Abstract
Background
Identifying sexually transmitted infections (STI) in HIV-infected individuals has potential to benefit individual and public health. There are few guidelines regarding routine STI screening in sub-Saharan African HIV programs. We determined sexual risk behavior and prevalence and correlates of STI in a national survey of large HIV treatment programs in Kenya.
Methods
A mobile screening team visited 39 (95%) of the 42 largest HIV care programs in Kenya and enrolled participants using population-proportionate systematic sampling. Participants provided behavioral and clinical data. Genital and blood specimens were tested for trichomoniasis, gonorrhea, chlamydia, syphilis, and CD4 T-lymphocyte counts.
Results
Among 1661 adults, 41% reported no sexual partners in the past 3 months. Among those who reported sex in the past 3 months, 63% of women reported condom use during this encounter compared with 77% of men (P < 0.001). Trichomoniasis was the most common STI in women (10.9%) and men (2.8%); prevalences of gonorrhea, chlamydia, and syphilis were low (<1%–2%). Among women, younger age (adjusted odds ratio [OR], 0.96 per year; 95% confidence interval [CI], 0.94–0.98) and primary school education or lower level (adjusted OR, 2.16; 95% CI, 1.37–3.40) were independently associated with trichomoniasis, whereas CD4 count, cotrimoxazole use, and reported condom use were not. Reported condom use at last sex was associated with reporting that the clinic provided condoms among both women (OR, 1.7; 95% CI, 1.17–2.35) and men (OR, 2.4; 95% CI, 1.18–4.82).
Conclusions
Women attending Kenyan HIV care programs had a 10.9% prevalence of trichomoniasis, suggesting that screening for this infection may be useful. Condom provision at clinics may enhance secondary HIV prevention efforts.
Treating sexually transmitted infections (STI) in HIV-infected individuals has potential to benefit both individual and public health.1 Some STIs may have increased incidence among HIV-infected compared with HIV-uninfected individuals.2 Sexually transmitted infections have been associated with increased genital HIV viral load,3–8 and observational data suggest that STIs facilitate HIV transmission.9 Researchers have hypothesized that preventing and treating STIs would reduce HIV incidence at population level. However, among 9 large trials investigating the effect of STI treatment on HIV incidence, only 1 demonstrated a significant reduction.10 Thus, some have questioned the importance of STI programs for HIV prevention,11 whereas others suggest that trial design, insufficient power, HIV epidemic phase, and STI prevalence explain null findings in these trials and that STI treatment should remain an HIV prevention tool.12
World Health Organization (WHO) guidelines recommend that STI assessment and treatment be an integral part of a comprehensive HIV strategy to improve the health of HIV patients, their partners, and families, as well as to reduce spread of HIV.13 Specifically, individuals newly diagnosed as having HIV should be asked about STI symptoms and receive laboratory screening for syphilis. When feasible, women should also receive testing for gonorrhea and chlamydial infection. Patients diagnosed as having an STI, as well as their sex partners, should receive treatment according to current guidelines. The role of further routine STI screening for individuals in HIV care programs is less clear.
The World Health Organization recommends that countries conduct national STI prevalence assessments every 3 to 5 years to inform STI treatment guidelines. These surveys, however, are expensive and rarely conducted in resource-constrained settings. The United States supports HIV programs through the President’s Emergency Plan for AIDS Relief. In Kenya in 2010, this funding was used to provide care to more than 1.3 million HIV-infected individuals, including antiretroviral therapy (ART) to more than 400,000.14 ART significantly decreases sexual HIV transmission, as most clearly demonstrated by the HIV Prevention Trials Network 052 study.15 However, STIs may alter genital viral load despite ART making STI screening in HIV programs potentially beneficial for individuals and for secondary prevention.16
To inform large HIV care programs about the potential use of routine STI screening, we conducted a national surveillance study in Kenya to assess the burden of STI among HIV-infected adults in HIV care programs. Our aims were to estimate STI prevalence in adults in HIV programs throughout Kenya, to determine recent sexual risk behavior, and identify cofactors associated with prevalent STI.
