Abstract
Accumulating evidence demonstrated that hydrogen sulfide (H2S) is highly involved in inflammation, oxidative stress, and apoptosis and contributes to the pathogenesis of kidney diseases. However, the role of H2S in cisplatin nephrotoxicity is still debatable. Here we investigated the effect of GYY4137, a novel slow-releasing H2S donor, on cisplatin nephrotoxicity in mice. Male C57BL/6 mice were pretreated with GYY4137 for 72 h prior to cisplatin injection. After cisplatin treatment for 72 h, mice developed obvious renal dysfunction and kidney injury as evidenced by elevated blood urea nitrogen (BUN) and histological damage. Consistently, these mice also showed increased proinflammatory cytokines such as TNF-α, IL-6, and IL-1β in circulation and/or kidney tissues. Meanwhile, circulating thiobarbituric aid-reactive substances (TBARS) and renal apoptotic indices including caspase-3, Bak, and Bax were all elevated. However, application of GYY4137 further aggravated renal dysfunction and kidney structural injury in line with promoted inflammation, oxidative stress, and apoptotic response following cisplatin treatment. Taken together, our results suggested that GYY4137 exacerbated cisplatin-induced nephrotoxicity in mice possibly through promoting inflammation, oxidative stress, and apoptotic response.
1. Introduction
cis-Diamminedichloroplatinum (cisplatin) is one of the most potent anticancer drugs and is widely used as the front-line therapy for the treatment of tumors of head, neck, lungs, and genitourinary tract [1]. However, the clinical application of cisplatin is limited by its serious adverse effects, particularly irreversible nephrotoxicity [2, 3], which occurred in about one-third of patients with cisplatin treatment [4]. Nephrotoxicity induced by cisplatin has been ascribed to several mechanisms, including inflammation, oxidative stress, and apoptosis [5, 6].
Hydrogen sulfide (H2S) is the third gasotransmitter besides nitric oxide (NO) and carbon monoxide (CO) and attracted more and more attention because of its pleiotropic physiological effects. Endogenous H2S is generated mainly from L-cysteine by two key enzymes named cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) [7]. Recently, emphasis has been placed on investigating whether H2S pathway is involved in different physiological or pathological processes by endogenous or exogenous perturbation. Exogenous H2S donor is reported to have renoprotective effects in prolonged warm renal ischemia-reperfusion injury [8], obstructive nephropathy [9], and gentamicin-induced renal injury [10]. The dysregulation of endogenous CBS or CSE also contributes to kidney ischemia-reperfusion injury [11] and diabetic nephropathy [12].
By reviewing the literatures, there exist inconsistent results about the effect of H2S on cisplatin-induced nephrotoxicity. One study showed that the inhibition of endogenous H2S production by DL-propargylglycine (PAG) could reduce renal damage induced by cisplatin through the restriction of inflammation in Wistar rats [13]. Oppositely, Ahangarpour et al. demonstrated that exogenous H2S donor sodium hydrosulfide (NaHS) was protective via antioxidant property in cisplatin-treated rats [14]. To address these controversies, we employed morpholin-4-ium 4-methoxyphenyl (morpholino) phosphinodithioate (GYY4137), a novel slow releasing H2S donor [15], to investigate the role of exogenous H2S in cisplatin-induced nephrotoxicity and the underlying mechanisms.
2. Materials and Methods
2.1. Materials
Cisplatin was purchased from Sigma-Aldrich (St. Louis, MO, USA). GYY4137 was purchased from Cayman Chemical Company (Ann Arbor, MI, USA), which was dissolved in 1 : 1 mixture of dimethyl sulfoxide (DMSO) and polyethylene glycol (PEG).
2.2. Animal Experiments
Male C57BL/6 mice (Jackson laboratory) aged 8–10 weeks old were maintained on a standard rodent chow with free access to food and water and were kept on a 12 h (light) : 12 h (dark) cycle. The animals were divided into three groups: control (CTR; n = 5), cisplatin alone (cp; n = 10), and cisplatin plus GYY4137 (cp + GYY4137, n = 10). Cisplatin was freshly prepared in ultrapure water at a concentration of 2 mg/mL and used to treat the mice by a single intraperitoneal (i.p.) injection (20 mg/kg), in both cp and cp + GYY4137 groups. GYY4137 was dissolved in 1 : 1 mixture of DMSO and PEG. The mice were pretreated for 72 h with a mixture of DMSO and PEG (CTR and cp groups) or GYY4137 (cp + GYY4137 group) at a dose of 21 mg/kg/d via a microosmotic pump (DURECT Corporation, Cupertino, CA, USA). To figure out the effect of GYY4137 alone on kidney morphology and function, another experiment was designed by the application of GYY4137 or vehicle to the mice for 6 days (n = 5 in each group). Animals were sacrificed after 72 h treatment of cisplatin or 6-day GYY4137 administration. Blood and kidney tissues were harvested for further analysis. All protocols employing mice were conducted in accordance with the principles and guidance of Sun Yat-Sen University Institutional Animal Care and Use Committee.
2.3. Renal Function and Histology
Blood urinary nitrogen (BUN) was determined to assess renal function. For histology, kidneys were fixed in 4% paraformaldehyde and stained with periodic acid-Schiff (PAS). The tissue damage was indicated by tubular lysis, dilation, disruption, necrosis, and cast formation. The degree of tissue damage was scored according to the percentage of damaged tubules as previously described: 0, no damage; 1, <25%; 2, 25–50%; 3, 50–75%; and 4, >75% [16].
2.4. Enzyme Immunoassay
The plasma TNF-α level was determined by an enzyme immunoassay kit (catalog number 559732, BD OptEIA, BD Biosciences, San Jose, CA, USA) according to the manufacturer's instructions.
