Abstract
Vestibular function laboratories utilize a multitude of diagnostic instruments to evaluate a dizzy patient. Caloric irrigators, oculomotor stimuli, and rotational chairs produce a stimulus whose accuracy is required for the patient response to be accurate. Careful attention to everything from cleanliness of equipment to threshold adjustments determine on a daily basis if patient data are going to be correct and useful. Instrumentation specifications that change with time such as speed and temperature must periodically be checked using calibrated instruments.
Keywords: Vestibular, eye-tracking, vestibular ocular reflex, visual angle, caloric
Learning Outcomes: As a result of this activity, the participant will be able to (1) determine if vestibular function devices are working properly, (2) list what can be calibrated locally and what needs professional attention, and (3) list the calibration instruments needed.
Any medical device that measures physiologic parameters should be checked periodically to make sure that what is being generated or measured is accurate and free of artifacts. Vestibular stimulation and measurement requires a significant amount of electromechanical hardware driven by custom software. Valid stimuli and measurements require attention to the setup for each patient and periodic preventative maintenance and calibrations.
Some Relevant Historical Clinical Background
In 1914, Robert Barany was awarded the Nobel Prize in Physiology or Medicine for his work on the vestibular system.1 Among his notable accomplishments, he introduced the bithermal caloric test as a measure of vestibular function. By carefully observing vestibular-induced nystagmus, he demonstrated how contemporary clinicians could qualitatively assess semicircular canal function in patients complaining of dizziness or giddiness.
In the early half of the 20th century, qualitative observational assessment of vestibular function could be meaningfully replaced with quantitative measurement. With each technological advancement, our ability to accurately measure vestibular-mediated reflexes improved, and we subsequently became able to recognize new manifestations of vestibular disease. Early electronic milestones, such as cathode ray tubes, led to the development of biologic amplifiers and ink-based strip chart recorders. These tools allowed investigators to measure the amplitude and time course of field potentials with sensitivity and precision that could not be matched by the human observer. The electroencephalogram, the electromyogram, and the electroretinogram all became feasible. (See Regan2 for an historical review.) The presence of the corneal retinal potential (CRP) was appreciated in the 1800s. However, when electroretinograms were first being recorded using biological amplifiers, it soon became apparent that changes in the amplitude of the recorded potential could be affected by room illumination and eye position.3 Capitalizing on the latter, the electro-oculogram (EOG) became a useful tool for recording eye movement.
By the early 1960s, the EOG recording methods were combined with the Barany's bithermal caloric test to create the electronystagmography (ENG) test and various rotational tests.4 Measuring the vestibulo-ocular reflex (VOR) using EOG methods offered the same advantages of earlier biological recording methods: increasing sensitivity and precision of measurement. For instance, the magnitude of a unilaterally weak semicircular canal response could be quantified as an asymmetry percentage using the Jongkees unilateral weakness formula. However, there were many sources of variation in the ENG recording. VOR-based eye movements could be easily suppressed or modified by volitional eye movements or changes in mental state. Additionally, factors that influenced the EOG (room illumination, electrode placement and coupling, differential amplification settings, bandpass filters, and gain characteristics and calibration), and the caloric response (temperature, stimulus duration, flow rate, and medium [water versus air]; head position; medications and alertness levels; anatomic variation of the temporal bone) all must be understood and, where possible, controlled by the examiner to achieve an accurate test result. In this sense, the bilateral, bithermal caloric response is one of the more challenging reflexes to reliably measure in the typical clinical setting, particularly when electrode-based EOG methods are employed.
Fortunately, over the past several decades computerized digital video-based recording systems (videonystagmography [VNG]) have become commonplace. Video recording systems remove many of the technical contaminates of the older EOG methodology (e.g., calibration changes and artifactual tracing drift from changes in electrode impedance or room illumination). When eye movements are digitized, binocular measurement of horizontal and vertical eye movements can be quantified with higher levels of precision than the older EOG technology. VNG-based measurements of torsional eye movements and pupil constriction state also become feasible. Faster sampling rates broaden the effective bandpass of the recording system. These enhancements offer improved resolution of small eye movements in three orthogonal axes and better characterization of eye movement ballistics. There is every indication that digital eye movement recording methods will continue to improve, leading to more automated measurements with higher resolution and accuracy. Yet, these improvements in data resolution must be matched by improvements in calibration to yield meaningful data. Calibration matters even more as technology improves.
Stimuli used to assess vestibular function fall into three general categories: caloric stimuli (warm and cold air or water media); stimuli that control or modify head position (tables, rotating chairs, etc.), and visual stimuli. The complexity of each stimulus has evolved with improvements in video eye movement recording. For instance, when qualitative observational judgments of slow component velocity (SCV) were all that was available, ice water stimuli provided an acceptable choice for detecting the presence or absence of semicircular canal function. With EOG-based recording systems, slow component velocities could be quantified, making it possible to more carefully compare responses to warm and cool caloric irrigations, leading to the standard Jongkees caloric asymmetry calculation. Current caloric irrigators have the ability to measure the temperature of the caloric stimulus at the tip of the ear irrigator, and manufacturers have taken great pains to improve the accuracy and stability of both water and air caloric stimuli. Substantial gains in test accuracy are enhanced so long as the equipment is operating as designed.
VOR responses to head movement, induced by chair rotation, or head movements, require careful calibration of motion sensors and feedback controls. With the miniaturization of accelerometers and other motion-sensitive transducers, eye movement responses to vertical canal and otolith stimulation can be correlated with head movements in ways that were cost prohibitive just a few years ago.
Traditionally, eye movements induced by visual stimulation were used to detect brain diseases affecting the gaze holding, saccadic, optokinetic, or pursuit systems. Although these continue to be an important focus, we are likely on the cusp of a revolution in our ability to measure how all of the systems that converge on the oculomotor system produce eye and head movements in everyday activities. Virtual reality methods are finding their way into the vestibular clinic. Systems that allow the investigator to reconstruct eye, head, and postural changes as a person behaves in everyday situations have developed rapidly. Visual and vestibular integration will take on new meanings as these technologies find their way into the clinic.
Cleanliness and Sanitation of Laboratory Equipment
Each year, 1.7 million health care-associated infections occur in the United States, resulting in an estimated 99,000 preventable deaths.5 The vestibular laboratory is not typically considered a high-risk environment for acquisition of health care-associated infection. However, there are some activities that occur in the vestibular laboratory that increase the risk of infection. Standard infection prevention methods include environmental control (ensuring clean surfaces and fluids in the laboratory), using barriers to mitigate contact with a potentially infectious host, and managing activities that increase the risk of exposure (such as activities that may involve contact with blood or contact with mucus membranes). Although a comprehensive review of laboratory infection-prevention methods is beyond the scope of this article, several equipment maintenance factors can influence the relative risk of exposure associate with any given procedure. The reader is encouraged to review the Centers for Disease Control and Prevention (CDC) Web page on health care-associated infections at www.cdc.gov/hai for a more comprehensive treatment of this important issue.
The most important thing any clinician can do to prevent disease spread is to employ strict hand hygiene—wash your hands! Wash your hands before and after every patient contact, before preparing any preparation (electrode gel) or device (electrode) that will touch the skin or enter the ear canal, after you remove gloves, and when moving from a contaminated site (such as taking electrodes off of the forehead) to a clean site (the surface where you unwrap a new electrocochleography [ECochG] electrode for insertion).
When the hands or gloves are not visibly soiled, it is recommended that an alcohol-based hand rub be used for hand washing. Overuse of scrubbing with soap and water can degrade skin integrity over the course of the day. Soap and water washing is required, however, when there is visible soil, after using the restroom, and before eating. One should wash for at least 15 seconds, regardless of whether soap and water or an alcohol rub is used. This is as much for your protection as the patients.
The second most important thing to do is to decontaminate equipment and laboratory surfaces between each patient. Cleaning and disinfection are two related though separate activities in the decontamination process. Cleaning is the mechanical removal of material on a surface. Disinfection is the death or destruction of pathogens (bacterial, viral, fungal, etc.). A surface can be visibly clean, but still harbor pathogens. The decontamination of surfaces in the laboratory (examination tables, rotary chair surfaces) should be accomplished using a Food and Drug Administration (FDA)-approved germicide, following the guidelines of the institution or the FDA (see: http://www.cdc.gov/HAI/prevent/sd_medicalEquip.html for details.) Bleach-based disinfectants can be effective but are corrosive and can damage laboratory equipment. For the cleaning and disinfection of laboratory equipment, know and follow manufacturer guidelines for the cleaning and maintenance of equipment.
Certain procedures commonly employed in the vestibular laboratory increase the risk of disease transmission. These activities include water caloric irrigations, EcochG, and evoked potential testing in general. Caloric irrigation exposes the ear canal to distilled water. Water can serve as a medium for pathogens to enter the ear canal. Thus it is important to ensure that water used in caloric irrigation is sterile. Fluid that remains after caloric irrigation can facilitate the growth of pathogens already in the ear canal. The ear canal is vulnerable to microabrasions (both preexisting and iatrogenic) due to the absence of a fat pad under the skin in the proximal regions of the structure. Understanding the risk is important. Performing a caloric irrigation following mechanical removal of earwax or when there is an observable abrasion in the ear canal increases the risk for infection. Along the same lines, it is better to perform caloric irrigations prior to cleaning the ear canal for ECochG. Small abrasions from inserting an instrument or electrode into the ear canal may not be visible on subsequent otoscopic inspection.
Acute otitis externa is the most common health care-associated infections that can develop after water caloric irrigation. Far less commonly, wax removal or ECochG electrode insertion may facilitate the development of external otitis. The most common pathogen is Pseudomonas aeruginosa, although other bacteria or fungal pathogens may also be encountered.