MATERIALS AND METHODS
Study Design and Sampling Framework
To recruit a representative sample of HIV programs with sufficient sample size to estimate STI prevalence, we conducted population-proportionate sampling of clinics. The number of patients on ART served as a proxy for clinic size. Of 321 clinics with more than 50 ART clients, the largest 42 were selected (all >1600 clients on ART). We recruited participants from 39 of these clinics: 2 of the original clinics had combined, 1 refused to participate, and 1 was inaccessible to the field team. These clinics were located in 7 of Kenya’s 8 provinces and accounted for an estimated 12% of all Kenyan HIV clinics and 51% of all ART clients.
Eligibility and Study Procedures
Four mobile study teams enrolled participants between February and September 2010. Participants were eligible if they were HIV infected, receiving care at one of the selected clinics, 18 years or older, and able and willing to provide informed consent. Because study procedures involved taking a vaginal swab, we excluded pregnant women who were more than 36 weeks’ gestation.
The number of patients enrolled per site was proportionate to the number of registered patients receiving ART at that clinic. An initial sample size of 1600 was allocated proportionate to the number of adults on ART across the study sites. Several small sites were oversampled to achieve a minimum sample size of 30 per site, and 1 very large site was oversampled for a final target sample size of 1780. Patients were systematically approached (i.e., every nth patient) and offered screening for eligibility. If a patient was eligible and consented, a trained study nurse administered a structured questionnaire, assessed the patient’s current STI symptoms, conducted a physical examination of the external genitalia, and collected specimens for laboratory testing. Study staff collected survey and clinic data on standardized paper forms and entered data into a Web-based database.
When symptoms and signs were present, the study nurse made a syndromic STI diagnosis and provided immediate treatment based on the current Kenya Ministry of Health guidelines. Once laboratory results were available, participants who were asymptomatic but diagnosed etiologically were called and asked to return to clinic for appropriate treatment. Participants who received STI treatment were given a referral card for their partners to seek STI care and HIV testing.
The study was approved by the institutional review boards of the 3 collaborating institutions including the Kenya Medical Research Institute, the University of Washington, and the US Centers for Disease Control and Prevention.
Survey Measures
The survey included questions about sociodemographic information, recent sexual risk behaviors, and HIV/medical history. We chose a subset of measures, a priori, as potential correlates of STI. Sociodemographic data included age, current marital status, education level, and the province in which the clinic was located. Participants indicated the number of sexual partners in the past 3 months, and if they reported 1 or more partners, they were asked a series of questions about each of their most recent partners (up to 3). These questions included whether the partner was a spouse (including polygamous marriages) or other type of partner, if last sex occurred within the past week or not, and whether or not a condom was used during the last sexual encounter. Participants also reported if their clinic provided condoms. Finally, we asked participants about when they were diagnosed as having HIV, current ART use, and current cotrimoxazole use.
Specimens and Laboratory Testing
We tested for Chlamydia trachomatis, Neisseria gonorrhoeae, Trichomonas vaginalis, and Treponema pallidum. Men provided a first-catch urine specimen, whereas women had vaginal swabs collected. Genital ulcer swabs were collected from participants with genital ulcers and tested for herpes simplex virus (HSV) 1, HSV-2, and T. pallidum. All participants had blood drawn for syphilis serology and CD4 T-lymphocyte counts.
First-catch urine and vaginal swab specimens were tested for C. trachomatis and N. gonorrhoeae by transcription-mediated amplification using the Gen-Probe APTIMA Combo 2GC/CT system; the Gen-Probe transcription-mediated amplification system was also used to test for the presence of T. vaginalis. We screened for syphilis using rapid plasma reagin assays for screening and T. pallidum hemagglutination assays for confirmation. For this analysis, participants with positive rapid plasma reagin and T. pallidum hemagglutination assay were categorized as having active syphilis. Real-time multiplex polymerase chain reaction was used to detect HSV-1, HSV-2, and T. pallidum from genital ulcers. CD4 T-lymphocyte counts were assessed using FACSCalibur.