2.5. Measurement of TBARS
The measurement of plasma thiobarbituric acid-reactive substances (TBARS) was based on the formation of malondialdehyde by using a commercially available TBARS Assay Kit (catalog number 10009055, Cayman Chemical) according to the manufacturer's instructions.
2.6. Western Blot Analysis
Isolated tissues were homogenized in ice-cold isolation solution with cocktail. The protein concentration was determined by Coomassie reagent. Protein lysates were denatured at 100°C for 10 min, separated by SDS-polyacrylamide gel electrophoresis, and transferred onto PVDF membranes. The bolts were blocked with 5% nonfat dry milk for 1 h and then probed with primary antibodies directed to CBS (Santa Cruz), CSE (Abcam), or β-actin (Sigma-Aldrich) overnight at 4°C, washed three times with Tris-buffered saline (TBS) containing 0.1% v/v Tween-20, and incubated for 1 h at room temperature (RT) with secondary antibodies (goat anti-rabbit IgG and goat anti-mouse IgG, Santa Cruz). The immunoreactive bands were visualized using chemiluminescent reagent (Thermo Scientific) and exposed to X-ray film. Resulting blots were scanned and quantified using Image-Pro Plus 6.0 software.
2.7. Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated using TRIzol (Invitrogen) and first-strand cDNAs were synthesized from 4 μg of total RNAs in a 20 μL reaction using Superscript (Invitrogen). The first-strand cDNAs served as the template for quantitative PCR (qPCR) performed in the Applied Biosystems 7900 Real Time PCR System using SYBR Green PCR reagent. Oligonucleotides were designed using Primer3 software (available at http://frodo.wi.mit.edu/primer3/) and the sequences are shown in Table 1. Cycling conditions were 95°C for 10 min, followed by 40 repeats of 95°C for 15 s, and 60°C for 1 min.
Table 1.
Sequences of qRT-PCR primers.
| Gene | Primer sequence | Accession number |
|---|---|---|
| GAPDH | 5′-GTCTTCACTACCATGGAGAAGG-3′ | M32599 |
| 5′-TCATGGATGACCTTGGCCAG-3′ | ||
|
| ||
| TNF-α | 5′-TCCCCAAAGGGATGAGAAG-3′ | NM_013693 |
| 5′-CACTTGGTGGTTTGCTACGA-3′ | ||
|
| ||
| IL-6 | 5′-CTTCCCTACTTCACAAGTCCGG-3′ | NM_031168 |
| 5′-GCCACTCCTTCTGTGACTCCAG-3′ | ||
|
| ||
| IL-1β | 5′-ACTGTGAAATGCCACCTTTTG-3′ | NM_008361 |
| 5′-TGTTGATGTGCTGCTGTGAG-3′ | ||
|
| ||
| SOD1 | 5′-AAGGCCGTGTGCGTGCTGAA-3′ | NM_921076 |
| 5′-CAGGTCTCCAACATGCCTCT-3′ | ||
|
| ||
| SOD2 | 5′-CGGCCTACGTGAACAATCTC-3′ | NM_013671 |
| 5′-GATAGCCTCCAGCAACTCTCC-3′ | ||
|
| ||
| SOD3 | 5′-TTCTTGTTCTACGGCTTGCTAC-3′ | NM_011435 |
| 5′-CTCCATCCAGATCTCCAGCACT-3′ | ||
|
| ||
| bak | 5′-CGCTACGACACAGAGTTCCA-3′ | NM_007523 |
| 5′-TCCATCTGGCGATGTAATGA-3′ | ||
|
| ||
| bax | 5′-TGCAGAGGATGATTGCTGAC-3′ | NM_007527 |
| 5′-GATCAGCTCGGGCACTTTAG-3′ | ||
|
| ||
| Caspase-3 | 5′-ACAGCACCTGGTTACTATTC-3′ | NM_009810 |
| 5′-CAGTTCTTTCGTGAGCAT-3′ | ||
|
| ||
| CBS | 5′-GGATTCCCCACATTACCACA-3′ | NM_012522 |
| 5′-CTGATGCGGTCCTTCACAC-3′ | ||
|
| ||
| CSE | 5′-ACCTTTGGCTCTGGGTGCT-3′ | NM_017074 |
| 5′-TCCTGAAGTGTTTCTCCATCC-3′ | ||
2.8. Statistical Analysis
All results were presented as means ± SE. The statistical analysis was performed using ANOVA followed by Bonferroni's test or unpaired Student's t-test with SPSS 13 statistical software. p < 0.05 was considered significant.
3. Results
3.1. GYY4137 Exacerbated Cisplatin-Induced Renal Dysfunction and Tubular Damage
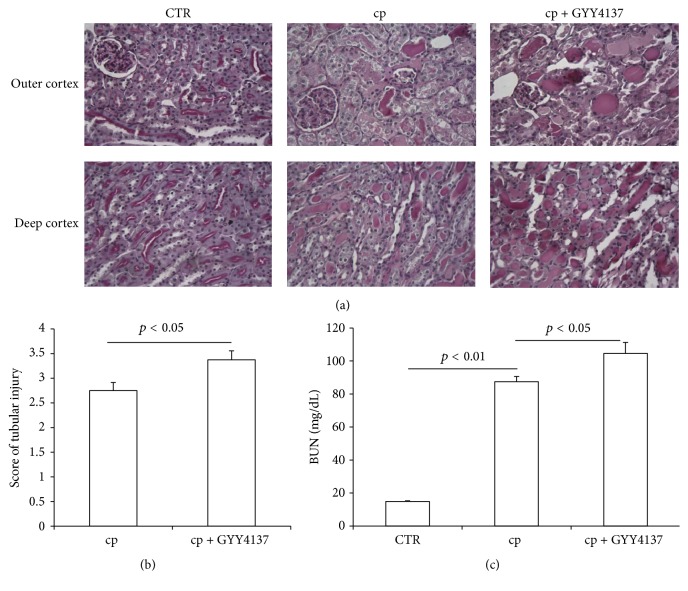
To evaluate the effects of GYY4137 on renal function in cisplatin-treated mice, we measured BUN level and found that BUN was robustly elevated in cisplatin-treated mice (cp: 87.4 ± 3.3 versus CTR: 14.8 ± 0.4 mg/dL, p < 0.01). However, GYY4137 administration resulted in a greater elevation of BUN (104.6 ± 6.6 mg/dL, p < 0.01, versus cp group) (Figure 1(c)). Next, we studied the tubular injury via a PAS staining. Microscopically, the mice treated with cisplatin displayed severe pathological changes, characterized by the distortion of the overall renal morphology, dilation of renal tubules, and appearance of protein casts. Remarkably, these histological changes were more severe in GYY4137-pretreated animals (Figures 1(a) and 1(b)). These data suggested that this H2S donor played a detrimental role in cisplatin-induced nephrotoxicity.