Traumatic rupture of the tympanic membrane (TM) may occur as a result of inadvertent trauma. High water pressure at the tip of the caloric irrigator is a common cause and should be considered in systems where a disposable irrigator tip must be manually cut to a specific size. In these cases, an inadvertently small lumen at the irrigator tip can result in a thin stream of high-pressure water striking the eardrum, causing a rupture and subsequent otitis media. Traumatic rupture may also occur from water irrigation in the setting of a monomeric TM. Such eardrums are prone to rupture even with slight water pressures. A pretest tympanogram and careful otoscopic inspection can be helpful in avoiding this pitfall.
The vestibular laboratory will be frequented by patients vulnerable to infection, and they will be exposed to procedures that can increase their risk for health care-associated infections. Understanding and mitigating health care-associated infection risk is the clinicians' responsibility. Following the guidelines in the manufacturer user manual and becoming familiar with the CDC recommendations for decontamination are the intelligent first steps in meeting this responsibility.
Relevant Standards
Table 1 shows those standards most directly relevant to the calibration of instrumentation used to assess vestibular function. Most of these International Electrotechnical Commission (IEC) standards address safety issues and are not really specific to vestibular assessment, but rather to many/most if not all medical devices. The sole standard currently available from American National Standards Institute (ANSI)/International Organization for Standardization (ISO)/IEC that is directly related to the assessment of vestibular function is ANSI S3.45 (2009). Indeed, the title is a bit of a misnomer. The standard specifies six different tests that elicit eye movement from vestibular or visual stimulation.
Table 1. ANSI and International Standards Relevant to Vestibular Testing.
| ANSI S3.45 2009 | American National Standard: Procedures for Testing Basic Vestibular Function |
| IEC 60601 1-12 2005 | Medical electrical equipment: Basic Safety and Essential Performance |
| IEC 60601 1-2 2007 | Medical electrical equipment: General Requirements for basic safety and essential performance. Collateral standard. Electromagnetic Compatibility. Requirements and Tests |
| IEC 60601 1-6 2010 | Medical electrical equipment: Basic Safety: Usability |
| IEC 14971 2012 | Medical devices–Application of risk management to medical devices |
A detailed description of this standard goes beyond the scope of this article. The six tests included in this standard are spontaneous nystagmus, gaze-evoked nystagmus, saccade test, pursuit testing, positioning and positional nystagmus, and caloric testing. The reader interested in a more detailed description of these tests should refer to Jacobson and Shepard6 or Desmond.7 The most recent edition of this standard includes a description of VOG, as well as EOG. The first edition only discussed EOG. The spontaneous nystagmus test evaluates eye movements when there is no visual or vestibular stimulation and the head is fixed in place. Gaze-evoked nystagmus evaluates eye movements when the patient looks in various directions (four eccentric positions). The saccade test evaluates eye movements in response to rapid, random changes in the location of a visual target. The visual pursuit test evaluates the ability of a patient to follow (with their eyes) a (visual) target moving predictably through their visual field. Positional testing assesses eye movement in response to static placement of the head in various positions (relative to the gravity vector). Positioning assesses eye movements in response to dynamic, typically rapid changes in head position. Finally, caloric testing involves the assessment of the magnitude (often slow-wave velocity) to water (or air) irrigation to warm and cool stimuli in each ear. The first edition of this standard expressed a strong preference for water calorics. The current edition still expresses a preference for water calorics, but includes an Annex concerning air calorics.
Calibration Parameters and Devices
The owners of the clinical equipment have several choices when it comes to calibration: (1) never mind, it is good enough (bad choice); (2) our biomed department takes care of it; (3) we hire a calibration service to come in and check it; or (4) I check the equipment myself. If you use the equipment every day, it is important that you, as the person responsible for producing clinically accurate results, trusts that your equipment is working properly. Can you tell by listening to the hum of the equipment? Touching the equipment to see if it is too hot or too cold? Comparing your clinical results with what you expected them to be? Yes, but that is not enough. You must also make measurements of outputs using known inputs. That is what calibration is all about. Calibration puts a signal or substance into the instrument and measures what comes out. You will know from the instrument specifications and operator/service training what the instrument should be doing with that signal or substance.
Basic Instrumentation used for Calibration
▪ Tape measure
▪ Digital thermometer
▪ Calibration software specific for device
▪ Calibration instructions
▪ Air flow meter
▪ Water volume cylinder
▪ Stop watch
Temperature
For example, you want to determine if the temperature of the water exiting from a caloric irrigator is what you expect. First, you look at the temperature displayed on the instrument to know what the instrument indicates it is producing. Then you measure the actual temperature of the water when it exits the irrigator tip. To make this measurement, you could just put an oral thermometer into a large coffee cup and read the temperature on the thermometer after you irrigated into the cup. But that would be a bad idea. Why? First, the water temperature should be accurate to within 1°C of the preset temperature for the duration of the irrigation. Starting out with a cold bolus of water in the ear canal and followed by a hot bolus will distort the caloric response. So instantaneous temperature is important and that measurement requires a fast reacting (not an averaging) digital thermometer. Second, if you put the irrigation water into a large, cold ceramic coffee mug, that mug will be heated by the irrigation water, which in turn causes the irrigation water to lose heat quickly and give you an erroneous reading. A Styrofoam cup with low thermal mass (i.e., a good insulator) would be a better water collection container. You must also be sure that the thermometer you are using has the responsiveness, physical size, accuracy, range, and resolution necessary (see Table 2). ANSI S3.45 (2009) specifies warm water temperature at 44 ± 0.5°C for both closed-loop and open-loop delivery systems. Cool water temperature is specified as 27 ± 0.5°C for closed-loop systems and 30 ± 0.5°C for open-loop systems.
Table 2. Thermometer Characteristics.
| Value | Responsiveness | Accuracy (°C) | Range (°C) | Resolution (°C) | Cost (U.S.$) |
|---|---|---|---|---|---|
| Bad | 1°C in 5 s | 1 | 35–40 | 0.1 | 10 |
| Better | 1°C in 1 s | 0.5 | 0–100 | 0.1 | 200 |
| Best | 1°C in 0.2 s | 0.1 | 0–100 | 0.1 | 300 |
Time
Timing is important to make sure that events occur according to specification. If your VNG computer is multitasking because you have several applications running, or if the hard drive is getting full, then what you think is a 10-second recording could be taking 12 or 15 seconds. That means a 20 or 50% error in your nystagmus velocity measurements! So running slow is not just an inconvenience, it will generate errors. Another example: If your irrigator now takes longer to reach temperature than it used to, it could that mean that the tubing is clogged and restricting flow or that your heater elements are corroded and therefore not transferring heat as quickly. Accurate timing is critical to proper operation. What is important for a timer: ease of use, accuracy, and cost (see Table 3).
Table 3. Timer Characteristics.
| Value | Device | Ease of Use | Accuracy (s) | Cost (U.S.$) |
|---|---|---|---|---|
| Bad | Clock secondhand | Hard, look up/down | 2 | 10 |
| Better | Smartphone stopwatch | May take two people | 0.2 | 400 |
| Best | Sports stopwatch | Best, one hand operation | 0.1 | 40 |
Distance and Angle
When determining eye movements, distance of the patient from the calibration source must be known for accurate calibrations. A calibration distance error of just 3 in. can result in a velocity measure error of 8%. Putting the patient at a 30-degree recline position for a caloric test puts the lateral semicircular canal approximately vertical and thus generating the strongest response. To calibrate your exam table to 30 degrees, you will need a tape measure (see Table 4) and some knowledge of trigonometry (see Appendix 1).
Table 4. Subtended Angle Measurements.
| Distance | Device | Negative | Positive | Cost (U.S.$) |
|---|---|---|---|---|
| Bad | Yardstick | Not long enough | Does not bend | 1 |
| Better | Marked string | Stretches | Same every time | 1 |
| Best | Metal tape in centimeters | Need trig | Accurate | 5 |
Flow
A caloric irrigator stimulus transfers heat from the air/water medium to the subject. Flow rate and temperature are two critical parameters. Pumps wear out, tubing or filters becomes clogged, and hoses develop air leaks. Because you cannot easily sense that these parameters are changing, you must periodically check the irrigator flow rate.
Water flow rate can also depend on the relative height difference between the irrigator tank and the patient's ear. If the irrigator must pump the water up hill, flow rates can be reduced. Fortunately, the right and left ears will normally be at the same height. However, you will be producing a weaker caloric stimulus if flow rate is below that desired. Thus, you should calibrate a water irrigator at your typical irrigator to patient height.
Water irrigators pump at a calibrated flow rate over a defined period of time to put a specific total volume of water into the ear. Air irrigators pump air at a calibrated flow rate over a period of time that can be extended beyond a prescribed period. Thus, air flow rate (liters per minute) is specified and measured. The various options for measuring water or air flow are shown in Table 5. ANSI S3.45 (2009) specifies a flow duration of 40 ± 1 seconds for both closed-loop and open-loop delivery systems. Flow rate is specified as 350 ± 35 ml/min for closed-loop systems, and 200 ± 20 ml/min for open-loop systems.