Statistical Analysis
Our analyses were designed, first, to estimate STI prevalence; second, to determine cofactors for STIs; and third, to provide sexual behavior data useful for secondary prevention programs. We compared sociodemographic, sexual behavior, and health-related differences between women and men using χ2 tests. Similarly, we calculated and compared the sex-specific counts and prevalences of individual STI. We weighted all estimates to reflect selection probability of each study site, and study site was also included in the data analysis to account for cluster effect. The study was powered to provide national estimates of prevalence of 4.5%, with 95% confidence intervals (CIs) of ±1.5%. Only trichomoniasis among women reached the 4.5% prevalence threshold; thus, we explored the bivariate correlates of trichomoniasis diagnosis among women using logistic regression and reported the corresponding odds ratios (ORs) and 95% CIs. Age was modeled as a continuous variable and education dichotomized. We further selected all variables in bivariate analysis with a P value of 0.10 or less and conducted a multiple logistic regression to assess the adjusted correlates between trichomoniasis and those variables. All analyses were conducted using Intercooled Stata 12 (College Station, TX).
RESULTS
Study Population
Of 1942 participants approached to participate, 281 (14%) declined participation and 1,661 (86%) were eligible, consented, and enrolled. As shown in Table 1, nearly two thirds (63.9%) were female, and women were younger, were less likely to be married, and had less education compared with men. The median age was 35 years (range, 18–70 years) among women and 40 years (range, 18–77 years) among men. Approximately 40% reported no sexual partners in the past 3 months, and this was significantly more common in women compared with men (P < 0.001). Among those who did have a recent partner, 95% reported only 1 partner in the past 3 months, more than half (59%) stated that they last had sex in the past week, and 63% of women reported using a condom during this encounter compared with 77% of men (P < 0.001). Reported condom use at last sex was significantly associated with reporting that the clinic provided condoms among both women (OR, 1.7; 95% CI, 1.17–2.35) and men (OR, 2.4; 95% CI, 1.18–4.82).
TABLE 1.
Sociodemographic, Sexual Behavior, and HIV/Medical Characteristics of HIV-Infected Adults in HIV Care Programs Throughout Kenya, 2010 (n = 1661)
| Characteristic | Women (n = 1063), Median (Range) or n (%) | Men (n = 598), Median (Range) or n (%) | P |
|---|---|---|---|
| Sociodemographic | |||
| Age, median (range), y | 35 (18–70) | 40 (18–77) | <0.001 |
| Currently married or cohabiting*, n (%) | 536 (50.5) | 476 (79.0) | <0.001 |
| Primary school education or less, n (%) | 556 (55.6) | 289 (48.9) | 0.013 |
| Sexual behavior, past 3 months | |||
| ≥1 partner in past 3 mo, n (%) | 587 (55.7) | 399 (67.5) | <0.001 |
| Current partner is spouse or live-in†, n (%) | 409 (72.5) | 361 (89.1) | <0.001 |
| ≤1 wk since last sex†, n (%) | 338 (57.2) | 248 (60.6) | 0.292 |
| Condom use at last sex†, n (%) | 377 (63.2) | 314 (76.6) | <0.001 |
| Reported that clinic provided condoms, n (%) | 560 (52.0) | 381 (62.1) | <0.001 |
| Medical/HIV | |||
| Diagnosed with HIV in last year, n (%) | 228 (22.0) | 142 (24.2) | 0.320 |
| Current ART use, n (%) | 773 (74.9) | 453 (77.7) | 0.322 |
| ART use if eligible, CD4 <350 cells/mm3‡, n (%) | 773 (85.6) | 453 (86.1) | 0.817 |
| ART use if eligible, CD4 <250 cells/mm3‡, n (%) | 773 (91.6) | 453 (89.4) | 0.186 |
| Cotrimoxazole use, n (%) | 626 (58.9) | 397 (66.9) | 0.039 |
| CD4 count, median (range), cells/mm3 | 344 (0–1383) | 326 (1–1368) | 0.060 |
Sampling weight and cluster information were taken into account in the analysis.
Includes polygamous marriages.
With most recent partner, among those who reported any partner in the past 3 months.
ART eligibility is based on recent (<250 cells/mm3) and current (<350 cells/mm3) Kenyan national guidelines. Participants categorized as ART eligible if currently on ART or if current CD4 count was less than 250 or less than 350 cells/mm3.
One quarter (23%) of participants had been newly diagnosed as having HIV in the past year. Among all participants, 76% were currently on ART, including 91% of those with CD4 T-lymphocyte counts less than 250 cells/mm3. Women had higher median CD4 T-lymphocyte counts than did men (344 vs 326 cells/mm3, respectively; P = 0.047). Men were more likely than women to be on cotrimoxazole (67% vs 59%, respectively; P < 0.001).
