SOICR, high concentrations (500 μM – 1 mM) of H2O2 caused both the release and termination thresholds to rise (see Fig. 3 B). This was followed by the complete loss of SOICR (FInhib) in 55.5 ± 3.44% of cells by 25 min (in 1 mM H2O2). In the absence of H2O2 there was no change in the thresholds for SOICR throughout the assay (Fig. 3 A). This suggests that prolonged oxidation can further modify the thresholds for SOICR and eventually lead to the complete loss of SOICR, which is consistent with the Fluo-4 data described previously (Fig. 1). Notably, when SOICR activity was lost, the level of luminal Ca2+ remains high, confirming that the loss of SOICR is not due to the depletion of luminal Ca2+ or inability of SERCA to fill the Ca2+ store. Given that SOICR is dependent on the activity of RyR2, store size, and filling, this shows that the loss of SOICR is attributable to the inactivity of RyR2. In the cells treated with high levels of H2O2, the presence of H2O2 increased the release threshold (FInhib) to the same extent as 2 mM tetracaine (maximum store level for cells treated with 2 mM tetracaine = 0.49 ± 0.02 versus maximum store level in cells with prolonged 1 mM H2O2 treatment and no tetracaine = 0.45 ± 0.03, p = 0.3), suggesting that prolonged RyR2 oxidation inhibits SOICR due to an increase in the release threshold for SOICR, a mechanism by which tetracaine itself inhibits RyR2 function and is also suggested to explain the loss of function of certain RyR2 mutations (2, 3, 25).
Figure 3.
Continued exposure to high concentrations of H2O2 increases the thresholds for SOICR. HEK293 cells stably expressing RyR2 were transfected with the luminally expressed FRET-based Ca2+ indicator; D1ER. The cells were superfused with KRH containing 1 or 2 mM Ca2+ with or without H2O2 (1 mM). At the end of each experiment cells were perfused with 20 mM caffeine to illustrate functional RyR2 channels. Representative traces of cells exposed to (A) 0 or (B) 1 mM H2O2; dashed lines represent the release (before: FSOICR and after: FSOICR∗ H2O2) and termination (before: FTermi and after: FTermi∗ H2O2) SOICR thresholds. FInhib (B) represents the store level after SOICR was inhibited by H2O2.
Brief exposure to H2O2 can result in an irreversible loss of SOICR
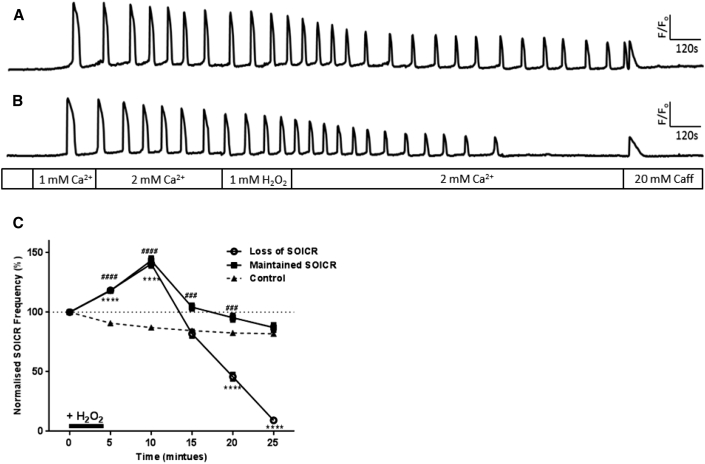
Because previous studies have found changes in RyR2 activity during oxidative stress to be partially reversible (11), we investigated if changes in SOICR mediated by short-term oxidation of RyR2 by high levels of H2O2 could be reversed when the oxidative challenge was removed. Cells were exposed to 1 mM H2O2 for 4.5 min as this previously resulted in a ∼25% increase in the frequency of SOICR (Fig. 1). As expected all cells exhibited a significant increase in SOICR frequency during the brief exposure to 1 mM H2O2 (Fig. 4). Following the increase in frequency, SOICR continued in 42.6 ± 8.38% of cells, whereas SOICR was completely inhibited in the remaining 57.4 ± 8.38% of cells (Fig. 4 C; Table 1). Again, given the distinctly different phenotypes we performed separate frequency analysis on each of the two cell populations. As can be seen in Fig. 4 both populations of cells showed a similar initial increase in the frequency of SOICR in response to H2O2. The increase in frequency peaked (143 ± 3.4% and 141 ± 3.2% in cells where SOICR continued or lost SOICR, respectively) ∼5 min following the removal of H2O2. This is very similar to the peak increase in frequency observed in cells continually exposed to H2O2 (154 ± 3.2%, Fig. 1). In those cells, which continued to experience SOICR, the frequency of SOICR returned to that of nontreated cells by 25 min post-H2O2 treatment. These data suggest that in roughly half of the cells, oxidation of RyR2 returned to control levels once H2O2 was removed, whereas in the other half the modification of RyR2 persisted.