Table 5. Options for the Measurement of Flow (Water and Air).
| Device | Negative | Positive | Cost (U.S.$) | |
|---|---|---|---|---|
| Water Flow | ||||
| Bad | Emesis basin | Not accurate | Available | 2 |
| Better | Measuring cup | Not accurate | Available | 2 |
| Best | Graduated cylinder | Must order | Accurate | 12 |
| Air Flow | ||||
| Bad | Listen to flow | Not accurate | Available | Free |
| Better | Anemometer | Difficult to use | Small flow stream | 150 |
| Best | Air flow meter | Difficult to use | Accurate | 50 |
Instructions and Training
There is a difference between determining that an instrument is operating within specifications and does not need calibrating as compared with actually calibrating an instrument. The former is the role that could and should be played by every responsible operator. Actual calibration where you must determine the cause of the mismatch between the specification and reality, and then fix it, requires more training and expertise. More often than not, that means your biomedical engineering department, factory-trained service technician, or the factory personnel must be used for repairs and calibrations. In the audiology field, vestibular included, service technicians are trained in schools and certified. Certification is granted after periodic training and testing by NASED (National Association of Special Equipment Distributors). More information about NASED can be found at www.nased.com.
Every device user's manual or product brochure includes device specifications including expected values and accuracy of those values. There are also common troubleshooting guides in user's manuals and service manuals to help users identify and fix many common problems that are more often user-caused than device-caused. There are also recommended cleaning and calibration intervals in the user's manual. Many device manufacturers also include troubleshooting software for users and calibration utilities for service technicians.
Calibration Instruments that Need Calibrating
Introduction
Instruments used to calibrate other instruments must also be calibrated. In other words, if you use a temperature meter to calibrate your caloric irrigator, then how do you know your temperature meter is accurate? The only way to know is to calibrate that temperature meter. That usually means sending the temperature meter back to the temperature meter factory on an annual basis for them to calibrate and to provide you with a certificate of calibration. Quality audit inspections by the FDA, consumer electronic regulatory bodies, and hospital joint commissions will check certificates of calibration.
Vestibular Calibration and Service Instruments
The following is a partial list of calibration devices that should be calibrated annually:
Voltmeters
Oscilloscopes
Temperature meters
Temperature sensors
Flowmeters
Specific Clinical Equipment
Water Caloric Irrigator
The water caloric irrigator basically heats and pumps water. During an ENG caloric test, the irrigator pumps a prescribed volume of water during a set time through a hose tip. Typically, commercial water caloric irrigators produce 250 mL of flow in 30 seconds. The examiner directs the water flow deep into the external ear canal to stimulate the lateral semicircular canal. Water temperatures used for testing are 7°C above (44°C) and 7°C below (30°C) normal body temperature (37°C). The warm water (relative to body temperature) stimulates and produces an upward convection current in the lateral semicircular canal, resulting in more rapid firing of hair cells neurons. These impulses increase in magnitude over a minute as heat permeates through the temporal bone. Impulses reach a peak response and then diminish in intensity; all in a matter of several minutes. This warm water stimulation is repeated for the opposite ear and the nystagmus response recorded. For comparison and to make sure the response can be reversed in direction, both ears are also stimulated with cool water. Peak nystagmus velocity measurements are compared using Jongkees formula to determine if the response from one ear is significantly weaker than the other ear.
Irrigator Functional Characteristics
Temperature accuracy
Temperature stability during irrigation
Time to temperature
Flow accuracy
The water caloric irrigator has historically been the preferred caloric stimulation medium (as compared with air) for decades because water is a great medium for transferring heat. The technical procedure of irrigation is quite straightforward because the water flushes into the external ear canal with no need for aiming in any apparent direction and the water simply drains out of the ear into a catch basin. Successful stimulation and determination of a weak ear is straightforward. However, the need for a water source, perforated eardrums, and the mess of spilled water makes water irrigation a second choice to air irrigation in some clinics. There will be more about air irrigators later.
Water Irrigator Use
The purpose of a water irrigator is to heat water to a precise temperature and to deliver that water through a hose into the external canal. Feedback to the operator from the irrigator includes: selected temperature, current temperature during heating, and a “ready” message indicating that the irrigator water temperature is stable. A valve starts and stops the flow of water and a timer tells the operator how much of the 30 seconds irrigation remains.
Calibration
Annual preventative maintenance and factory calibration of water irrigators is recommended. You want to make sure that the temperature and flow rate meet specifications. Water that is too warm or too cool may cause an asymmetrical warm/cool response or be uncomfortable to the patient. Although a temperature mismatch would not typically result in an errant caloric test interpretation, it does indicate a need for cleaning or calibration of the irrigator. Debris in the water tanks can clog tubing and filters, reducing water flow rate and resulting in a weak caloric stimulus.
In-House Calibration Checks
Water irrigators are easier to check for proper temperature and flow than an air irrigator. You the user will not be able to calibrate the water irrigator, but you can verify stimulus accuracy. Calibration verification equipment includes a graduated cylinder, a stopwatch, and a digital thermometer.
Using a stopwatch, determine how long it takes for the irrigator to heat the water to the desired temperature. Check that time against irrigator specs. A time that is too long may indicate a problem with the irrigator or possibly your water was very cold to start, thus requiring a longer heat cycle.
For both warm and cool irrigations and with the graduated cylinder positioned at a typical supine patient ear height, measure the flow volume produced during a complete irrigation; measure in seconds how long it takes to fill the cylinder up to the prescribed level. Compare that value with the irrigator specification. Repeat for the other temperature.
Water temperature in the ear should be stable and accurate. Some older model irrigators purged water from the hose before irrigation to bring the heated water up to the tip where it exits the hose. Newer irrigator designs pump the heated water up to the tip and then recirculate it back to the internal irrigator tank on a continuous basis before irritation. When the irrigation starts, the water in the handle is already at the correct temperature and is simply diverted into the patient's ear.
To check calibration using an insulated catch container, place the temperature probe in the container and when the irrigator has reached temperature and is ready, fill the container. The thermometer should have the capacity to measure temperature from 30 to 44°C in 0.1°C increments and to react quickly to temperature changes. Watch the temperature readout and note the temperature midway and after the irrigation is completed. The temperature should be within 1°C of the expected temperature at both points in time. If not, then the irrigator needs calibration. Repeat for the other temperature. One temperature point may be accurate and the other temperature point may be off. Sometimes a cold exam room or cold catch container may cool the warm water faster, indicating that the irrigator temperature is not correct. Repeat the calibration test keeping this in mind.
Air Caloric Irrigator
The air irrigator heats or cools room air and pumps the air through an ear speculum, directed by the examiner into the external ear canal while the examiner views the TM and aims the air stream. Air temperatures selected are 13°C below (24°C) and 13°C above (50°C) normal body temperature (37°C). In some irrigators, 48°C is used instead of 50°C for patient comfort. Air stimulus temperatures are more extreme than water and irrigations take longer to produce a nystagmus response equal to water. Air is a poorer medium for conducting heat. Similar to a water caloric irrigator, the clinical air caloric examination consists of a cool irrigation but for 60 seconds in each ear while measuring the nystagmus peak response for each ear followed by the warm air irrigation in each ear. Peak nystagmus velocities are compared to identify a weaker ear.
Functional Characteristics
Temperature accuracy
Temperature stability during irrigation
Time to temperature
Flow accuracy
Visibility of TM
The air caloric irrigator is a common and popular alternative to the water caloric irrigator. Early air caloric irrigator designs had difficulty cooling air below room temperature (and thus were not able to produce a cool caloric stimulus in a warm room), had difficulty maintaining accurate temperatures of the air where it entered the ear canal, and were large and noisy. Newer designs use Peltier electronic heaters and coolers in the irrigator handles and temperature sensors at the tip to regulate stimulation temperature. Air caloric irrigators are preferred over water in patients who may have a perforation of the TM to prevent contamination of the middle ear. Air caloric irrigators do not require a water supply or a means of catching or disposing of water after it drains from the ear canal. However, because air is a poorer heat transfer medium relative to water, the caloric irrigation requires a longer stimulation time to transfer the same amount of heat and the air must be accurately directed at the TM during the whole irrigation period. A damp external ear canal from draining can result in a reversed nystagmus from inadvertent cooling during warm air irrigations. A lighted otoscope with magnification as part of the irrigator handle makes directing the air stream easier. Because the air must be directed toward the TM during irrigation, the examiner must view each ear canal orientation and identify any wax impaction using the irrigator otoscope without air flow.
Air Irrigator Use
The purpose of an air irrigator is to cool or heat room air to a precise temperature and to deliver that air at a constant flow rate through a speculum directed by the examiner at the TM for a fixed period of time. The operator can also change the set temperature, raising or lowering it (within safety limits) to achieve the desired stimulus. Feedback to the operator includes the actual air temperature, when the exiting air temperature is correct and stable, and how much time has elapsed during the irrigation. Air flows continuously after the irrigator temperature has been selected and stimulation can start and be continued after the elapsed time if the examiner desires—for example, if the air stream temporarily moved off the TM. You do not want to stimulate the ear with air when inspecting the canal for wax impaction. So turn off the air and use only a lighted otoscope for inspection.
Calibration
Annual preventative maintenance and calibration is recommended for air caloric irrigators. You want to make sure that the air flow rate and temperature are within specifications. An internal water tank with a water pump is part of the Peltier heater/cooler in the irrigator handle. This tank is filled only with distilled water to prevent sediment, oxidation, and clogging of internal pumps and tubes. An in-line water filter traps debris, and this filter must be checked and replaced when sufficient debris reduces flow. Water is retained in this closed system and only loses volume with osmotic evaporation. Thus, the distilled water level should be checked and topped off as needed.
In-House Calibration Checks
Air caloric irrigators are difficult to check in the field. Air flow rate should be measured with a flowmeter tube connected to the irrigator speculum. The flowmeter typically uses a small ball that floats in an air stream with gravity pulling the ball down and air flow pushing the ball up. Markings on the wall of the flowmeter indicate the flow rate. The flowmeter should indicate flow rates up to 20 L per minute in 0.5 L/min increments. Care should be taken to have a good air seal so all of the air from the speculum goes through the flowmeter.