Prevalence of STI
Among the 1063 HIV-infected women, 10.9% (95% CI, 7.8–14.0) had an etiologic diagnosis of trichomoniasis. Seventeen women were diagnosed as having gonorrhea, 6 with chlamydia, and 3 with syphilis. Only 6 women had 2 of these STI diagnosed concurrently. The 598 HIV-infected men in our study had significantly lower prevalences of most STI when compared with women. Only 2.8% (95% CI, 1.3–4.2) of men had laboratory-confirmed trichomoniasis. Five men were diagnosed as having urethral gonorrhea and 5 with syphilis; no men had chlamydia or more than 1 of these STIs.
Among the 68 participants from whom genital ulcer swabs were collected, HSV-1 was detected in 3 of these swabs, HSV-2 was detected in 46, and T. palladium was detected in 1. Eighteen specimens were negative for the 3 pathogens studied.
Individuals who reported any sex partner in the past 3 months likely represent individuals at higher risk for recent infection. We found somewhat higher prevalences in this subgroup—for example, the prevalence of trichomoniasis was 12.3% (95% CI, 8.5–16.1) among women and 3.4% (95% CI, 1.4–5.4) among men.
Correlates of Trichomoniasis Diagnosis Among Women
Among women, we explored correlates of laboratory-confirmed trichomoniasis diagnosis (Table 2). Younger age, lower education level, and diagnosis of HIV within the past year were significantly associated with higher prevalence of trichomoniasis. In the multiple regression model (Table 2), younger age and lower education level remained independently associated with trichomoniasis. Similarly, the association of recent HIV diagnosis remained independently associated with trichomoniasis, after adjusting for age.
TABLE 2.
Bivariate and Multivariate Sociodemographic, Sexual Behavior, and HIV/Medical Correlates of Laboratory-Confirmed Trichomoniasis Diagnosis Among HIV-Infected Women in HIV Care Programs Throughout Kenya, 2010 (n = 1063)
| Characteristic | Trichomoniasis Diagnosis
|
Bivariate Analysis
|
Multivariable Analysis
|
|||||
|---|---|---|---|---|---|---|---|---|
| Yes (n = 115), wt% | No (n = 941), wt% | OR | 95% CI | P | OR adjusted* | 95% CI | P | |
| Sociodemographic | ||||||||
| Age | 33† | 35† 0.96 |
0.96 0.93–0.99 |
0.94–0.98 | <0.001 0.014§ |
0.96 | 0.94–0.98 | 0.007‡ |
| Currently married or cohabiting|| | 47.6 | 50.8 | 0.88 | 0.63–1.23 | 0.448 | |||
| Primary school education or less | 71.7 | 53.9 | 2.16 | 1.37–3.41 | <0.001 | 2.16 | 1.37–3.40 | 0.009‡ |
| Sexual behavior, past 3 mo | ||||||||
| ≥1 partner in past 3 mo | 62.8 | 55.0 | 1.38 | 0.86–2.22 | 0.180 | |||
| Current partner is spouse/live-in¶ | 65.8 | 73.2 | 0.70 | 0.49–1.00 | 0.154 | |||
| ≤1 wk since last sex¶ | 53.7 | 58.0 | 0.84 | 0.51–1.38 | 0.491 | |||
| Condom use at last sex¶ | 63.2 | 63.1 | 1.00 | 0.65–1.55 | 0.980 | |||
| Reported clinic provides condoms | 47.8 | 52.5 | 0.83 | 0.56–1.21 | 0.326 | |||
| Medical/HIV | ||||||||
| Diagnosed with HIV in last year | 25.6 | 8.3 | 3.79 | 1.64–8.77 | 0.002 | 3.82 | 1.59–9.21 | 0.0028§ |
| Current ART use | 71.8 | 75.5 | 0.83 | 0.55–1.25 | 0.365 | |||
| Cotrimoxazole use | 64.3 | 58.1 | 1.30 | 0.72–2.35 | 0.389 | |||
| CD4 count | 296† | 350† | 0.96# | 0.88–1.05 | 0.354 | |||
Sampling weight and cluster information were taken into account in the analysis.
Each correlate is adjusted for other variables in bivariate analysis with a P value less than 0.1.
Median.
Multivariable model including age and primary school education.
Multivariable model including age and HIV diagnosed within last year.
Includes polygamous marriages.