Figure 4.
Short exposure to high [H2O2] leads to the inhibition of SOICR. HEK293 cells stably expressing RyR2 were loaded with Fluo-4 in KRH buffer. The cells were then transiently superfused with KRH containing 0, 1, or 2 mM Ca2+ and 1 mM H2O2 (4.5 min) followed by KRH alone (21.5 min). At the end of each experiment cells were perfused with 20 mM caffeine. Representative traces from cells where SOICR (A) was maintained or (B) was inhibited. (C) The frequency of SOICR events, per 5 min bin, normalized to the frequency of events before the application of H2O2. Cells treated with H2O2 are displayed as those where SOICR events were maintained or where SOICR ceased by the end of the experiment. Control n = 158, Loss of SOICR n = 148, Maintained SOICR n = 106. Data shown are mean ± SE. ∗∗∗∗ Loss of SOICR, p < 0.0001; ### and #### Maintained SOICR, p < 0.001 and <0.0001, respectively, compared to control (no H2O2).
Table 1.
Summary of H2O2-Mediated Inhibition of SOICR
| Persistent H2O2 | Removal of H2O2 | Posttreatment with DTT | Pretreatment with DTT | |
|---|---|---|---|---|
| Time to loss of SOICR (minutes) | 14.41 ± 0.74 | 17.23 ± 0.64a | 18.15 ± 0.85b | 12.32 ± 0.55, not significant |
| Loss of SOICR (% cells) | 85.8 ± 6.89 n = 5 | 57.4 ± 8.38, not significant, n = 5 | 72.52 ± 10.04, not significant, n = 5 | 73.9 ± 11.7, not significant, n = 8 |
Values are mean ± SE. Time and percentage of cells experiencing complete inhibition of SOICR. Time is given from the application of H2O2. Means and statistical analysis were calculated from the number of assays performed (n = 5 or 8) Total cells per condition were: 234, 148, 188, and 203 for persistent removal of H2O2, posttreatment with DTT, and pretreatment with DTT, respectively.
p < 0.05
p < 0.01 versus persistent H2O2
Oxidation-induced loss of SOICR cannot be reversed or prevented by reducing agents
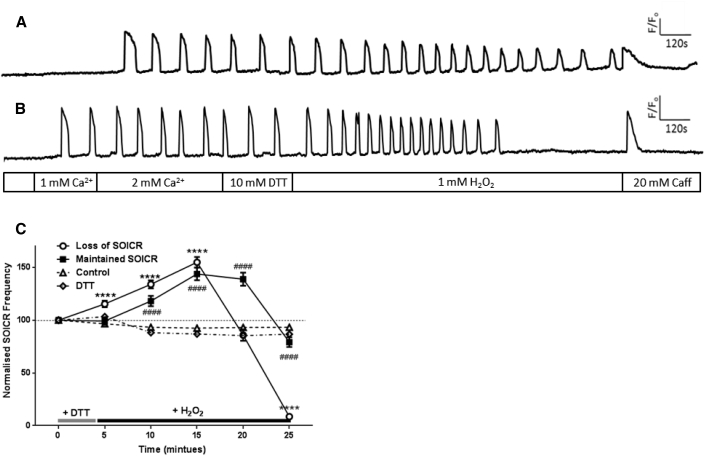
SOICR was inhibited in roughly half of the cells treated with H2O2 for 4.5 min, suggesting that endogenous cellular reducing agents were unable to reverse RyR2 oxidation. Thus, we next investigated whether the application of an exogenous reducing agent could prevent the loss of SOICR in these cells. Accordingly, cells were either treated with the reducing agent DTT immediately after H2O2 or were pretreated with DTT before H2O2.