Irrigator air temperature is measured at the speculum tip in the center of the air stream. This requires a very small fast-reacting resistance temperature detectors (RTD) sensor and a digital temperature display with readings in 0.1°C increments. Care must be made to position the RTD in the air stream and mechanically hold it there during the measurement. Check both warm and cool temperatures. Calibration room temperature should be around 72°F (22°C) and humidity below 80% when making these calibration measurements. If the irrigator appears to be out of specifications, send the irrigator to the factory for checks and calibration.
Perform temperature and flow checks twice a year or if a problem is suspected. Flow rates can change if the irrigator handle is dropped and the air seal in the handle breaks and leaks air. To identify leaks, put your ear on the handle and listen for air leaks as you gently push the otoscope head to the side. Leaks must be repaired in the factory.
Use a stopwatch and measure how long it takes for the irrigator to heat the air to the desired, stable temperature. Check that time against irrigator specifications. Too long may indicate a problem with the irrigator.
Inspect the hose and handle for wear. The hose contains air and water tubes and wires that carry power and signals. The handle contains electronic circuits. Thus damage to the hose or handle should preclude clinical use. In other words, repair the irrigator.
Speculums are single use. One large speculum may be needed to view the TM and a smaller speculum to finely direct air flow. To reduce the spread of infection, dispose of used speculums after each patient.
Electro-Oculography Amplifier
EOG records eye movements in the horizontal and vertical directions using skin electrodes that pick up and amplify the eye's CRP. The EOG is becoming a legacy device with the acceptance of video goggles that track eye movements. However, in some patients, especially the very young and very old, the EOG is the only technology that can successfully be used to obtain eye movement recordings. The most common configuration of the EOG utilizes independent left and right eye horizontal electrode pairs and one pair of vertical electrodes above and below one eye with a common lead on the forehead. Lead wires consist of a safety end where the metal pin is not exposed and thus cannot inadvertently be plugged into a dangerous alternating current (AC) outlet. The other electrode wire end has a snap for attachment to a disposable one-use adhesive skin electrode. One reason that EOG is not used more frequently is the cost of the disposable electrodes, the skin prep time, and eye movement signals that are contaminated with muscle noise artifacts. However, there is a place for these EOG devices and the current ANSI ENG standard (ANSI S3.45 2009) specifies their functional requirements and correct application.
Functional Characteristics
Electrodes—disposables
Upper cutoff frequencies (24, 40 Hz)
Lower cutoff frequency (direct current [DC], 0.005 Hz)
Drift correction
Common mode rejection ratio (CMRR)
Eye movement resolution
Electrode impedance measurement
Much debate surrounded correct specifications and use of EOG amplifiers in the 1980s and 1990s. The vertical channel is important for recording eye blinks so as not to mistake eye blinks that contaminate the horizontal channel as actual nystagmus. Two horizontal channels were recommended if the patient is suspected of disconjugate eye movements; otherwise, a bitemporal recording with one horizontal channel is sufficient. Amplifier bandwidth settings are important too. The lower setting should pass DC signals to produce a steady eye position trace when a patient is gazing at an offset target. Changing from DC to a very low-frequency (0.005 Hz) AC high-pass filter can be used to reduce electrode drift. Drift is a common problem with electrodes especially if the skin impedance is high from poor skin prep. On the top end, the high-pass filter is commonly set to 24 Hz for every test except the saccade test. For that test, the high-pass filter is increased to 40 Hz, allowing a more accurate representation of saccade waveform morphology. Environmental electrical noise is everywhere, especially the 60-Hz alternating current (AC) from power lines. In many parts of the world, AC power is 50 Hz. To circumvent noise, EOG amplifiers use a differential amplifier on the front end with the ability to reject high-amplitude environmental noise that is common to both lead wires but still amplify the differential eye signal on those same two lead wires. This amplifier spec is the CMRR and is measured in decibels. The higher the CMRR, the better.
The eye position signal is never 0 V, even if the patient is looking straight ahead due to the half-cell1 offset potential on the electrodes. With amplifiers set to a DC low-pass filter setting, the half-cell potential is electronically subtracted by a sample-and-hold amplifier early in the amplifier before the offset is amplified too much. This subtraction is the result of the user telling the amplifier to center the trace by the push of a button or with a software-generated centering signal. Centering is also called “zeroing the trace.” Centering the trace is also used to remove the constant drift of the signal in some patients. Drift looks like a long nystagmus beat and it contaminates the waveform and makes interpretation difficult. Drift is often caused by one electrode of a pair that is not making good skin contact. Electrode impedance testing is a way to identify these problematic electrodes. Most amplifiers have impedance measurement functions built in. The amplifier sequentially passes a small 10-Hz square wave constant current from the common electrode sequentially through each of the other applied electrodes. Impedance of that electrode is the measured voltage drop (EOG signal amplitude) across the electrode divided by the current amplitude. A good electrode impedance is less than 5 kΩ. Anything higher is typically corrected by scrubbing the skin and applying a new electrode, then waiting 5 minutes for the half-cell potential to “settle” and diminish drift. (Note: Half-cell potential is the inherent varying voltage between the electrode and the skin when negative and positive ions migrate through the electrode gel as the skin-electrode interface attempts to chemically stabilize.)
Eye Calibration
The EOG amplifier amplifies the CRP, producing a voltage that corresponds to the patient's gaze angle. This voltage is scaled to volts/degree of eye movement in the calibration section of an ENG protocol. The calibration value is then used for each test to produce traces whose amplitude converted to degrees is used to measure the response to a stimulus. Because the CRP is the result of metabolic activity of the retina, room light and patient physiology will often produce significant changes to CRP during the course of an examination. When the patient closes their eyes, eye movements can still be recorded. However, the CRP also changes with reduced light hitting the retina. Thus, to maintain accurate recordings, room lights must be subdued and calibration must be repeated frequently during the course of an examination. Even calibrate before each of the four caloric stimulations. To speed up the calibration process and not require sitting up, a second calibration bar can be placed above the examination table for use by a supine or caloric-positioned patient.
Electro-oculogram Amplifier Calibration
According to amplifier specifications, the signal gain must be around 5,000 and the bandwidth from DC 40 Hz. Aging of electronic components will affect these specifications. Capacitors are used in all electronic filters and they tend to age and change values more than the other components. Fortunately, the amplifier does not have to have exact and consistent specifications over its lifetime because the output of the amplifier, if stable, is used to scale the eye signal relative to a gaze angle. Thus, if the amplifier gain changed from 5,000 to 4,000 after 10 years of use, it could still be used with measurement accuracy. If the filter settings changed from 24 Hz to 28 Hz due to aging, the nystagmus waveform might have a little different shape but still be identifiable and measureable.
Troubleshooting Electro-oculogram
How do you know if your EOG is working properly or not and if it needs calibration or repair? First, drifting or uninterpretable eye traces are indicators that something is wrong. If you get traces that appear to correlate with eye movements, then the amplifier is powered on (have patient shake their head slowly left and right then up and down while you watch the traces). If one channel is giving you problems, then most likely the electrodes or lead wires are at fault. Lead wires get bent a lot and can develop intermittent open circuits (breaks). To isolate, swap these lead wires with known good ones. Check the electrode impedance, re-prepare the skin, and replace the offending electrodes. If one channel drifts constantly and the electrodes and lead wires have been replaced, then swap the lead wires at the patient cable so that the left eye goes into the right channel and vice versa. Does the drift move to the new channel? If so, then the problem is not with the EOG amplifier. If one eye signal is noisy and you replaced the electrodes, then swap the L/R leads to see if the noise changes channels too. If it does, the problem is not in the amplifier.
There are times when the amplifier hardware has a problem and it needs factory repair and recalibration. During repair, EOG bandpass filters can be checked and adjusted to match specs. CMRR and zeroing can be checked and parts replaced if the original specifications are not met. Your EOG amplifier should function or be repairable for many years or decades, and with calibration it can match original specifications.
Digital Light Bar
In a vestibular laboratory, eye movements are evaluated by asking a patient to look at a stationary or a moving visual target. One of the most common devices for presenting a target is a digital light bar (DLB)—digital, because the target is a discrete light-emitting diode (LED). Target motion is produced by sequentially lighting different LEDs. For example, pendulum movement is produced by lighting each LED from a left point to a right point with proper delay between turning the light on and off and the next light on and off. Optokinetic patterns are produced by switching on three spaced LEDs in rapid sequence so the patient thinks the three are all lighted at once. Then jumping to the next LED trio to make it look like a series of three bars moved over one position. Constant-velocity or sinusoidal-velocity patterns can be created with proper timing. Sequencing of these LEDs is important for perception of smooth motion by the patient. Often a microcontroller in the DLB controls timing.
Eye movements are recorded to determine if the patient can maintain their fovea on the target (sans head motion). Each DLB has several LEDs with a physical width of each LED. These LEDs are stacked side by side to form a light bar of perhaps 256 lights. Each LED has an address for the software to turn it on and off. The LED has a visual angle relative to the patient so turning on LED m presents a target for the patient at an angle of n degrees off their gaze center. The one variable we have to calibrate is distance from the eye to the target, the rest is trigonometry.
Calibration of the Digital Light Bar
For eye movement calibration, the DLB must be positioned at a defined distance from the patient and at the proper height to match level gaze. If the patient distance is 1 m (39.37 in.) from their temple to the center of the DLB, then the light must move 27 cm (10.5 in.) from center for a change of 15 degrees in visual angle. For 10 degrees, the target must move 17 cm (6.7 in.). Refer to Appendix 1 for a summary of the trigonometry underlying these calculations. The best way to measure patient distance is with a tape measure or a string of 1-m length. If the patient cannot be 1 m from the DLB, then the VNG software can be programmed to compensate for the different distance and turn on a different LED at 15 degrees. Note that moving the patient too close results in convergence of the two eyes and too far away they may not be able to see the smaller target.