Among those who reported any partner in the past 3 months.
The OR reflects a 100 cells/mm3-increase in CD4 count.
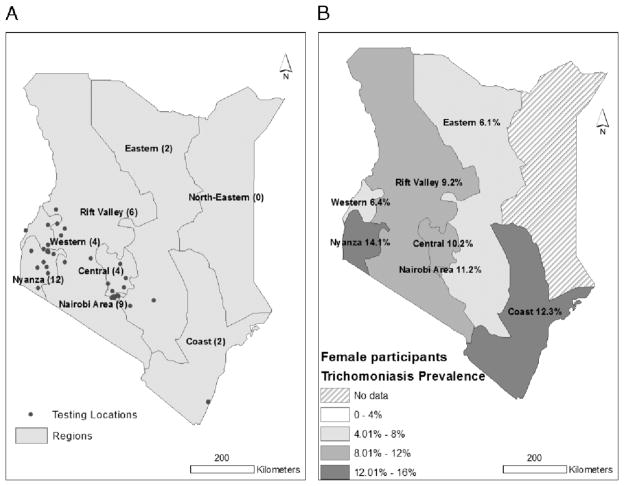
There were no significant regional differences in trichomonas prevalence among women. The provinces with the highest prevalence among women were Nyanza (14.1%) and Coast (12.3%) (Fig. 1).
Figure 1.
Maps of participating clinics (A) and regional prevalence of trichomoniasis (B) among HIV-infected women in HIV care programs throughout Kenya, 2010 (n = 1063).
DISCUSSION
This was the first large, representative study in sub-Saharan Africa to estimate the prevalence of multiple STIs and other genital infections among HIV-infected adults in HIV care programs. Approximately 11% of women had laboratory-confirmed trichomoniasis, but prevalences of other STIs—including gonorrhea, chlamydia, and syphilis—were relatively low (<1%–2%), consistent with relatively low levels of reported sexual risk behavior. In addition, 4% of individuals had genital ulcers, two thirds due to HSV-2. Currently, WHO recommends STI screening at enrollment into HIV programs,17 but many programs do not implement STI screening. Our study suggests that in large HIV care programs in Kenya, screening for trichomoniasis may be the most useful in women, whereas testing for other STIs might be less beneficial.
To our knowledge, there have been only 2 other large STI surveillance studies among HIV-infected adults in sub-Saharan Africa, neither in HIV care programs. A 1993 to 1997 study of male factory workers in Zimbabwe found that HIV-positive men had an incidence of self-reported STI syndromes (including urethral discharge, genital ulcer, and genital warts) of 16.8 per 100 person-years.18 Because of the high-risk population and the self-reported approach to identifying STI, it is difficult to compare these findings with ours. The other study enrolled HIV-infected pregnant women in Malawi, Tanzania, and Zambia between 2001 and 2003 and reported prevalence for trichomoniasis (18.8%), chlamydia (2.6%), genital ulcers (2.2%), and gonorrhea (1.7%).19 These prevalences were higher than what we observed. These differences may be attributable to regional or historic differences between populations, or to the greater likelihood of recent unprotected sex among pregnant women compared with our sample of HIV clinics, in which a third of participants reported no sexual partner in the past 3 months.
Similar to US-based studies of HIV-infected women,20 trichomoniasis had the highest prevalence among nonviral STIs assessed. Consistent with the broader epidemiology of trichomoniasis, we observed a lower prevalence among men than women. Among men, the consequences of infection are less severe. Among women, trichomoniasis can lead to birth complications including low birth weight and premature birth. Furthermore, among HIV-infected women, trichomoniasis is associated with increased vaginal HIV shedding.20 Consequently, a United States–based expert panel recommends periodic screening for trichomoniasis among HIV-infected women.21 In addition, a recent modeling study among HIV-infected women in the United States estimated that, in populations with a high prevalence of T. vaginalis, nearly a quarter of HIV infections transmitted from women to men are attributable to T. vaginalis co-infection in the female partner.22 Our study suggests that periodic screening for T. vaginalis in HIV programs in Africa may be beneficial.