As shown in Table 1, treatment of the cells with 10 mM DTT before or after exposure to H2O2 was not able to prevent the loss of SOICR with 72.52 ± 10.04% or 73.9 ± 11.7% of cells treated with DTT (post or pre, respectively) experiencing the complete loss of SOICR by 25 min. Although lower, the percentage of cells experiencing loss of SOICR was not significantly different in either condition to those cells persistently exposed to H2O2 (85.8 ± 6.89%, p > 0.05). Again, given the two distinctly different phenotypes (cells where SOICR ceased or continued) we analyzed each group separately (Figs. 5 and 6). In cells where SOICR ceased, treatment with DTT following H2O2 had no effect on the mean time to inhibition compared to cells where H2O2 was removed but no reducing agent was applied (18.15 ± 0.85 vs. 17.23 ± 0.64 min for cells treated with and without DTT, respectively, p > 0.05) (Table 1). However, compared to cells where H2O2 was present throughout the assay both removal of H2O2 and application of DTT delayed the mean time to inhibition of SOICR (Table 1). Interestingly, pretreatment of cells with DTT before the application of H2O2 did not protect against the loss of SOICR as the mean time to SOICR inhibition in the continued presence of H2O2 following DTT treatment was not different to cells exposed to H2O2 without DTT pretreatment (14.41 ± 0.74 vs. 12.32 ± 0.55 min, respectively) (Table 1). Because DTT had no effect on SOICR, we confirmed that the concentration of DTT used (10 mM) was sufficient to reduce other intracellular proteins. This was achieved by coexpressing the oxidation sensitive fluorescent protein roGFP2-Orp1 (26). Fig. S1 in the Supporting Material shows that 10 mM DTT rapidly reduces roGFP2-Orp1, indicating that the lack of effect on SOICR is not due to insufficient DTT (reducing capacity) entering the cell.
Figure 5.
Short exposure to high [H2O2] leads to irreversible inhibition of SOICR. HEK293 cells stably expressing RyR2 were loaded with Fluo-4 in KRH buffer. The cells were then transiently (4.5 min) superfused with KRH containing 0, 1, or 2 mM Ca2+ and 1 mM H2O2 followed by 10 mM DTT (21.5 min). At the end of each experiment cells were perfused with 20 mM caffeine. Representative traces from cells where SOICR (A) was maintained or (B) was inhibited. (C) The frequency of SOICR events, per 5 min bin, normalized to the frequency of events before the application of H2O2. Cells treated with H2O2 and DTT are displayed as those where SOICR events were maintained or where SOICR ceased by the end of the experiment (Loss of SOICR). Control n = 158, Loss of SOICR n = 188, Maintained SOICR n = 75. Data shown are mean ± SE. ∗∗∗∗ Loss of SOICR, p < 0.0001; ### and #### Maintained SOICR, p < 0.001 and <0.0001, respectively, compared to control (no H2O2).
Figure 6.