What to Look Out For
If the DLB is mounted upside down, the target will move right instead of left. If the DLB is horizontal during a vertical calibration, then vertical eye calibration will be wrong (almost zero). If the patient does not pay attention to the first move left, then calibration will be half normal and waveforms will be 2× normal size.
Digital Light Bar Features
LED color—red is easier to see, but yellow-green recommended in the ANSI standard
LED brightness—easy to see in a dimly lit laboratory
LED spacing (resolution)—number of LEDs/degree of visual angle
Target angles available—how far the LEDs range from center?
Calibration distance range—allowable distance for ± 15 degrees
Target motion—how smooth is the motion?
Height adjustable—should be at level gaze height
Orientations—horizontal and vertical
Video Goggles
Every vestibular laboratory must record eye movements, either for observation or to measure how the eyes move. Video goggles must provide a high-resolution video eye image captured at a fast enough rate to accurately portray the kind of eye movements presented by dizzy patients. The goggles must also record without visible light so the patient cannot fixate and suppress eye movements. The goggles must not impede vision so the patient can see a visual target at different points within their visual field. The goggles must be sanitary to prevent the spread of disease between patients.
As a user, you are responsible to make sure that your video goggles are functioning properly and how to adjust them for each patient. You should also know the functional limitations of your goggles.
Functional Characteristics
Before you put your goggles on a patient, you should think about goggles sanitation and cleaning of the mirrors. Also check the mechanical integrity of the cables, looking for wear. Eye-tracking algorithms use pupil contrast relative to the iris to find the pupil center or pupil edge depending on the tracking method. Patients with eye makeup, especially eyeliner, and droopy eye lids might have eye-tracking issues. Narrow eye slits, blindness, reduced visual acuity, macular degeneration, eye muscle surgery, and cataract lens replacement surgery can affect recordings and should be noted in the patient's record. Fine, slick hair might cause the goggles strap to slip during head movement.
Application and Maintenance
Observation
Much clinical information can be obtained just by viewing eye movement videos recorded during patient positioning maneuvers. These videos are also useful for patient education. The human visual nervous system can only discriminate frame rates below ∼20 frames per second, and at higher rates the frames appear continuous. However, the visual nervous system can identify frames that are not synchronized with the action or with audio. Audio that does not match the video is easy to identify as are videos of each eye that do not appear linked in time. Thus, calibration of video recording should include subjective analysis of synchronicity. The goggles themselves do not need to be calibrated because no measurements are being made.
Traces
Further assessment of eye movements requires measurement of eye position, in other words, traces of horizontal and vertical eye position on a computer screen. These traces are relative to the head. If the head moves, then the eye traces are subject to misinterpretation because they are contaminated with head movements. For example, a patient creates VOR nystagmus traces normally just by rotating their head or lying down. The best way to calibrate eye movements without contamination by head movement is to keep the patient's head stationary with a head support or head strap to a fixed head support. If not available, then hold the top of the patient's head and or instruct them to use their eyes to follow a target and not move their head. Because measurements are being made, the eye movements must be calibrated for each patient. Eye movements are calibrated by the change in pupil position in the video image. A video image is made up of discrete pixels in a two-dimensional (2D) matrix. The “typical” size of a video matrix in today's cameras ranges from 320 horizontal (H) × 240 vertical (V) to 640 H × 480 V. When the patient looks at a target moving in their visual field, the pupil center will move from one pixel location (H1, V1) to another position (H2, V2). If the calibration is purely horizontal, then vertically V1 = V2 or at least that change should be small and can be ignored. If the eye moves 30 degrees during calibration, meaning that the patient looks 15 degrees to the left followed by 15 degrees to the right and the pupil center on the video moved from (100, 60) to (200, 60) then the calibration is (200 − 100)/30 degrees or 100/30 = 3.3 pixels/degree. The calibration can be assumed to be accurate for eye movements of up to 30 degrees off center. Now, the eye movement has been calibrated horizontally for that patient and this calibration value can be used to scale the traces as they are plotted on the y-axis on the computer screen. (Recall time is on the x-axis.) Likewise, the vertical eye movement is calibrated using the same method. Each eye is calibrated independently because each eye moves independently. Each patient must be calibrated due mainly to the eye image size on the computer screen. For example, large cheekbones push the cameras away from the face resulting in a smaller eye image and thus fewer pixels of eye movement for the same calibration angle. If the patient cannot see the calibration targets, or if they do not understand the instructions for calibration, default calibration values are available. Default calibration will introduce measurement errors of up to 25%, so the measurement results are more qualitative than quantitative.
Camera Physical Characteristics
Color Versus Black and White
Only special black-and-white video cameras are used for vestibular testing because they must operate in the dark with enhanced sensitivity to infrared (IR) light wavelengths. These black-and-white cameras will produce a better, clearer image because they are sensitive to low levels of IR. Dark means that the patient cannot see any light but the cameras can still see the eyes. This is possible because the eyes are illuminated with IR light that is outside the range of what the human eye can see. Black-and-white cameras used in goggles have a special filter to block all visible light and transfer only IR light. That filter maintains the same brightness and contrast in the eye image under all outside ambient lighting conditions. You should make sure that the goggles do not have any visible light when vision is removed. Thus, the room light should be dim and the goggles snug to prevent outside light from entering the goggles in an amount that the patient can fixate and suppress the nystagmus you are trying to measure.
IR Illumination Levels
Because IR light is invisible, you cannot tell how bright it is by looking at it. With visible light, your pupil constricts to reduce the amount of light hitting the retina. Thus, there are safety standards for the amount of IR illumination exposure that is safe for the human eye. This standard takes into account intensity and duration of IR exposure. VNG tests are of short duration (less than 2 hours) and exposure is typically less than 1 mW/cm2. These specifications are provided by the goggle manufacturer and they should be reviewed by the customer. You can also request a report from the manufacturer showing how they measured (and not just calculated) the IR intensity in their goggles.
Camera Resolution Available
The higher the camera's spatial resolution (more pixels), the better the details in the eye image and the greater will be the sensitivity in eye position measurements. A camera will have 640 × 480 or better resolution in year 2014 and that resolution should get higher as technology advances. The resolution parameter is supplied by the camera manufacturer and cannot be calibrated or improved.
Frame Rate
The higher the camera's frame rate (frames per second or hertz), the more accurate you can measure fast eye movements such as saccades. Analog cameras according to the National Television System Committee standard produce signals for televisions video inputs. The TV requires 30 Hz frames split into two overlapping fields (interlaced images). This field rate is sometimes reported as a camera of 60 Hz. Eye tracking can be from each frame or each field. The eye images can be captured for display on a computer by conversion of each frame to a 2D gray scale matrix and then that matrix is transferred into the computer via a universal serial bus (USB) connection. Thus, there must be an analog-to-digital conversion of the video frame before it can be displayed on the computer screen or analyzed by the software. Contrasting that are pure digital cameras where there is no National Television System Committee standard and the eye image can be transferred at a much higher frame rate. Typical modern digital cameras can easily produce a 100-Hz frame rate and by reducing the vertical height of the eye image (reduced scan lines), frame rate can increase to 250 Hz or more. Frame rate can fall depending on shared functions by the computer. These are called dropped frames and will appear as jitter in the video image. There are utilities available from the manufacturer that display frame rate and can be used during calibration to check if the specifications are being met and if there are any dropped frame issues.
Focus Range
Eye image clarity is important to identify and measure subtle eye torsion and for a well-defined pupil edge for eye tracking algorithms. All cameras us an optical lens that focuses the eye image on the camera image sensor. Because the eye is so close to the camera sensor (inches), the lens must focus the eye for each patient. Lens focal length is apparent when you look at what parts of the eye are in focus at the same time. Because the eye is a globe, when the pupil is rotated toward the canthi, the pupil may not still be in focus. Interestingly, IR light focuses at a different focus setting than does visible light. Thus if a camera does not have a visible light-blocking filter and you focus the camera in a lighted room then use only IR light for testing, the eye may not be in focus. As a calibration or function check, make sure you have a good focus adjustment range working in the camera. If not, the camera should be repaired by the factory.
Goggles Mask
The goggles purpose is to hold the camera so the eye image remains in the same position even when the patients move their head. The goggle's face seal and headband are the two most important components that need periodic maintenance. A light-tight seal between the goggles and the face is produced by a flexible rubber mask or by foam rubber between the mask and the face. The mask should be cleaned with manufacturer-supplied sanitation wipes. Cleaning the rubber mask with alcohol will dry out and harden the rubber material and shorten the functional life of the mask. Maintenance requires the user to check for light leaks and broken or worn mask components. Most goggles have replaceable components that can be applied with instructions by almost anyone with good dexterity and fine motor skills. The elastic strap holding the goggles in place is one component that can and should be replaced yearly.
Mirrors
IR reflective (hot) mirrors reflect IR light but allow visible light to pass through. These mirrors are placed in front of the patient's eyes so they can look through them at the target. Cameras are mounted above to see the reflection of the eyes in the mirrors and to stay out of the visual field. Clean mirrors produce a sharp eye image. If your mirrors have fingerprints or smudges, the eyes will not track well. Use a mirror cleaning cloth to wipe the mirrors. Do not use a spray cleaner in goggles as that cleaner can get on the camera lens. Do not use window cleaner as that can damage the IR coating. Normally, the camera lens will stay clean but it too can be wiped with the lens cloth.
Camera Cables
Most cameras today connect to a VNG computer via wires rather than wirelessly for technical reasons including signal integrity, maximum bandwidth, and camera/LED power. The drawback is that wires get broken or damaged, resulting in intermittent or lost video signals. You can prevent cable problems by treating them as breakable objects. If an eye image does become intermittent, most likely the problem is with a cable. Identify the defective cable by substitution or swapping cables until the eye image returns to normal.