We noted an appreciable prevalence of genital ulcer disease (4%), mostly due to HSV-2. The World Health Organization recommends episodic acyclovir treatment to accelerate healing and decrease HIV shedding in ulcers.13 Genital ulcer disease may not be noted in routine follow-up at HIV care programs without examination or questions regarding ulcers. Our study yielded a lower prevalence of syphilis (<1%) compared with the Kenya AIDS Indicator Survey (4.2% among HIV-infected adults), a community-based survey.23 In contrast to our study of HIV care program attendees, most HIV-infected KAIS respondents were unaware of their HIV status. Participants in HIV care programs may have less risky sexual behavior than a community-based cohort. In addition, given their attendance in a clinic-based program, our participants may also have been more likely to receive treatment for symptomatic STI and routine cotrimoxazole prophylaxis, which may have some effect on bacterial STIs.
Consistent with other sub-Saharan African studies,19,24 we found that less education and younger age were independently associated with an increased risk of trichomoniasis in women. Antenatal surveillance data from South Africa have shown a significant association between lower educational attainment and HIV risk.25 This reflects a trend observed throughout Africa in which HIV risk transitioned from being associated with higher education early in the epidemic to an association with less education in more mature epidemics.26 Likewise, it is plausible that the least educated HIV-infected women lacked appropriate knowledge and awareness of risk or symptoms for other STI. A recent analysis of data pooled from 4 female microbicide trials also found that younger age was associated with multiple incident STI outcomes (although not trichomoniasis).27 Younger women with a higher likelihood of cervical columnar ectopy may be at increased risk for STI due to age-discordant mixing, where younger women have older male partners, among whom STI prevalence is high. This pattern is consistent with other research from African that has found that age-discordant mixing is both prevalent28 and a risk factor for HIV infection.29 We did not collect data on partner age, so we were unable to look at age mixing directly in our data.
We found that a relatively high proportion of both HIV-infected women (65%) and men (78%) reported condom use at last sex. These estimates are consistent with estimates from a recent cross-sectional study of HIV-infected Kenyans living in the Kibera settlement, which found that 82% of men and 65% of women reported consistent condom use.30 We observed that women and men who reported availability of condoms at the HIV clinic had a 1.7- to 2.4-fold increased likelihood of reporting condom use at last sex, suggesting that provision of condoms and advertising their availability may promote their use.
The primary strength of this study was the inclusion of a sample of the largest HIV care programs across Kenya. Coupled with the systematic sampling scheme, the STI prevalence estimates from this study are generalizable to HIV-infected adults receiving care at large facilities throughout Kenya. Our study protocol included etiologic testing for several STIs, which is superior to syndromic diagnosis that may fail to detect asymptomatic infections or lead to misclassification of STI. Finally, our study is relevant to prevention interventions in HIV care programs. We anticipate that our data will inform cost-effectiveness assessments to determine the merit of annual STI screening within HIV programs.
Our study also has limitations. First, given the relatively low overall prevalence of STI we observed, we were unable to evaluate correlates of individual STI other than trichomoniasis in women. We used urine detection for male trichomoniasis and would have had higher detection with the addition of semen and urethral swabs.31 The purpose of our study was to determine the use of STI screening in HIV care programs; hence, we did not sample individuals in the community. We elected to broadly sampled large HIV programs, which represent most HIV-infected adults in care programs. It is possible that smaller clinics may have different STI prevalence; however, we do not have reasons to believe that STI prevalence in smaller clinics would differ. Finally, the sexual behavior measures we assessed may have been subject to social desirability bias if participants who engaged in high-risk sexual behaviors were less likely to report this. Similarly, social desirability bias may have influenced our observed association between condom provision and reported condom use.
Integrating STI treatment into routine HIV care may benefit individual health, and its potential impact on population-level transmission is undefined. Our study suggests that based on the STI prevalence among HIV-infected adults, annual screening for trichomoniasis in HIV-infected women might be beneficial. The relatively older ages of women in this study suggest that many younger women are not enrolled in HIV care, pointing to a gap in services. Presumably, many younger HIV-infected women diagnosed in prevention-of-mother-to-child-transmission programs did not enroll in HIV care after delivery. Our data suggest that these women may benefit most from trichomoniasis screening. Further studies to determine relative benefits of targeted screening or syndromic management will be important to help HIV programs decide which approaches jointly and sustainably optimize individual, program, and population outcomes.
Acknowledgments
The authors thank Jonathan Glick for creating the maps used in this manuscript.