Figure 1.
Effects of GYY4137 on renal function and tubular injury following cisplatin treatment. (a) Representative images of periodic acid-Schiff staining (×400) of kidneys. (b) Tubular injury score. (c) BUN levels. CTR: control, n = 5; cp: cisplatin, n = 10; and cp + GYY4137: cisplatin + GYY4137, n = 10. Data are means ± SE.
3.2. GYY4137 Promoted Cisplatin-Induced Inflammation
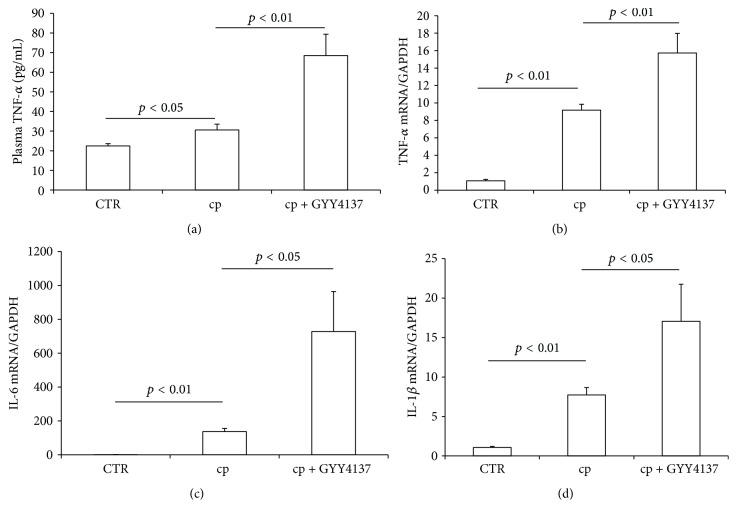
It is well known that the activation of inflammation was involved in the pathogenesis of cisplatin nephrotoxicity. Particularly, tumor necrosis factor-α (TNF-α) has shown a central role in mediating cisplatin-induced inflammation [17]. Therefore we measured the expression of renal TNF-α mRNA and circulating TNF-α level. As shown by the data, renal TNF-α mRNA was increased by 9.2-fold in cisplatin group as compared to the control mice, while GYY4137 pretreatment resulted in a much higher renal TNF-α mRNA expression than cisplatin alone group (Figure 2(b)). Consistent with the regulation of TNF-α in kidney, GYY4137 also promoted circulating TNF-α level after cisplatin treatment (Figure 2(a)). Meanwhile, the expressions of renal interleukin-6 (IL-6) and IL-1β mRNA showed similar patterns as renal TNF-α regulation (Figures 2(c) and 2(d)).
Figure 2.
Effects of GYY4137 on cisplatin-induced inflammatory response. (a) Enzyme-linked immunosorbent assay analysis of circulating TNF-α. (b) qRT-PCR analysis of renal TNF-α mRNA expression. (c) qRT-PCR analysis of renal IL-6 mRNA expression. (d) qRT-PCR analysis of renal IL-1β mRNA expression. CTR: control, n = 5; cp: cisplatin, n = 10; and cp + GYY4137: cisplatin + GYY4137, n = 10. Data are means ± SE.
3.3. GYY4137 Aggravated Cisplatin-Induced Oxidative Stress
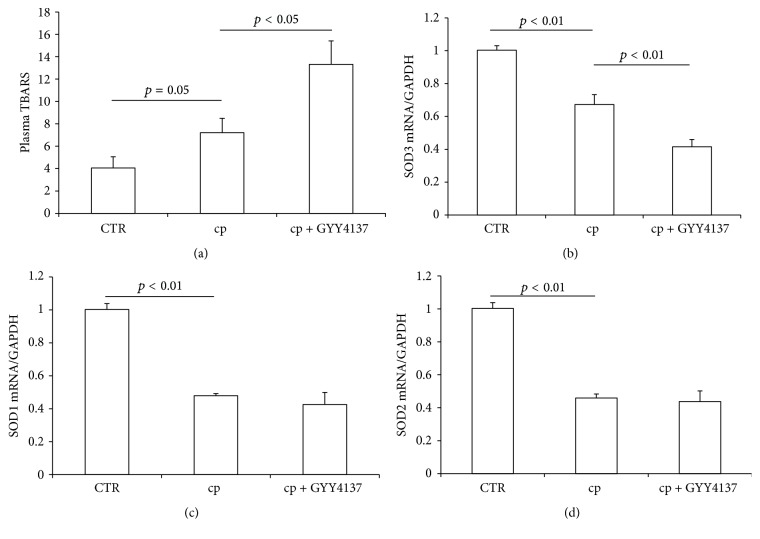
Oxidative stress is another important factor responsible for cisplatin nephrotoxicity besides inflammation [18, 19]. Therefore we assessed the effect of GYY4137 pretreatment on cisplatin-induced oxidative stress. The level of plasma TBARS, a marker of oxidative stress, was increased from 4.1 ± 1.0 (control group) to 7.2 ± 1.3 (cisplatin alone group), while the TBARS level was further increased to 13.3 ± 2.1 in GYY4137 plus cisplatin group (Figure 3(a)). Moreover, we found that the cisplatin-induced oxidative stress was associated with the downregulation of antioxidative enzymes superoxide dismutase 1 (SOD1), SOD2, and SOD3, among which SOD3 was further decreased by GYY4137. These findings indicated that GYY4137 pretreatment aggravated cisplatin-induced oxidative stress possibly via a suppression of SOD3 following a challenge of cisplatin.