Software Characteristics
Eye Tracking
This is the method of converting gaze direction to horizontal and vertical eye position traces. Another way of looking at this is the eye image is a 2D digital image with size of, for example, 640 H × 480 V pixels. The goal of the eye tracker is to determine the x, y position of the pupil center in that 2D matrix, with the upper left corner of the 2D matrix having coordinates of 0,0. Then if x increases, the subject is looking more to the left (mirror image) and if y increases, the patient is looking more downward. Nystagmus is just a change in that pupil position over time. To convert x, y pupil position to degrees of gaze, have the patient sit in front of a target (see sections on DLB and television) that moves a lighted spot 15 degrees in each direction from center gaze; then the VNG software records the x1, y1 for the left gaze position and x2, y2 for the right gaze position. If the patient does not have his or her head tilted to the side, then y1 = y2 and x1 − x2 would be the horizontal difference in pupil position within the 2D matrix that would correspond to 30 degrees of eye movement. That is the way the cameras are calibrated for each patient. Calibration will vary between patients as a result of eye image size. For example, a deeply set eye socket will have a smaller eye image and a flatter face will have a larger eye image.
Camera Resolution
Eye calibration depends on the size of the eye image. A bigger eye image will have higher spatial resolution because the eye image fills more camera pixels. However, the pupil must remain fully in the image over all possible gaze angles. If the pupil slides out of the frame, for example, if the patient looks far left, then there is no way to get an accurate pupil center measurement.
Pupil Centering
Some digital cameras use a region of interest to select only part of the camera field for eye tracking. This is like looking at the world through a window. By moving the window in front of you, you can see different parts of the world. Likewise, if the software moves the region of interest, then a different part of the eye image is shown. This is a way the camera manufacturer has designed a way to “window the world” without moving the camera physically. If the goggles slide up, the window can be changed to bring the eyes back to center. As the person responsible for eye calibration, you must aim the cameras to keep the pupil within the camera field at all times. This can be done mechanically or electronically. Pupil centering is required for each patient and should be adjusted during the examination as needed.
Camera Synchronization
We have all seen videos where the sound did not match the speakers lip movements. That lack of synchronization is disturbing. If voice and video are not synchronized during a VNG examination, there is a problem with the technology that needs to be repaired or adjusted. Likewise, if the two cameras capturing the left and right eye are not synchronized, then it appears the eyes are disconjugate even when they are not. This is a problem with camera video capture timing and can be detected by observation of the video during an optokinetic or saccade test. This is a hardware or software issue that should be fixed to prevent an erroneous diagnosis.
Video Goggles Summary
Eye tracking with video goggles is the most important part of the technology to get right. Making sure you have a functional and well-maintained video goggles will go a long way toward ensuring that your examination produces high-quality results. Maintenance involves cleaning the mask and mirrors, adjusting the headband to keep the mask snug against the patient's face, focusing the cameras to produce clear eye images, centering the pupil in the video frame to allow for full range tracking, and adjusting the illumination threshold for proper pupil tracking. Eye calibration using gaze targets allows for accurate measurement of eye movements and is done once per patient at the start of the examination. Default calibrations, when selected, will only produce a qualitative measurement.
Vng Exam Chair
A VNG test requires the patient to be seated, supine or supine with head elevated. For the seated tests, the patient has eye movements recorded with vision and without vision.
Features
Side arm chair or clinical exam chair
Calibration distance
Height relative to calibration stimulus
Patient stability
Adjustments
Because patients may be dizzy at the time of testing, it is important that they are stable, supported, and safe. Swivel chairs that can roll out from under patients when they are trying to sit or get up are not a good option. A movable chair changes the distance to the calibration screen. Another bad option is a secretarial swivel chair without armrests. A third bad option is with the patient seated on the side of an exam table with their legs dangling off the side. There is no back support so the distance to the oculomotor stimulus can vary depending on how they lean or slouch. A four-legged, side-arm chair on the floor 1 m from the oculomotor stimulus provides the most stability and safety. A clinical exam chair can also be used for seated tests, but it may not be appropriate for supine tests.
The patient's height affects the height of the stimulus target. Patients should be looking straight ahead at the calibration stimulus. DLBs may contain an adjustable floor stand to position the light bar at the height of the patient's eyes while seated. Televisions and LCD projectors producing the oculomotor stimulus may have their center target adjustable with software to the patient height or the projector can be tilted up or down. Laser target projectors can be tilted vertically to position the target at the correct height.
Exam Table
The purpose of an ENG examination table is to place the patient in a seated, supine or head-elevated position. The table model should be selected for safety, function, and ease of use. Simple ENG exam tables hinge in the middle (at the waist) with pneumatic-assisted lifts that slowly lower the back to a supine position. The back can also be raised to place the patient in a 30-degree elevated position for caloric testing and with the head hanging over the end (with head supported) for Dix-Hallpike positioning tests. The table should be wide enough that larger patients may safely roll and lie on their side for positional tests. Safety of the examiners and the functional integrity of their back, arms, and legs are also important when the examiner is maneuvering and holding patients. Electrically operated tables still in use incorporate motors with screw drives that raise and lower the back support and simultaneously move the knee/leg support.
Care must be taken if a patient is expected to sit on the edge of the exam table during ENG calibration, gaze, and oculomotor tests. An elderly or dizzy patient sitting on the edge of a table with their legs dangling is not comfortable or stable. Sitting on a table edge also changes the distance from the patient to the light bar or television as they slouch or lean back. A stable nonswivel chair with four legs and arm rests is a better position for calibration, gaze, and oculomotor tests. Then the patient can be moved to the exam table for positional, positioning, and caloric tests.
Operation Checks and Calibration
A piece of clinical furniture like an examination table may be 30 years old and still in use. Finding a dedicated VNG/ENG exam table to purchase may be difficult. Most VNG/ENG manufacturers suggest using an adjustable physical therapy treatment table. Like any furniture, the table should be periodically checked for loose or worn parts. The table should be cleaned and sanitized. If the pneumatic tube no longer slows the movement of the back support, it should be replaced. Instructions for performing the Dix-Hallpike maneuver have been updated recently. Letting the patient's head hang off the table unsupported is no longer recommended. Rather, an examiner-supported head or just maintaining the patient supine with the head on the table will produce useful results and protect the neck from injury. Speed of descent in the Dix-Hallpike test is no longer believed to be as critical as was once taught. Noisy electric motors on tables may create interference in EOG signals and audio recordings.
LCD Projector or TV Stimulus
Calibration, gaze, and oculomotor targets can be generated using a wall-mounted TV or ceiling-mounted LCD projector. These devices are replacing the DLB stimulus because a user can obtain vertical stimuli without rotating a light bar and 2D patterns can be generated for optokinetic stimuli.
TV Features
Background and target colors
Target selection
Target angles available
Calibration distances
Screen size
Screen resolution
Mounting
Positioning
The LCD projector can easily produce a 48-in wide display for target presentation. That size is approaching full visual field optokinetic stimulation. The projector must be ceiling mounted and project down over the patient's head who is seated in a chair in front of a projection screen. Care must be taken that a tall patient will not interfere with the projection. The patient is typically one meter from the screen.
A LCD television with a flat screen is wall-mounted 1 m in front of the patient. If the TV is a 32-in class, then at that distance, a ± 15-degree horizontal and a ± 10-degree vertical target deflection can be created. Larger TV screens can produce greater target angles. For example, a 42-in class TV will allow a ± 20-degree horizontal and a ± 15-degree vertical deflection (see Appendix 1).
A question arises as to how to position the patient such that center gaze does not require them to look up or down at the center target. One way to resolve that calibration issue is to move the center target up or down in software to match the height of the patient. Another way is to physically move the TV or aim the projector up or down or raise/lower the exam chair.
Calibration Configuration
The screen ratio may be 4:3 (normal screen) or 16:9 (wide screen) depending on the device used. The latter is preferable for a wider horizontal target range. For the target to move 15 degrees (10.5 in. with patient at 39 in.), then the screen must be set to display with the proper resolution and width. Use a tape measure to confirm that the target is moving the proper distance. Check that the furthest target remains on the screen and is not shifted off from the TV display that was configured to stretch mode. Set the display to provide a target to background with contrast to match the ambient light in the room. Too strong of contrast may reduce the ability of the subject to maintain concentration on the moving optokinetic pattern for the whole test. Consider changing the background and target colors to obtain the optimum stimulus. For children, choose a more interesting target like a smiley face to display rather than a simple circle. Make sure the target is not so large visually that the patient will look around the edges of the target rather than tracking the center of the target. Patients cannot wear their eyeglasses with video goggles, which is acceptable because the target must be trackable but not necessarily in clear focus.
Control Inputs
Vestibular function measurement devices are electric devices that have electric switches that control power or send remote control signals to the device.
Control Functions
Power switch
Emergency stop
IR remote
Radiofrequency remote
Foot switch
Computer keyboard
Mouse
In-House Function Checks
One of the main reported functional system failures stems from lack of power. Fuses rarely blow except in situations of catastrophic electrical failure and if that happens, it should prompt the user to contact a specialist to find out why. More frequently, the user forgets to plug in one of the cables: USB, FireWire, or AC will always result in a failure of function. Also, check if the power switches are turned on or the computer is in standby or sleep mode. Is the Emergency Stop button pressed in and thus preventing the chair from rotating? Is the reclining rotating chair reclined and thus preventing the chair from rotating? Rarely is a switch bad; more likely it is a switch that is in the wrong position. If the control is an IR remote, then a direct line of sight from the remote to the receiver is required. If the control is a radiofrequency remote, then the receiver must be plugged into the USB port. A foot switch must be plugged into the correct connector.