Supported by the Centers for Disease Control and Prevention (U62/CCU024512) and the President’s Emergency Plan for AIDS Relief through the Centers for Disease Control and Prevention under the terms of cooperative agreement U62PS024512-05.
Footnotes
The authors state no conflicts of interest.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the US Centers for Disease Control and Prevention. The use of trade names is for identification purposes only and does not constitute endorsement by the US Centers for Disease Control and Prevention or the Department of Health and Human Services.
Author contributions: Sara Nelson Glick, Benson Singa, Naomi Bock, and Grace John-Stewart conceptualized the present analysis. Sara Nelson Glick conducted the statistical analyses and wrote the manuscript. Grace John-Stewart and Naomi Bock conceptualized and designed the study and obtained funding. Benson Singa and Gaston Djomand designed study procedures, and Benson Singa oversaw field implementation of the study procedures. Judd Walson, James Odek, and R. Scott McClelland contributed to the design, conduct, and interpretation of study results. Linda Chaba and Hongjiang Gao provided data management and statistical analysis support. All authors contributed reviewed, edited, and approved the final version of this article.
References
- 1.Wasserheit JN. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 2.McClelland RS, Lavreys L, Katingima C, et al. Contribution of HIV-1 infection to acquisition of sexually transmitted disease: A 10-year prospective study. J Infect Dis. 2005;191:333–338. doi: 10.1086/427262. [DOI] [PubMed] [Google Scholar]
- 3.Kreiss J, Willerford DM, Hensel M, et al. Association between cervical inflammation and cervical shedding of human immunodeficiency virus DNA. J Infect Dis. 1994;170:1597–1601. doi: 10.1093/infdis/170.6.1597. [DOI] [PubMed] [Google Scholar]
- 4.Mostad SB, Overbaugh J, DeVange DM, et al. Hormonal contraception, vitamin A deficiency, and other risk factors for shedding of HIV-1 infected cells from the cervix and vagina. Lancet. 1997;350:922–927. doi: 10.1016/S0140-6736(97)04240-2. [DOI] [PubMed] [Google Scholar]
- 5.Wright TC, Jr, Subbarao S, Ellerbrock TV, et al. Human immunodeficiency virus 1 expression in the female genital tract in association with cervical inflammation and ulceration. Am J Obstet Gynecol. 2001;184:279–285. doi: 10.1067/mob.2001.108999. [DOI] [PubMed] [Google Scholar]
- 6.Ghys PD, Fransen K, Diallo MO, et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Cote d’Ivoire. AIDS. 1997;11:F85–93. doi: 10.1097/00002030-199712000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Cu-Uvin S, Hogan JW, Caliendo AM, et al. Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. Clin Infect Dis. 2001;33:894–896. doi: 10.1086/322613. [DOI] [PubMed] [Google Scholar]
- 8.Sha BE, Zariffard MR, Wang QJ, et al. Female genital-tract HIV load correlates inversely with Lactobacillus species but positively with bacterial vaginosis and Mycoplasma hominis. J Infect Dis. 2005;191:25–32. doi: 10.1086/426394. [DOI] [PubMed] [Google Scholar]
- 9.Fleming DT, Wasserheit JN. From epidemiological synergy to public health policy and practice: The contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosskurth H, Mosha F, Todd J, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: Randomised controlled trial. Lancet. 1995;346:530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 11.Gray RH, Wawer MJ. Reassessing the hypothesis on STI control for HIV prevention. Lancet. 2008;371:2064–2065. doi: 10.1016/S0140-6736(08)60896-X. [DOI] [PubMed] [Google Scholar]
- 12.Hayes R, Watson-Jones D, Celum C, et al. Treatment of sexually transmitted infections for HIV prevention: End of the road or new beginning? AIDS. 2010;24(suppl 4):S15–S26. doi: 10.1097/01.aids.0000390704.35642.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. [Accessed December 19, 2012];Essential Prevention and Care Interventions for Adults and Adolescents Living With HIV in Resource-Limited Settings. 2008 Available at: http://www.who.int/entity/hiv/pub/prev_care/OMS_EPP_AFF_en.pdf.
- 14.President’s Emergency Plan for AIDS Relief. [Accessed December 19, 2012];Partnership to Fight HIV/AIDS in Kenya. 2011 Available at: http://www.pepfar.gov/countries/kenya/index.htm.