Pretreatment with DTT does not prevent H2O2-mediated inhibition of SOICR. HEK293 cells stably expressing RyR2 were loaded with Fluo-4 in KRH buffer. The cells were then transiently superfused with KRH containing 0, 1, or 2 mM Ca2+ and 10 mM DTT (4.5 min) followed by 1 mM H2O2 (21.5 min). Some cells were superfused throughout the assay with 10 mM DTT (DTT). At the end of each experiment cells were perfused with 20 mM caffeine. Representative traces from cells where SOICR (A) was maintained or (B) was inhibited. (C) The frequency of SOICR events, per 5 min bin, normalized to the frequency of events before the application of DTT. Cells treated with both DTT and H2O2 are displayed as those where SOICR events were maintained or where SOICR ceased by the end of the experiment (Loss of SOICR). Control n = 234, Loss of SOICR n = 203, Maintained n = 82, Persistent DTT n = 203. Data shown are mean ± SE. ∗∗∗∗ Loss of SOICR, p < 0.0001; ### and #### Maintained SOICR, p < 0.001 and <0.0001, respectively, compared to control (no H2O2).
Discussion
Acute oxidation of RyR2 has recently been shown to increase Ca2+ leak due to a reduction in the release threshold for SOICR (20). However, how chronic oxidation of RyR2 impacts SOICR is unknown. In this study we assessed the effect of acute and prolonged exposure to H2O2 on cells expressing RyR2. Our data demonstrate for, to the best of knowledge, the first time that prolonged oxidation of RyR2 leads to an increase in the release threshold for SOICR, which prevents further SOICR events. Interestingly, our data also show that even brief exposure to high concentrations of H2O2 can lead to loss of SOICR. This oxidation-mediated inhibition of SOICR was irreversible in most cells even when treated with DTT. Our data suggest that a high level of RyR2 oxidation leads to an irreversible increase in the release threshold for SOICR and underlies the inhibition of Ca2+ leak seen during extreme oxidative stress.
A major finding of this study is that the previously observed biphasic effect of oxidation of RyR2 (13, 15) in myocytes is due to a decrease and subsequent increase in the release threshold for SOICR. These data suggest that oxidation of RyR2 can modify RyR2 activity by a similar mechanism as mutations within RyR2, which depending on their location are known to increase or decrease the release threshold for SOICR (3, 25). Several molecular models have been proposed to explain the initial increase and then decrease in channel function due to oxidation. Salama and colleagues (17) have proposed that there are three closely related thiol groups. Disulphide formation between two groups leads to an increase in channel activity, whereas further oxidation leads to a switch in disulphide formation to a different pair of the three free thiols and closes the channel. Dulhunty and colleagues (16) suggest that RyR2 contains thiol groups within the channel pore that activate the channel (SHa) and a second group of thiols within the transmembrane domain that inhibit the channel (SHi). Zhang et al. offer an alternative model consisting of four states. Rapid oxidation of fast but low-affinity thiols leads to an increase in activity; oxidation of slow but high-affinity thiols has no impact on channel function but primes the channel for further oxidation (27). Although different mechanisms have been proposed, these molecular models all support that continued oxidation of RyR2 can lead to closure of the channel, which is also demonstrated by this study. However, here we propose that prolonged oxidation appears to increase the release threshold for SOICR leading to an eventual inhibition of SOICR, rather than an immediate cessation of SOICR. Our data suggest that extensive oxidation of RyR2 does not close the channel, rather it increases the release threshold for SOICR to such an extent that store Ca2+ is no longer able to drive SOICR events. This mechanism of RyR2 channel inhibition is consistent with that of tetracaine, which also prevents SOICR by increasing the release threshold for release due to a reduction in RyR2 open probability (2, 28).
The locations of the different thiol groups responsible for the changes in RyR2 activity remain elusive. The lack of crystal structure for RyR2 makes locating these sites difficult. By mutating cysteine 3602 to alanine, we were recently able to determine that this residue is not important in the effects of oxidizing agents on RyR2 (20). However, given the predicted number of free thiols within RyR2 (at least 21 (7)) this approach would require a concerted effort to individually characterize each potential site.