Figure 3.
Effects of GYY4137 on cisplatin-induced oxidative stress. (a) Measurement of circulating thiobarbituric acid-reactive substances (TBARS) levels. (b) qRT-PCR analysis of renal SOD3 mRNA expression. (c) qRT-PCR analysis of renal SOD1 mRNA expression. (d) qRT-PCR analysis of renal SOD2 mRNA expression. CTR: control, n = 5; cp: cisplatin, n = 10; and cp + GYY4137: cisplatin + GYY4137, n = 10. Data are means ± SE.
3.4. GYY4137 Aggravated Cisplatin-Induced Apoptotic Response in the Kidney
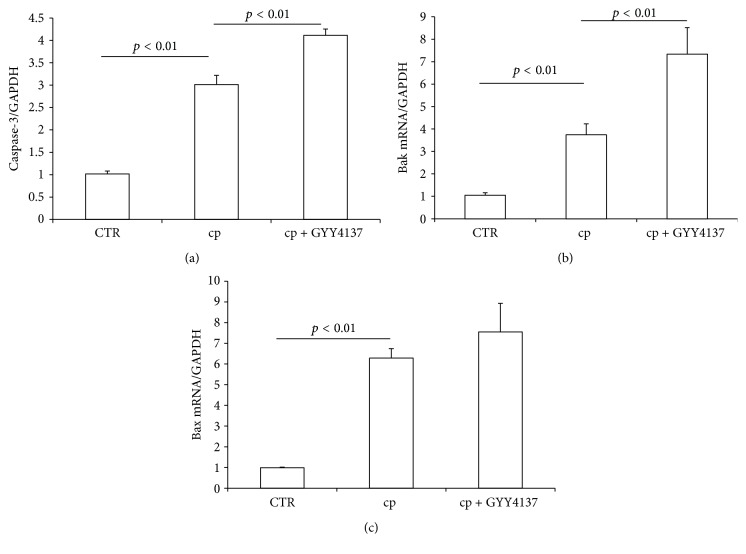
Apoptotic pathway is also reported to be a molecular mechanism of cisplatin-induced nephrotoxicity, and the Bak and caspase activation served as potential key elements [20, 21]. Here we measured renal mRNA expression of caspase-3, Bak, and Bax in mice from different groups. We found the enhanced mRNA expressions of caspase-3 and Bak after cisplatin treatment were further increased in GYY4137-pretreated animals (Figures 4(a) and 4(b)). In contrast, the induction of Bax showed no difference between cisplatin alone group and GYY4137 plus cisplatin group (Figure 4(c)). Overall, these data suggested that GYY4137 could aggravate apoptotic response in cisplatin nephrotoxicity.
Figure 4.
Effects of GYY4137 on cisplatin-induced apoptotic response. (a) qRT-PCR analysis of caspase-3 mRNA expression in kidney. (b) qRT-PCR analysis of Bak mRNA expression in kidney. (c) qRT-PCR analysis of Bax mRNA expression in kidney. CTR: control, n = 5; cp: cisplatin, n = 10; and cp + GYY4137: cisplatin + GYY4137, n = 10. Data are means ± SE.
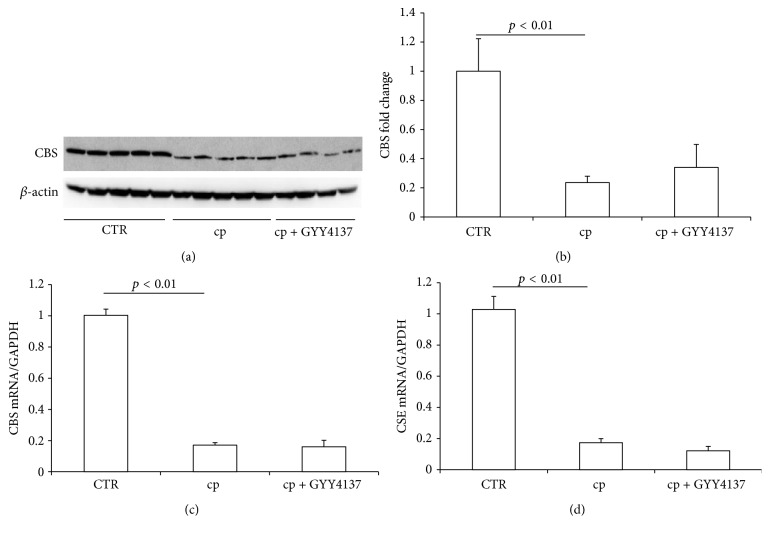
3.5. GYY4137 Treatment Had No Effect on the Downregulation of Renal CBS and CSE after Cisplatin Administration
To demonstrate whether GYY4137 affected the expression of endogenous H2S-producing enzymes, we detected the mRNA and/or protein levels of CBS and CSE in the kidney. Both CBS (Figures 5(a)–5(c)) and CSE (Figure 5(d)) were significantly decreased after cisplatin treatment, which was unaffected by GYY4137. Thus the reduction of CBS and CSE might act as a protective mechanism against cisplatin-induced renal injury.
Figure 5.