In-House Calibration
Test each of the buttons on the remote control to make sure they are detected and functional. Push the Emergency Stop button on the rotational chair to make sure the chair stops as desired. Press the escape or abort key on the keyboard during a test to make sure it properly aborts a test as desired. Make sure the mouse pointer moves smoothly and accurately across the computer screen and that the mouse buttons click and select appropriately. If any of these devices do not work as they used to, check batteries and clean surfaces. If they are still not working, simply replace with new devices. These devices are rarely repairable and must be replaced.
Eye Movement Waveforms
Waveforms or traces display relative changes in gaze up, down, left and right. Because most eye movement is horizontal or vertical as compared with oblique, there are typically two traces for each eye. One trace displays scaled horizontal eye movements and another trace displays scaled vertical eye movements. Traces are recorded for each eye separately in case both eyes are not moving together or one eye is not recording well due to recording noise or tracking artifacts. Additional eye traces that are less commonly recorded are torsional eye movements and pupil diameter.
Features and Calibrations
For historic reasons going back to strip chart recorders, eye movement traces are scaled so they can be measured in time and amplitude using a ruler. One centimeter in time represents 1 second and 1 cm in amplitude represents 10 degrees. Measuring eye velocity, especially of the slow component of nystagmus, can confirm automatic VNG software measurements. For example, a nystagmus trace that moves 10 degrees in 1 second would have a velocity of 10 degrees/s. This is basically the slope of the line. Horizontal gaze traces that go below the center line on the graph are considered left of gaze center. Vertical gaze traces that go below the center line indicate downward gaze.
Torsional eye movements are measured in degrees of eye rotation with traces below the center line deemed counterclockwise and above clockwise.
Pupil diameter recordings are currently not scaled in millimeters but are used to show relative changes from time to time and eye to eye. These relative changes are calculated as a percent diameter change over a selected period.
VNG Software
Prior to computers recording eye movements during vestibular function tests, we had strip chart recorders displaying horizontal and vertical eye movements that were then hand measured or eyeballed for normalcy. Computerized VNG/ENG has the same purpose as a strip chart recorder: to display eye position traces in a graphical form that can either be hand measured on a printout (for verification) or computer measured. How do you know the computer measurements are accurate? Does that confidence level require calibration or verification of the computer and software?
Software Features
Eye movement waveforms
Number of channels visible
Trace resolution
Scale factor on printout
Pupil diameter
Torsion
Eye blink rejection
Eye makeup rejection
Nystagmus SCV measurement and averaging method
Saccade measurements
Eye video recording
Pursuit measurement
Torsion measurement
Pupil measurement
Geometric compensation
Spontaneous nystagmus identification
Age-matched norms
Software Functions
The purpose of VNG software is to produce a record of eye movements during vestibular function tests. These eye movements are analyzed by the software and verified by the operator, then included in the patient's medical record for interpretation by a physician in conjunction with the patient's history and physical examination. The measurements are compared with normative thresholds to determine if the patient is normal or abnormal in that particular test for their age.
Measurements
Separate measurements are made for horizontal and vertical eye movements. For gaze, positional and caloric tests, the vestibular or slow component of the nystagmus waveform is measured (degrees per second). That measurement requires accurate timing of the recording and accurate amplitude scaling. Scaling goes back to eye calibration for each patient prior to the first test. For pursuit tracking, optokinetic, and VOR tests, the gain is measured. Gain is the ratio of the eye velocity to target (or chair) velocity. Again, accurate amplitude scaling and time recording is important. For saccade tests, eye latency, velocity, and accuracy is measured. These measurements require accurate amplitude and timing records at a higher sample rate and bandwidth than nystagmus.
Noise and Other Artifacts
Measurements are complicated by environmental and physiologic signal artifacts. Eye blinks, squinting, and lack of attention by the patient will cause periods of the recording to be unmeasurable garbage. That is one reason why recordings continue for 30 seconds or more to get a good data set that you can believe in. Filtering of the signals removes some of the artifacts. Software algorithms identify nystagmus based on a combination of a fast and a slow component for every beat. However, the experienced human brain can pick out nystagmus better than any algorithm. Thus, a quality review of the waveforms is important along with some beat editing to remove misidentified nystagmus.
Age-Matched Norms
Young children have underdeveloped oculomotor function that results in reduced pursuit gain. Older patients lose the ability to pursue a target without injecting catch-up saccades. Thus, when measured results are compared with norms, it is important to know the age of the patient and what is normal for that age. In general, older patients will have slower reflexes and less smooth tracking eye movements.
Trace Measurement Checks
Eye trace recordings in a printed report will be scaled for hand measurement verification of the computer measurement. For visual comparison, it is also important that the scale factor on a report does not change between tests. Thus a 10 degrees/s nystagmus looks the same if it is from a gaze test or a caloric test.
To check the accuracy of a nystagmus measurement, you will need a ruler and a calculator. Identify the scaling on the paper. Traditionally it is 10 degrees/cm and 1 s/cm. Using a pencil, align the ruler on an SCV nystagmus beat and draw a line parallel to that SCV. The slope of that line is the nystagmus velocity. For example, if the line changes 1 cm in each direction (basically corner to corner on a 1 × 1-cm grid) then the nystagmus beat is 10 degrees/s. If the line covers 2 cm vertical for each 1-cm horizontal, then the nystagmus velocity is 20 degrees/s. This is how you would check the calibration of your VNG.
Measurement Confidence
To build confidence in your VNG, it is recommended that you test normal subjects prior to testing patients. One problem may be finding a “normal.” It is important to ask health questions of the normal volunteer before testing. A history of concussion, migraine, motion sickness, ear surgery, or eye muscle surgery would remove them from the normal category. Low nystagmus velocity response on a caloric test can be due to wax impaction or poor alerting. Any normal subject testing should be conducted as if the subject was a patient, complete with a health history, physical examination, and proper test methods. Don't be surprised if you find something abnormal in your normal, especially spontaneous nystagmus or low VOR gain on a caloric or rotational chair.
Retesting
False-positives are often considered worse than false-negative vestibular test results. If the result of your test does not match the patient's history, then repeat the test after reinstructing the patient telling them what you expect him or her to do. Use the most normal test from that patient in your report. Comment on why a particular test may have abnormal results that are not related to pathology.
Rotational Vestibular Chair
The purpose of a rotational chair is to passively rotate a patient at a precise velocity to stimulate their lateral semicircular canal and elicit a VOR response. Vision must be eliminated for this VOR test because vision (pursuit and optokinetic) act to keep the eyes on a visual target and you want to eliminate vision for pure VOR tests. Contrasting the VOR are two tests where you want to use vision. The vision-enhanced VOR test and the vision suppression of the VOR test. In those last two tests, the patient looks at earth stationary targets as they rotate, thus increasing VOR gain or looking at an object that is rotating with them while suppressing their VOR response.
In a clinical setting, rotational chairs produce a sinusoidal harmonic acceleration (SHA) profile at frequencies from 0.01 to 0.64 Hz in seven steps.
Peak chair velocity at each frequency should be set to garner a reasonable VOR nystagmus velocity realizing that VOR gain increases with frequency in normal individuals. Peak stimulus velocity (degrees per second) can be set at 20 degrees/s to 100 degrees/s at each frequency. However, doubling the test frequency while maintaining the same peak sinusoidal velocity (e.g., 60 degrees/s) will double the torque required to turn that patient.
Functional Characteristics
Speed
Drift
Accuracy
Patient weight effects
Frequencies and velocities
Maximum velocities
Safety
VOR stimulation is with either an SHA profile or a trapezoidal velocity profile (aka step). The patient VOR response is a nystagmus with an SCV that varies in amplitude over time. Sinusoidal VOR gain, gain symmetry, and phase are measured at each of seven stimulus frequencies. Step velocity gain and the associated exponential decay time constant of the nystagmus response are measured for the acceleration and deceleration components of the stimulus. Because we are quantifying the VOR response, the rotational chair stimulus must be accurate and repeatable for each patient.
Calibration
In-house calibration and preventative maintenance is recommended every 6 months. You want to make sure that the chair is rotating at the proper speed and will stop safely. In case the patient becomes ill during rotation, you should practice being able to stop the test slowly or quickly. Patient harnesses restrain the patient during rotation and should be checked for correct operation weekly. For higher-speed rotation, ankle straps should be used. The chair should be stopped for patient entry and exit and for eye calibration. Feedback on how fast the chair is actually rotating comes from a tachometer on the chair motor. This tachometer signal is used by the chair electronics to apply more or less torque to the motor to maintain the desired chair speed. Thus, even heavy patients can be rotated at a precise velocity. This is similar to the speed control on your car. You can maintain your speed up a mountain highway if your engine has enough torque. The steeper the road, the greater the required torque. Rotational chairs cannot downshift like a car, so there is a limit to how much torque can be applied to keep the chair turning at the proper speed. High SHA frequencies at high velocities require the most torque from the rapid accelerations with each change of direction.
In-House Chair Calibration Checks
All chairs have a tachometer for velocity feedback and some chairs also have position feedback. If a chair is rotating slightly when it is enabled to rotate but commanded to stop, then that undesirable drift should be removed via chair calibration, either electronically or through calibration software that adds or subtracts a small signal. Chair speed is measured using a digital timer in the PC to count the time it takes for the chair to make one full revolution at a constant speed. For example, if it takes 12 seconds to make a complete revolution, then the chair speed is 360/12 = 30 degrees/s. Calibration software will adjust the control signal to set chair speed at the desired speed. The same software will scale the tachometer signal to match the actual speed. Thus if a 1-V chair rate signal causes the chair to rotate at 30 degrees/s and produces a 1.5-V tachometer signal, then the rate and tachometer signal can be scaled to both represent 30 degrees/s in the test traces. If the traces deviate from the presets, then the software can warn the user that the chair is out of calibration.