A second finding of this study is that the increase in the release threshold for SOICR following prolonged exposure to H2O2 is irreversible. In the majority of cells the increase in the release threshold and corresponding inhibition of SOICR could not be reversed even in the presence of high concentrations of a reducing agent (DTT). The inability of DTT to reverse the effect of oxidation suggests that the structural changes mediated by oxidation are to such an extent they cannot be reversed, this may be due to the inaccessible nature of these sites (11, 16). Interestingly, the inaccessibility of these sites suggests they could be buried within the protein. Methionine residues are commonly buried within a proteins structure due to the hydrophobic nature of their thiol-ether side chain; however, they are still readily oxidized by H2O2 in a pattern similar to that seen in vivo (29, 30). Therefore, although only cysteines have been implicated in RyR2 oxidation, the role of methionines should not be overlooked. Although direct regulation of RyR2 by methionine oxidation has not been reported, calmodulin (CaM) a protein that associates and regulates RyR2 is known to undergo methionine oxidation (31). The oxidation of CaM reduces its affinity for RyR2 leading to dissociation, destabilization of RyR2, and a subsequent increase in RyR2 activity (32). HEK293 endogenously express CaM (33), which would likely be dissociated by the high concentrations of H2O2 in our assays. However, the association of CaM with RyR2 appears to have no effect on the release threshold for SOICR (34), suggesting it is not responsible for any of the changes reported here. Additionally, HEK293 cells do not express other RyR2 interacting proteins known to alter RyR2 function (e.g., FKBP12.6 and calsequestrin); therefore, combined with the close agreement with single-channel studies of RyR2 thiol modification, we can be confident our data unveil the direct effect of RyR2 oxidation in an intact cell model.
In this study the increase in the threshold for SOICR was primarily observed at supraphysiological concentrations of H2O2, similar to those used by others studying RyR1 single channel function who also observed a biphasic response (18). However, the presence of endogenous reducing agents, such as glutathione, in our intact cell model will reduce the concentration of H2O2 to which RyR2 is exposed. Therefore, the changes in the threshold for SOICR are likely to be occurring at lower concentrations of H2O2 within the cell. The presence of endogenous reducing agents, particularly with respect to the reversibility of the effect of oxidation, highlights the importance of extending previous single channel data on RyR2 thiol modifications (by doxorubicin (19, 35)) and RyR1 oxidation (by H2O2 (18)) to an intact cell model, and suggests that thiol modification induced irreversible inhibition of RyRs observed at the single-channel level is not an artifact of working with purified RyR channels. Notwithstanding, the irreversible nature of oxidation-induced SOICR inhibition in situ suggests it may be a pathological form of regulation, because RyR2 activity could only be recovered through the replacement of the oxidized channels. Under normal levels of oxidative stress it is more likely to result in an increase in activity due to the decrease in release threshold. This is consistent with the greater levels of cytoplasmic Ca2+ seen in heart failure models where ROS production is increased. The increase in the release threshold for SOICR is only likely to occur where high level short term or prolonged exposure to oxidizing agents occurs. This effect has been reported to be one mechanism of anthracycline cardiotoxicity (35).
Another finding of our study is that even short-term exposure to high levels of oxidative stress can irreversibly inhibit SOICR. Interestingly, the timescale of the inhibition of SOICR due to the acute exposure to H2O2 does not appear to be much greater than that seen in cells chronically exposed to H2O2, occurring after the removal of H2O2. Why this delay between exposure to H2O2 and SOICR inhibition occurs is unclear because the removal of H2O2 should minimize continued RyR2 oxidation. However, given the large oxidative insult applied the delayed response could be attributable to ROS-induced ROS release (RIRR) (36). RIRR is known to occur following the application of exogenous oxidizing agents to cells (37). This RIRR response to acute oxidative stress has major implications because it suggests that the duration of the oxidative injury to the heart may not need to be sustained for long periods of time for irreversible modification of RyR2 to occur.