- 15.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Politch JA, Mayer KH, Welles SL, et al. Highly active antiretroviral therapy does not completely suppress HIV in semen of sexually active HIV-infected men who have sex with men. AIDS. 2012;26:1535–1543. doi: 10.1097/QAD.0b013e328353b11b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO. Sexual and Reproductive Health of Women Living With HIV/AIDS: Guidelines on Care, Treatment and Support for Women Living With HIV/AIDS and Their Children in Resource-Constrained Settings. Geneva, Switzerland: 2006. [Accessed December 19, 2012]. Available at: HYPERLINK http://www.who.int/hiv/pub/guidelines/sexualreproductivehealth.pdf. [Google Scholar]
- 18.Machekano RN, Bassett MT, Zhou PS, et al. Report of sexually transmitted diseases by HIV infected men during follow up: Time to target the HIV infected? Sex Transm Infect. 2000;76:188–192. doi: 10.1136/sti.76.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aboud S, Msamanga G, Read JS, et al. Genital tract infections among HIV-infected pregnant women in Malawi, Tanzania and Zambia. Int J STD AIDS. 2008;19:824–832. doi: 10.1258/ijsa.2008.008067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kissinger P, Amedee A, Clark RA, et al. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex Transm Dis. 2009;36:11–16. doi: 10.1097/OLQ.0b013e318186decf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aberg JA, Kaplan JE, Libman H, et al. Primary care guidelines for the management of persons infected with human immunodeficiency virus: 2009 Update by the HIV medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:651–681. doi: 10.1086/605292. [DOI] [PubMed] [Google Scholar]
- 22.Quinlivan EB, Patel SN, Grodensky CA, et al. Modeling the impact of Trichomonas vaginalis infection of HIV transmission in HIV-infected individuals in medical care. Sex Transm Dis. 2012;39:671–377. doi: 10.1097/OLQ.0b013e3182593839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NASCOP. 2007 Kenya AIDS Indicator Survey: Final Report. Nairobi, Kenya: National AIDS and STI Control Programme; 2009. [Google Scholar]
- 24.Buve A, Weiss HA, Laga M, et al. The epidemiology of trichomoniasis in women in four African cities. AIDS. 2001;15(suppl 4):S89–S96. doi: 10.1097/00002030-200108004-00010. [DOI] [PubMed] [Google Scholar]
- 25.Johnson LF, Dorrington RE, Bradshaw D, et al. The effect of educational attainment and other factors on HIV risk in South African women: Results from antenatal surveillance, 2000–2005. AIDS. 2009;23:1583–1588. doi: 10.1097/QAD.0b013e32832d407e. [DOI] [PubMed] [Google Scholar]
- 26.Hargreaves JR, Bonell CP, Boler T, et al. Systematic review exploring time trends in the association between educational attainment and risk of HIV infection in sub-Saharan Africa. AIDS. 2008;22:403–414. doi: 10.1097/QAD.0b013e3282f2aac3. [DOI] [PubMed] [Google Scholar]
- 27.Feldblum PJ, Lie CC, Weaver MA, et al. Baseline factors associated with incident HIV and STI in four microbicide trials. Sex Transm Dis. 2010;37:594–601. [PubMed] [Google Scholar]
- 28.Ott MQ, Barnighausen T, Tanser F, et al. Age-gaps in sexual partnerships: seeing beyond ‘sugar daddies’. AIDS. 2011;25:861–863. doi: 10.1097/QAD.0b013e32834344c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly RJ, Gray RH, Sewankambo NK, et al. Age differences in sexual partners and risk of HIV-1 infection in rural Uganda. J Acquir Immune Defic Syndr. 2003;32:446–451. doi: 10.1097/00126334-200304010-00016. [DOI] [PubMed] [Google Scholar]
- 30.Ragnarsson A, Ekstrom AM, Carter J, et al. Sexual risk taking among patients on antiretroviral therapy in an urban informal settlement in Kenya: A cross-sectional survey. J Int AIDS Soc. 2011;14:20. doi: 10.1186/1758-2652-14-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaydos-Daniels SC, Miller WC, Hoffman I, et al. The use of specimens from various genitourinary sites in men, to detect Trichomonas vaginalis infection. J Infect Dis. 2004;189:1926–1931. doi: 10.1086/386309. [DOI] [PubMed] [Google Scholar]