Effects of GYY4137 on CBS and CSE expression after cisplatin treatment. (a) Western blot analyses of CBS and β-actin in kidney. (b) Densitometry of CBS. A densitometric ratio between the densitometry of CBS and β-actin was calculated, and data are expressed in comparison with the controls. (c) qRT-PCR analysis of CBS. (d) qRT-PCR analysis of CSE. CTR: control, n = 5; cp: cisplatin, n = 10; and cp + GYY4137: cisplatin + GYY4137, n = 10. Data are means ± SE.
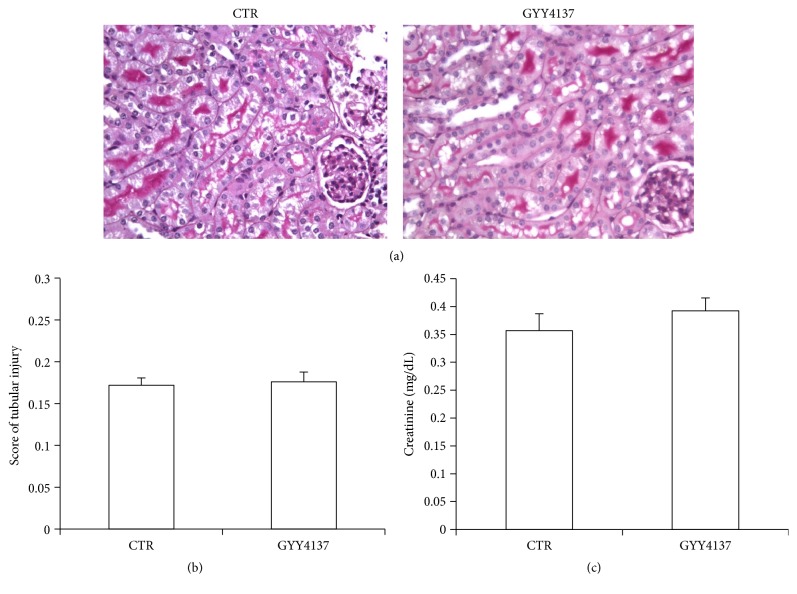
3.6. GYY4137 Alone Did Not Result in Renal Injury
To exclude the effect of the drug alone on renal structure and function, we set up the GYY4137 alone group and measured plasma creatinine and observed the morphological changes. The plasma creatinine level in GYY4137 group was not significantly different from that in CTR group (GYY4137: 0.392 ± 0.023 versus CTR: 0.357 ± 0.030 mg/dL, p > 0.05). Next, we studied the tubular injury via a PAS staining. Microscopically, the mice treated with GYY4137 did not display any pathological changes (Figures 6(a) and 6(b)). These data suggested that GYY4137 alone did not result in obvious nephrotoxicity.
Figure 6.
Effects of GYY4137 alone on renal morphology and function. (a) Representative images of periodic acid-Schiff staining (×400) of kidneys. (b) Tubular injury score. (c) Plasma creatinine levels. n = 5 in each group. Data are means ± SE.
4. Discussion
Hydrogen sulfide (H2S) has been known as a toxic gas with “rotten egg” smell for a long period of time. It shows its toxicity possibly via inhibiting mitochondrial cytochrome c oxidase (CcO) and oxidative phosphorylation and thus decreasing the production of adenosine triphosphate (ATP). However, growing evidence has demonstrated the biological and physiological importance of H2S. Its physiological relevance was firstly reported by Abe and Kimura where endogenous H2S serves as a neuromodulator in the brain and facilitates the hippocampal long-term potentiation by enhancing NMDA receptor-mediated responses [22]. In addition, H2S also displays important functions in cardiovascular system, kidney, liver, gastrointestinal, and endocrine systems [23–27]. In this regard, H2S has been widely acknowledged as the third gasotransmitter besides NO and CO. In kidney, H2S promotes urinary sodium excretion via both tubular and vascular mechanisms [25]. H2S can also inhibit renin-angiotensin system (RAS) by decreasing reactive oxygen species (ROS) generation [28] or cyclic adenosine monophosphate (cAMP) generation [29]. Meanwhile, H2S modulates renal oxidative stress response through upregulating antioxidant haem oxygenase-1 (HO-1) in human mesangial cells [30] and acts as an oxygen sensor in renal medulla [31]. Overall, accumulating evidence clearly demonstrated important roles of H2S in the kidney. Therefore, aberrant regulation of renal H2S might contribute to the pathogenesis of kidney diseases and thus modulation of endogenous or exogenous H2S could be potentially effective for treating renal diseases.
CBS and CSE are two major enzymes responsible for H2S production. Watanabe et al. reported that homozygous CBS mutants have about 40 times plasma homocysteine levels as normal and a majority of them died within 5 weeks after birth [32]. Mutant mice lacking CSE displayed pronounced hypertension in line with diminished endothelium-dependent vasorelaxation [33]. Decreased endogenous H2S production was reported to be associated with various diseases. The aortic H2S production was decreased by half in spontaneously hypertensive rats (SHRs) compared to normotensive rats and exogenous NaHS supplement attenuated hypertensive vascular collagen remodeling [34]. H2S deficiency also contributed to the progression of renal fibrosis. Endogenous H2S production was decreased during obstructive nephropathy and NaHS treatment attenuated unilateral ureteral obstruction- (UUO-) induced renal fibrosis [9, 35]. Moreover, recent report demonstrated that suppressed CSE/H2S pathway contributed to the pathogenesis of streptozotocin- (STZ-) induced DN [36]. Consistently, Zhou et al.'s results proved that H2S alleviated STZ-induced DN via attenuating oxidative stress and inflammation and inhibiting renin-angiotensin system activity [37].