The basal oxidized state of RyR2 is controversial. Hanna et al. have shown that to mimic in situ channel activity single-channel recordings must be performed in mildly oxidizing conditions (38). This observation suggests that the dyad contains a greater level of ROS than the bulk cytosol, which is perhaps unsurprising given the localized production of superoxide by NADPH oxidase 2 (39) and the severely restricted diffusion of the dyad (40). Our data show that the threshold for SOICR can be both increased and decreased by ROS and indicate that in vivo changes in the localized ROS content will be able to tune the activity of RyR2 by causing small increases or decreases in the threshold for SOICR. This mechanism is akin to the proposed mode of RyR2 regulation by phosphorylation. Consistent with a lack of change in store Ca2+ content in healthy myocytes treated with DTT (11), the persistent application of DTT did not alter the frequency of SOICR (Fig. 6) or elicit a change in the release threshold for SOICR (data not shown) suggesting that, in our cell model and healthy myocytes, the basal levels of RyR2 oxidation are negligible or are at least insufficient to alter the activity of RyR2. This is also consistent with isolated RyR2 protein studies where the application of DTT does not alter RyR2 single-channel activity (35, 38). Similarly, pretreatment with DTT also had no impact on the effect on the subsequent addition of H2O2 (Fig. 6). This strengthens the notion that the basal levels of RyR2 oxidation are negligible in our cell model, because an already low oxidation state of RyR2 would not be meaningfully altered by DTT pretreatment.
In summary, we demonstrate that changes in RyR2 function observed at the single-channel level result in alterations in the release threshold for SOICR, which likely underlies both the increase and decrease in RyR2 function observed in cardiac myocytes during oxidative stress. Our results indicate that oxidation regulates RyR2 via the same mechanism as phosphorylation, methylxanthines, and RyR2 mutations, further supporting the importance of the thresholds for SOICR in regulating the channel.
Author Contributions
H.M.M.W. and P.P.J. designed the research; H.M.M.W., K.J.H., J.J.M., and J.C.M. performed the research; H.M.M.W., J.Z.Z., K.J.H., J.J.M., J.C.M., and P.P.J. analyzed the data and H.M.M.W., J.Z.Z., and P.P.J. wrote the article.
This work was supported by research grants from the Marsden Fund administered by the Royal Society of New Zealand [10-UOO-205] and the Lottery Health Research Board to P.P.J.
Acknowledgments
We thank Dr. Tobias P. Dick for providing the roGFP2-Orp1 cDNA.
Editor: Godfrey Smith.
Footnotes
Supporting Materials and Methods and one figure are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30239-9.
Supporting Material
References
- 1.Bers D.M. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu. Rev. Physiol. 2014;76:107–127. doi: 10.1146/annurev-physiol-020911-153308. [DOI] [PubMed] [Google Scholar]
- 2.Jiang D., Xiao B., Chen S.R. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc. Natl. Acad. Sci. USA. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones P.P., Jiang D., Chen S.R. Endoplasmic reticulum Ca2+ measurements reveal that the cardiac ryanodine receptor mutations linked to cardiac arrhythmia and sudden death alter the threshold for store-overload-induced Ca2+ release. Biochem. J. 2008;412:171–178. doi: 10.1042/BJ20071287. [DOI] [PubMed] [Google Scholar]
- 4.Kong H., Jones P.P., Chen S.R. Caffeine induces Ca2+ release by reducing the threshold for luminal Ca2+ activation of the ryanodine receptor. Biochem. J. 2008;414:441–452. doi: 10.1042/BJ20080489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J.Z., Waddell H.M., Jones P.P. Regulation of RYR2 by sarcoplasmic reticulum Ca(2+) Clin. Exp. Pharmacol. Physiol. 2015;42:720–726. doi: 10.1111/1440-1681.12364. [DOI] [PubMed] [Google Scholar]