Drive belts from the motor to the chair main shaft will stretch with time and should be checked for proper tension. This can be done by enabling the chair servomechanism with the chair stopped then grabbing the chair seat with both hands and attempting to rotate the chair. If the chair rotates, then the belts are slipping and they need to be tightened (with power turned off). Refer to manufacturer's instructions for belt tightening. Other preventative maintenance checks include visual and functional checks of the patient restraints (seat and ankle belts) and adjustability of the head support.
Laser Target Projector
For calibration, gaze, saccade, and pursuit target presentation, a low-power red laser beam on the chair is deflected using small galvanometer motors to move reflecting mirrors. The XY laser consists of two galvanometers with mirrors, two beam-positioning galvanometer controllers, and one laser beam that reflects off the moving mirrors to move the laser dot. These galvanometers are designed to quickly and accurately move the laser beam without overshoot or undershoot to the appropriate point on the booth wall surrounding the chair.
In-House Laser Calibration Checks
Observation and measurement of target position can be done in-house. Using trigonometry (see Appendix 1), we know that if the patient is 39.4 in from the target, then for a 15-degree target deflection, the laser spot must move 10.5 in. That number is calculated as 39.4 × tan (15d) = 10.5 in. This is the linear distance, not the curve distance. Note that vertical target deflection is slightly more difficult to measure. If the target center position is the same height as the laser from the floor, then a 15-degree deflection will move 10.5 in. But if the starting position of the laser is lower on the wall, then a 15-degree deflection down will be slightly further distance than a 15-degree up deflection. But the overall distance up and down will be close to the proper distance. Thus, we suggest taking the sum of the two (21 in.) to determine if the total angle is correct. Any calibration changes to the laser may indicate bigger problems and are best handled by a factory-trained service technician.
Laser Subjective Visual Vertical
By rapidly oscillating the X mirror of a galvanometer, a laser beam will trace a horizontal path. If the oscillation is of sufficient frequency, then the beam will move fast enough that a person cannot discern a point but rather sees a line. If the Y mirror alone is oscillated, a vertical line is generated. Then by driving both X and Y at the same time, a diagonal line is created. If the amplitude and timing relationship between the signals to the X and Y mirrors is changed, then the line will rotate about a center point to a different visual angle, anywhere from pure vertical to pure horizontal. This phenomenon is used to produce a line that rotates in front of the patient. The patient is asked to adjust the tilted laser line to vertical in a subjective visual vertical test.
In-House Subjective Visual Vertical Calibration Checks
The angle that the static subjective visual vertical (SVV) varies from true vertical during this test is an indication of patient's otolith imbalance and degree of abnormal function. Thus, accurate SVV angle indication down to 0.1 degrees is important for correct clinical interpretation. Software determines SVV voltages and the desired angle. There is no tachometer or position feedback to the computer so the angle should be checked at several easy to measure angles (45 degrees has equal X and Y deflections). If the SVV angle is correct, the measurement should be noted and a follow-up measurement performed every 6 months. If the measurement is in error, then a service technician will need to make a factory-authorized adjustment.
Optokinetic Drum
The optokinetic drum projects vertical stripes or dots on the curved booth walls. These stripes rotate in a precise fashion using a motor whose speed is controlled by software. Just like the rotational chair, the motor has a tachometer that tells the software how fast it is turning and in what direction. Optokinetic stimuli can be either sinusoidal or constant velocity. A true optokinetic stimulus produces circular vection or the sense of self-motion after ∼30 seconds of observation of a full-field visual stimulus.
In-House OKN Calibration Checks
The optokinetic drum motor is calibrated the same way as the rotational chair. When the drum should be stationary, it should not drift. This drift can be removed by adjusting the speed signal to the drum from the software or with a potentiometer at the motor servomechanism amplifier. The former can be done by the operator and the latter by a service technician. Drum speed is determined by measuring the time for a complete rotation. That requires built-in software with a pulse-per-revolution signal or a stopwatch and observation of a complete rotation of a drum projecting stripes. The rate signal voltage to the drum can then be adjusted to set drum speed.
The lamp that creates the optokinetic stripes without shadows requires a special lamp that acts as a point source of light. These lamps also have a brightness that is controlled by lamp voltage. If the voltage is too high, then the stripe contrast is too much and the lamp will have a short life. Measuring the lamp voltage with a lighted lamp is the only way to know if the voltage is within specifications. That measurement is usually done by a factory-trained service technician if there are indications that there is a problem. Typically a short lamp life (less than 1 year) or poor dark–light contrast in the stripes indicates a problem.
Chair Booth
The function of the chair booth (aka enclosure) is to provide a light-tight environment for testing the VOR of patients with their eyes open. This booth is nominally 6 ft in diameter and 8 ft in height with the chair mounted in the center. A door to the booth allows for patient entry and exit. Communication between the examiner and patient is via an intercom and observation of the patient in the dark with a camera and IR illumination. The patient must remain alerted with tasking in this quiet dark booth to obtain the best clinical results. For that reason, clear audible communication is important. Observation of the patient is important for the examiner to detect if the patient is under duress because of claustrophobia or the result of chair or visual stimuli. The intercom speakers are located in the ceiling to keep the subject from localizing the sound source and thus possibly interfering with test results.
In-House Booth Calibration Checks
A light-tight booth means that after 5 minutes of sitting in the dark, you cannot see the booth wall or your hand in front of your face. It is really dark even after dark adaptation. If not dark, you must either turn off lights outside the booth or find and fix the light leak. Light leaks will increase VOR gain as the patient watches the booth walls when they rotate. Although this may seem obvious, make sure the booth interior light is turned off during VOR testing. Leaving the lights on during a VOR test will result in a gain close to 1.0 and a phase close to 0.
Patient comfort during a 30-minute chair booth experience is important for many reasons including getting good test results. A booth ventilation fan draws air in from the floor and removes it slowly through the roof. Air flow is the result of small box fans in the ceiling. These fans should be quiet and not interfere with the patient hearing your questions over the intercom. Noisy fans should be replaced. Booth lighting is either an incandescent bulb such as a 15-W appliance bulb or white LED lights. The bulb can burn out and should be replaced, but the LED lighting if bad, must be repaired by a service technician.
During the winter when humidity is low, the examiner can shuffle on the floor and generate electrostatic discharge that can damage electronics, attract dust, or zap a patient. Newer booths have antistatic vinyl flooring. Older booths have carpet that can be sprayed with a fabric antistatic spray if electrostatic discharge is a problem. As with all medical devices that contact a patient, sanitation is important. Routinely clean the head support of any skin oils or makeup. Wipe off chair and arm surfaces with a cleaning wipe.
Conclusion
Vestibular function equipment requires proper configuration, calibration, and maintenance. The expected life of this PC-based equipment is typically 5 years before the technology changes. Manufacturers guarantee they will have spare parts for 5 years of service and will make best attempts to repair and calibrate the equipment for longer periods. It is your responsibility to make sure you provide the dizzy patient with good test results and that requires attention to proper application of the technology and your equipment that is in good working order.
Appendix 1
How to calculate the desired position of calibration targets on a flat surface: To calibrate eye movements through a VNG or ENG recording system, we need to establish the relationship between the recorded eye movements and the angle of eye rotation (expressed in degrees of arc subtended) produced by those movements. In most cases, we have a calibration angle in mind that we want to use. So say we have a patient facing a wall that is 39 in. away and we want the patient to move his or her eyes horizontally ± 15 degrees to establish calibration. How far should the targets be placed on the wall to cause the eyes to subtend a 15-degree arc? The problem is set up in the following equation, where x = distance from the forehead to the wall, eyes focused on a gazing point directly in front, and y = distance from a midline target on the wall to a lateral target to produce a 15-degree change in lateral gaze.
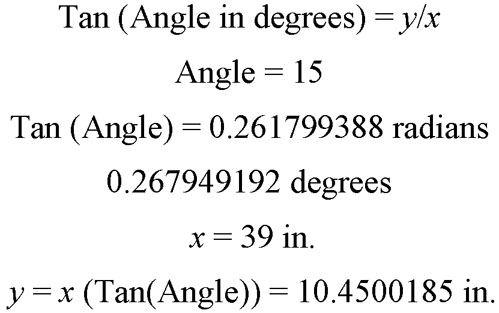 |
In Microsoft Excel, the formula would be: =TAN(RADIANS(Angle in degs)) * distance from forehead to center gazing point. Thus, we would want to place each target 10.45 in. on either side of the center gazing point.
References
- 1.Bárány R Nobel lecture: some new methods for functional testing of the vestibular apparatus and the cerebellum Nobel Media AB 2014. November 30, 2014. Available at: http://www.nobelprize.org/nobel_prizes/medicine/laureates/1914/barany-lecture.html. Accessed March 18, 2014
- 2.Regan D. New York, NY: Elsevier; 1989. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. [Google Scholar]
- 3.Arden G. St. Louis, MO: Mosby; 1991. History of electro-oculography. [Google Scholar]
- 4.Jongkees L BW Philipszoon A J Electronystagmography Acta Otolaryngol Suppl 1964189(Supplement):1–111. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Healthcare-associated infections Available at: http://www.cdc.gov/ncidod/dhqp/hai.html. Accessed December 18, 2014
- 6.Jacobson G, Shepard N. San Diego, CA: Plural; 2008. Balance Function Assessment and Management. [Google Scholar]
- 7.Desmond A. New York, NY: Thieme; 2004. Vestibular Function: Evaluation and Treatment. [Google Scholar]