Considering the anti-inflammatory and antioxidant effects of H2S, we tend to expect protective effects of H2S in kidney diseases. However, the facts are not that simple as expected. Inhibition of endogenous H2S formation by PAG (an irreversible inhibitor of CSE) could attenuate both cisplatin- and gentamicin-induced nephrotoxicities in rat models [13, 38–40], which suggested that endogenous H2S may aggravate kidney injury. In contrast, exogenous H2S donors, including NaHS and sodium thiosulfate (STS), were reported to protect against both cisplatin- and gentamicin-induced nephrotoxicities [10, 14, 41]. In consideration of these conflicting results, the role of H2S in kidney injury is hard to conclude and thus deserves further evaluation. In the present study, we employed a novel H2S donor, GYY4137, to study its role in cisplatin-induced nephrotoxicity. Our results showed that GYY4137 further exacerbated cisplatin-induced renal injury by aggravating inflammation, oxidative stress, and apoptosis in the kidney.
It is well accepted that inflammation is involved in the pathogenesis of cisplatin nephrotoxicity. Renal, circulatory, and urinary tumor necrosis factor-α (TNF-α) and other proinflammatory cytokines including interleukin 1β (IL-1β) were known to be upregulated by cisplatin injection [17, 42, 43]. TNF-α seemed to play a central role in the activation of inflammatory cascade since that inhibition of TNF-α via genetic or pharmacological approach strikingly attenuated cisplatin nephrotoxicity [17]. Our results showed a significant increase of circulating TNF-α and renal TNF-α mRNA expression after cisplatin administration. Meanwhile, renal IL-1β and IL-6 mRNA expression were remarkably increased in cisplatin group. These increments of inflammatory cytokines were further increased in cisplatin plus GYY4137 group, which indicated that GYY4137, a H2S donor, exacerbated the inflammatory response induced by cisplatin.
Oxidative stress is another known contributor of cisplatin-induced renal injury. Antioxidants are shown to be protective against cisplatin treatment both in cultured renal tubular cells [19, 44] and in animal models [45, 46]. Our results also showed an elevation of plasma TBARS level accompanied by significantly decreased renal expressions of SOD1, SOD2, and SOD3. Similar to GYY4137 effects on inflammation, this H2S donor aggravated oxidative stress in cisplatin-treated animals in line with a further reduction of SOD3. In agreement with promoted inflammation and oxidative stress, GYY4137 further enhanced the mRNA expressions of caspase-3 and Bak, suggesting that apoptotic response was also deteriorated.
Considering the aggravated effect of this H2S donor on cisplatin-induced renal injury, we detected the expression of endogenous H2S-producing enzymes in the kidney. The mRNA expressions of CBS and CSE were significantly decreased after cisplatin treatment, which was not affected by GYY4137. The protein level of CBS further confirmed this result. The previous study demonstrated the consistent results that GYY4137 increased the levels of H2S but had little effect on H2S-synthesizing activity [47]. The apparent downregulation of CBS and CSE probably would result in reduced H2S production, which might be a protective mechanism against cisplatin injury. In agreement with our findings, previous study showed that inhibition of endogenous H2S formation ameliorated the injury after cisplatin administration [13].
GYY4137 is a novel slow-releasing H2S donor while the conventional donors such as NaHS release H2S instantaneously in aqueous solution. Ahangarpour et al. reported that NaHS ameliorated the kidney dysfunction and damage in cisplatin-induced nephrotoxicity in Sprague-Dawley rats [14], which disagreed with our results. We think the difference of the species (mouse and rat) and/or H2S donors might result in the discrepancies. Bolus intravenous or intraperitoneal administration of GYY4137 to anesthetized rats increased plasma H2S concentration at 30 minutes and remained elevated during the 180-minute time course, while NaHS delivery to rats did not elevate plasma H2S levels [15]. Thus it is reasonable to expect that plasma H2S was increased and remained at a steady level in the current experiment with the use of microosmotic pump for GYY4137 delivery.
In summary, this study firstly examined the role of a novel exogenous H2S donor GYY4137 in a mouse model of cisplatin nephrotoxicity. Following cisplatin administration, the mice showed severe renal injury accompanied with increased oxidative stress, inflammation, and apoptotic response, which was further aggravated by GYY4137, suggesting a detrimental role of exogenous H2S in cisplatin-induced kidney injury in mouse.
Acknowledgments
This work was supported by grant from Scientist Development Grant from the American Heart Association (11SDG7480006), National Institutes of Health Grants DK104072 and DK094956, and VA Merit Review from the Department of Veterans Affairs.
Disclosure
Tianxin Yang is a Research Career Scientist in the Department of Veterans Affairs.
Competing Interests
The authors have declared that no conflict of interests exists.