- 6.Xiao B., Tian X., Chen S.R. Functional consequence of protein kinase A-dependent phosphorylation of the cardiac ryanodine receptor: sensitization of store overload-induced Ca2+ release. J. Biol. Chem. 2007;282:30256–30264. doi: 10.1074/jbc.M703510200. [DOI] [PubMed] [Google Scholar]
- 7.Xu L., Eu J.P., Stamler J.S. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 8.Marx S.O., Marks A.R. Dysfunctional ryanodine receptors in the heart: new insights into complex cardiovascular diseases. J. Mol. Cell. Cardiol. 2013;58:225–231. doi: 10.1016/j.yjmcc.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho H.T., Stevens S.C., Györke S. Arrhythmogenic adverse effects of cardiac glycosides are mediated by redox modification of ryanodine receptors. J. Physiol. 2011;589:4697–4708. doi: 10.1113/jphysiol.2011.210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belevych A.E., Terentyev D., Gyorke S. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc. Res. 2009;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terentyev D., Györke I., Györke S. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ. Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eager K.R., Roden L.D., Dulhunty A.F. Actions of sulfhydryl reagents on single ryanodine receptor Ca(2+)-release channels from sheep myocardium. Am. J. Physiol. 1997;272:C1908–C1918. doi: 10.1152/ajpcell.1997.272.6.C1908. [DOI] [PubMed] [Google Scholar]
- 13.Yan Y., Liu J., Cheng H. Bidirectional regulation of Ca2+ sparks by mitochondria-derived reactive oxygen species in cardiac myocytes. Cardiovasc. Res. 2008;77:432–441. doi: 10.1093/cvr/cvm047. [DOI] [PubMed] [Google Scholar]
- 14.Boraso A., Williams A.J. Modification of the gating of the cardiac sarcoplasmic reticulum Ca(2+)-release channel by H2O2 and dithiothreitol. Am. J. Physiol. 1994;267:H1010–H1016. doi: 10.1152/ajpheart.1994.267.3.H1010. [DOI] [PubMed] [Google Scholar]
- 15.Xie H., Zhu P.H. Biphasic modulation of ryanodine receptors by sulfhydryl oxidation in rat ventricular myocytes. Biophys. J. 2006;91:2882–2891. doi: 10.1529/biophysj.106.087338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eager K.R., Dulhunty A.F. Cardiac ryanodine receptor activity is altered by oxidizing reagents in either the luminal or cytoplasmic solution. J. Membr. Biol. 1999;167:205–214. doi: 10.1007/s002329900484. [DOI] [PubMed] [Google Scholar]
- 17.Abramson J.J., Salama G. Critical sulfhydryls regulate calcium release from sarcoplasmic reticulum. J. Bioenerg. Biomembr. 1989;21:283–294. doi: 10.1007/BF00812073. [DOI] [PubMed] [Google Scholar]
- 18.Favero T.G., Zable A.C., Abramson J.J. Hydrogen peroxide stimulates the Ca2+ release channel from skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1995;270:25557–25563. doi: 10.1074/jbc.270.43.25557. [DOI] [PubMed] [Google Scholar]
- 19.Ondrias K., Borgatta L., Ehrlich B.E. Biphasic effects of doxorubicin on the calcium release channel from sarcoplasmic reticulum of cardiac muscle. Circ. Res. 1990;67:1167–1174. doi: 10.1161/01.res.67.5.1167. [DOI] [PubMed] [Google Scholar]