References
- 1.Cohen S. M., Lippard S. J. Cisplatin: from DNA damage to cancer chemotherapy. Progress in Nucleic Acid Research and Molecular Biology. 2001;67:93–130. doi: 10.1016/s0079-6603(01)67026-0. [DOI] [PubMed] [Google Scholar]
- 2.Osanto S., Bukman A., Van Hoek F., Sterk P. J., De Laat J. A. P. M., Hermans J. Long-term effects of chemotherapy in patients with testicular cancer. Journal of Clinical Oncology. 1992;10(4):574–579. doi: 10.1200/JCO.1992.10.4.574. [DOI] [PubMed] [Google Scholar]
- 3.Meyer K. B., Madias N. E. Cisplatin nephrotoxicity. Mineral and Electrolyte Metabolism. 1994;20(4):201–213. [PubMed] [Google Scholar]
- 4.Beyer J., Rick O., Weinknecht S., Kingreen D., Lenz K., Siegert W. Nephrotoxicity after high-dose carboplatin, etoposide and ifosfamide in germ-cell tumors: incidence and implications for hematologic recovery and clinical outcome. Bone Marrow Transplantation. 1997;20(10):813–819. doi: 10.1038/sj.bmt.1700980. [DOI] [PubMed] [Google Scholar]
- 5.Matsushima H., Yonemura K., Ohishi K., Hishida A. The role of oxygen free radicals in cisplatin-induced acute renal failure in rats. Journal of Laboratory and Clinical Medicine. 1998;131(6):518–526. doi: 10.1016/S0022-2143(98)90060-9. [DOI] [PubMed] [Google Scholar]
- 6.Pabla N., Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney International. 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 7.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41(1):113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 8.Lobb I., Zhu J., Liu W., Haig A., Lan Z., Sener A. Hydrogen sulfide treatment ameliorates long-term renal dysfunction resulting from prolonged warm renal ischemia-reperfusion injury. Journal of the Canadian Urological Association. 2014;8(5-6):E413–E418. doi: 10.5489/cuaj.1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song K., Wang F., Li Q., et al. Hydrogen sulfide inhibits the renal fibrosis of obstructive nephropathy. Kidney International. 2014;85(6):1318–1329. doi: 10.1038/ki.2013.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Otunctemur A., Ozbek E., Dursun M., et al. Protective effect of hydrogen sulfide on gentamicin-induced renal injury. Renal Failure. 2014;36(6):925–931. doi: 10.3109/0886022X.2014.900599. [DOI] [PubMed] [Google Scholar]
- 11.Wang P., Isaak C. K., Siow Y. L., O K. Downregulation of cystathionine β-synthase and cystathionine γ-lyase expression stimulates inflammation in kidney ischemia-reperfusion injury. Physiological Reports. 2014;2(12) doi: 10.14814/phy2.12251.e12251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto J., Sato W., Kosugi T., et al. Distribution of hydrogen sulfide (H2S)-producing enzymes and the roles of the H2S donor sodium hydrosulfide in diabetic nephropathy. Clinical and Experimental Nephrology. 2013;17(1):32–40. doi: 10.1007/s10157-012-0670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francescato H. D. C., Cunha F. Q., Costa R. S., et al. Inhibition of hydrogen sulphide formation reduces cisplatin-induced renal damage. Nephrology Dialysis Transplantation. 2011;26(2):479–488. doi: 10.1093/ndt/gfq447. [DOI] [PubMed] [Google Scholar]
- 14.Ahangarpour A., Abdollahzade Fard A., Gharibnaseri M. K., Jalali T., Rashidi I. Hydrogen sulfide ameliorates the kidney dysfunction and damage in cisplatin-induced nephrotoxicity in rat. Veterinary Research Forum. 2014;5(2):121–127. [PMC free article] [PubMed] [Google Scholar]
- 15.Li L., Whiteman M., Guan Y. Y., et al. Characterization of a novel, water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide. Circulation. 2008;117(18):2351–2360. doi: 10.1161/circulationaha.107.753467. [DOI] [PubMed] [Google Scholar]
- 16.Wei Q., Dong G., Franklin J., Dong Z. The pathological role of Bax in cisplatin nephrotoxicity. Kidney International. 2007;72(1):53–62. doi: 10.1038/sj.ki.5002256. [DOI] [PubMed] [Google Scholar]
- 17.Ramesh G., Reeves W. B. TNF-α mediates chemokine and cytokine expression and renal injury in cisplatin nephrotoxicity. The Journal of Clinical Investigation. 2002;110(6):835–842. doi: 10.1172/jci200215606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agarwal A., Balla J., Alam J., Croatt A. J., Nath K. A. Induction of heme oxygenase in toxic renal injury: a protective role in cisplatin nephrotoxicity in the rat. Kidney International. 1995;48(4):1298–1307. doi: 10.1038/ki.1995.414. [DOI] [PubMed] [Google Scholar]
- 19.Schaaf G. J., Maas R. F. M., De Groene E. M., Fink-Gremmels J. Management of oxidative stress by heme oxygenase-1 in cisplatin-induced toxicity in renal tubular cells. Free Radical Research. 2002;36(8):835–843. doi: 10.1080/1071576021000005267. [DOI] [PubMed] [Google Scholar]
- 20.Park M. S., De Leon M., Devarajan P. Cisplatin induces apoptosis in LLC-PK1 cells via activation of mitochondrial pathways. Journal of the American Society of Nephrology. 2002;13(4):858–865. doi: 10.1681/ASN.V134858. [DOI] [PubMed] [Google Scholar]
- 21.Jiang M., Wang C.-Y., Huang S., Yang T., Dong Z. Cisplatin-induced apoptosis in p53-deficient renal cells via the intrinsic mitochondrial pathway. American Journal of Physiology—Renal Physiology. 2009;296(5):F983–F993. doi: 10.1152/ajprenal.90579.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abe K., Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. The Journal of Neuroscience. 1996;16(3):1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y.-H., Yan C.-D., Bian J.-S. Hydrogen sulfide: a novel signaling molecule in the vascular system. Journal of Cardiovascular Pharmacology. 2011;58(6):560–569. doi: 10.1097/fjc.0b013e31820eb7a1. [DOI] [PubMed] [Google Scholar]
- 24.Łowicka E., Bełtowski J. Hydrogen sulfide (H2S)—the third gas of interest for pharmacologists. Pharmacological Reports. 2007;59(1):4–24. [PubMed] [Google Scholar]
- 25.Xia M., Chen L., Muh R. W., Li P.-L., Li N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. Journal of Pharmacology and Experimental Therapeutics. 2009;329(3):1056–1062. doi: 10.1124/jpet.108.149963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magierowski M., Jasnos K., Kwiecień S., Brzozowski T. Role of hydrogen sulfide in the physiology of gastrointestinal tract and in the mechanism of gastroprotection. Postepy Higieny I Medycyny Doswiadczalnej. 2013;67:150–156. doi: 10.5604/17322693.1038356. [DOI] [PubMed] [Google Scholar]
- 27.Gai J.-W., Wahafu W., Guo H., et al. Further evidence of endogenous hydrogen sulphide as a mediator of relaxation in human and rat bladder. Asian Journal of Andrology. 2013;15(5):692–696. doi: 10.1038/aja.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue H., Yuan P., Ni J., et al. H2S inhibits hyperglycemia-induced intrarenal renin-angiotensin system activation via attenuation of reactive oxygen species generation. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0074366.e74366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu M., Liu Y.-H., Goh H. S., et al. Hydrogen sulfide inhibits plasma renin activity. Journal of the American Society of Nephrology. 2010;21(6):993–1002. doi: 10.1681/ASN.2009090949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D'Araio E., Shaw N., Millward A., Demaine A., Whiteman M., Hodgkinson A. Hydrogen sulfide induces heme oxygenase-1 in human kidney cells. Acta Diabetologica. 2014;51(1):155–157. doi: 10.1007/s00592-013-0501-y. [DOI] [PubMed] [Google Scholar]
- 31.Bełtowski J. Hypoxia in the renal medulla: implications for hydrogen sulfide signaling. Journal of Pharmacology and Experimental Therapeutics. 2010;334(2):358–363. doi: 10.1124/jpet.110.166637. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe M., Osada J., Aratani Y., et al. Mice deficient in cystathionine β-synthase: animal models for mild and severe homocyst(e)inemia. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(5):1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang G., Wu L., Jiang B., et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322(5901):587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao X., Zhang L.-K., Zhang C.-Y., et al. Regulatory effect of hydrogen sulfide on vascular collagen content in spontaneously hypertensive rats. Hypertension Research. 2008;31(8):1619–1630. doi: 10.1291/hypres.31.1619. [DOI] [PubMed] [Google Scholar]
- 35.Jung K.-J., Jang H.-S., Kim J. I., Han S. J., Park J.-W., Park K. M. Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochimica et Biophysica Acta. 2013;1832(12):1989–1997. doi: 10.1016/j.bbadis.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 36.Yuan P., Xue H., Zhou L., et al. Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrology Dialysis Transplantation. 2011;26(7):2119–2126. doi: 10.1093/ndt/gfq749. [DOI] [PubMed] [Google Scholar]
- 37.Zhou X., Feng Y., Zhan Z., Chen J. Hydrogen sulfide alleviates diabetic nephropathy in a streptozotocin-induced diabetic rat model. The Journal of Biological Chemistry. 2014;289(42):28827–28834. doi: 10.1074/jbc.m114.596593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Francescato H. D. C., Marin E. C. S., De Queiroz Cunha F., Costa R. S., Da Silva C. G. A., Coimbra T. M. Role of endogenous hydrogen sulfide on renal damage induced by adriamycin injection. Archives of Toxicology. 2011;85(12):1597–1606. doi: 10.1007/s00204-011-0717-y. [DOI] [PubMed] [Google Scholar]
- 39.Francescato H. D. C., Chierice J. R. A., Marin E. C. S., et al. Effect of endogenous hydrogen sulfide inhibition on structural and functional renal disturbances induced by gentamicins. Brazilian Journal of Medical and Biological Research. 2012;45(3):244–249. doi: 10.1590/S0100-879X2012007500016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dam V. P., Scott J. L., Ross A., Kinobe R. T. Inhibition of cystathionine gamma-lyase and the biosynthesis of endogenous hydrogen sulphide ameliorates gentamicin-induced nephrotoxicity. European Journal of Pharmacology. 2012;685(1–3):165–173. doi: 10.1016/j.ejphar.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 41.Dickey D. T., Wu Y. J., Muldoon L. L., Neuwelt E. A. Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. Journal of Pharmacology and Experimental Therapeutics. 2005;314(3):1052–1058. doi: 10.1124/jpet.105.087601. [DOI] [PubMed] [Google Scholar]
- 42.Li S., Gokden N., Okusa M. D., Bhatt R., Portilla D. Anti-inflammatory effect of fibrate protects from cisplatin-induced ARF. American Journal of Physiology—Renal Physiology. 2005;289(2):F469–F480. doi: 10.1152/ajprenal.00038.2005. [DOI] [PubMed] [Google Scholar]
- 43.Yousef M. I., Hussien H. M. Cisplatin-induced renal toxicity via tumor necrosis factor-α, interleukin 6, tumor suppressor P53, DNA damage, xanthine oxidase, histological changes, oxidative stress and nitric oxide in rats: protective effect of ginseng. Food and Chemical Toxicology. 2015;78:17–25. doi: 10.1016/j.fct.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 44.Xiao T., Choudhary S., Zhang W., Ansari N. H., Salahudeen A. Possible involvement of oxidative stress in cisplatin-induced apoptosis in LLC-PK1 cells. Journal of Toxicology and Environmental Health A. 2003;66(5):469–479. doi: 10.1080/15287390306449. [DOI] [PubMed] [Google Scholar]
- 45.Kuhad A., Tirkey N., Pilkhwal S., Chopra K. Renoprotective effect of Spirulina fusiformis on cisplatin-induced oxidative stress and renal dysfunction in rats. Renal Failure. 2006;28(3):247–254. doi: 10.1080/08860220600580399. [DOI] [PubMed] [Google Scholar]
- 46.Fatima S., Al-Mohaimeed N., Arjumand S., Banu N., Al-Jameil N., Al-Shaikh Y. Effect of pre- and post-combined multidoses of epigallocatechin gallate and coenzyme Q10 on cisplatin-induced oxidative stress in rat kidney. Journal of Biochemical and Molecular Toxicology. 2015;29(2):91–97. doi: 10.1002/jbt.21671. [DOI] [PubMed] [Google Scholar]
- 47.Tang B., Ma L., Yao X., et al. Hydrogen sulfide ameliorates acute lung injury induced by infrarenal aortic cross-clamping by inhibiting inflammation and angiopoietin 2 release. Journal of Vascular Surgery. 2016 doi: 10.1016/j.jvs.2015.10.010. [DOI] [PubMed] [Google Scholar]