- 20.Mi T., Xiao Z., Chen S.R. Role of Cys3602 in the function and regulation of the cardiac ryanodine receptor. Biochem. J. 2015;467:177–190. doi: 10.1042/BJ20141263. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J.Z., McLay J.C., Jones P.P. The arrhythmogenic human HRC point mutation S96A leads to spontaneous Ca(2+) release due to an impaired ability to buffer store Ca(2+) J. Mol. Cell. Cardiol. 2014;74:22–31. doi: 10.1016/j.yjmcc.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y., Tian X., Chen S.R. Abnormal termination of Ca2+ release is a common defect of RyR2 mutations associated with cardiomyopathies. Circ. Res. 2012;110:968–977. doi: 10.1161/CIRCRESAHA.111.256560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zima A.V., Picht E., Blatter L.A. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ. Res. 2008;103:e105–e115. doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukyanenko V., Wiesner T.F., Gyorke S. Termination of Ca2+ release during Ca2+ sparks in rat ventricular myocytes. J. Physiol. 1998;507:667–677. doi: 10.1111/j.1469-7793.1998.667bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang D., Chen W., Chen S.R.W. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc. Natl. Acad. Sci. USA. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutscher M., Sobotta M.C., Dick T.P. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H., Gomez A.M., Cheng H. ROS regulation of microdomain Ca(2+) signalling at the dyads. Cardiovasc. Res. 2013;98:248–258. doi: 10.1093/cvr/cvt050. [DOI] [PubMed] [Google Scholar]
- 28.Trafford A.W., Sibbring G.C., Eisner D.A. The effects of low concentrations of caffeine on spontaneous Ca release in isolated rat ventricular myocytes. Cell Calcium. 2000;28:269–276. doi: 10.1054/ceca.2000.0156. [DOI] [PubMed] [Google Scholar]
- 29.Keck R.G. The use of t-butyl hydroperoxide as a probe for methionine oxidation in proteins. Anal. Biochem. 1996;236:56–62. doi: 10.1006/abio.1996.0131. [DOI] [PubMed] [Google Scholar]
- 30.Hoshi T., Heinemann S. Regulation of cell function by methionine oxidation and reduction. J. Physiol. 2001;531:1–11. doi: 10.1111/j.1469-7793.2001.0001j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao J., Yin D.H., Squier T.C. Loss of conformational stability in calmodulin upon methionine oxidation. Biophys. J. 1998;74:1115–1134. doi: 10.1016/S0006-3495(98)77830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balog E.M., Norton L.E., Fruen B.R. Role of calmodulin methionine residues in mediating productive association with cardiac ryanodine receptors. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H794–H799. doi: 10.1152/ajpheart.00706.2005. [DOI] [PubMed] [Google Scholar]
- 33.Jung J., Nam J.H., Lee M.G. Dynamic modulation of ANO1/TMEM16A HCO3(-) permeability by Ca2+/calmodulin. Proc. Natl. Acad. Sci. USA. 2013;110:360–365. doi: 10.1073/pnas.1211594110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tian X., Tang Y., Chen S.R. Calmodulin modulates the termination threshold for cardiac ryanodine receptor-mediated Ca2+ release. Biochem. J. 2013;455:367–375. doi: 10.1042/BJ20130805. [DOI] [PubMed] [Google Scholar]
- 35.Hanna A.D., Lam A., Beard N.A. Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Mol. Pharmacol. 2014;86:438–449. doi: 10.1124/mol.114.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zorov D.B., Filburn C.R., Sollott S.J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieminen A.L., Byrne A.M., Lemasters J.J. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am. J. Physiol. 1997;272:C1286–C1294. doi: 10.1152/ajpcell.1997.272.4.C1286. [DOI] [PubMed] [Google Scholar]
- 38.Hanna A.D., Lam A., Dulhunty A.F. Cardiac ryanodine receptor activation by a high Ca2+ store load is reversed in a reducing cytoplasmic redox environment. J. Cell Sci. 2014;127:4531–4541. doi: 10.1242/jcs.156760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prosser B.L., Ward C.W., Lederer W.J. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 40.Jones P.P., Bazzazi H., Colyer J. Inhibition of cAMP-dependent protein kinase under conditions occurring in the cardiac dyad during a Ca2+ transient. Biophys. J. 2006;91:433–443. doi: 10.1529/biophysj.106.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.