Abstract
Objective
Although concern about chronic ketamine abuse has grown, the characteristic symptomatology of chronic ketamine users has yet to be examined. This study aims to measure the psychotic, depressive and anxiety symptoms in chronic ketamine users.
Methods
A group of chronic ketamine users in Guangzhou, China were evaluated. The socio-demographic and drug use characteristics of subjects were documented. Symptoms of psychosis, depression, anxiety were evaluated by the Positive and Negative Syndrome Scale (PANSS), Beck Depression Inventory (BDI) and Beck Anxiety Inventory (BAI). The severity of the symptoms was identified by standard severity cutoffs.
Results
The PANSS total score, positive symptom, negative symptom, general psychopathology subscale score were 45.3±8.4, 8.0±1.7, 13.2± 3.9 and 24.2± 4.9 respectively. BDI and BAI score was 13.1±6.5 and 15.7±9.6 respectively. 77.5% and 46.0% of the subjects showed moderate to severe depressive symptoms and anxiety symptoms respectively. The BDI score was positively correlated with ketamine use frequency. The BAI score was positively correlated with ketamine use frequency.
Conclusions
Depressive symptoms were commonly presented in chronic ketamine users. The higher ketamine use frequency and dosage were associated with more severe depressive symptoms.
Keywords: Ketamine, Symptoms, Depression, Psychosis
1. Introduction
Ketamine, an N-methyl-D-aspartate (NMDA) receptor antagonist was developed in the 1960s and is widely used in medicine for anesthesia and pain management. Its use as a recreational drug of abuse has become widespread, in recent decades, particularly in Europe and Southern China. Ketamine has also attracted the attention of researchers as a potential model of psychopathology because it provokes distinctive psychological symptoms in humans and rodents (Becker et al., 2003; Aalto et al., 2005; Frohlich and Van Horn, 2014). Krystal et al. (1994) reported that a single intravenous dose of ketamine given to healthy volunteers produced acute psychotic symptoms as well as impairment of memory. Recreational ketamine use has been reported to produce psychotic symptoms similar to those observed in schizophrenia (Morgan et al., 2004b).
In addition to these short-term experimental studies, clinicians have observed a broad array of clinical symptoms in chronic ketamine users, although few systematic studies of symptom patterns associated with chronic ketamine use have been published. It was reported that chronic ketamine users had higher levels of subthreshold psychotic symptoms (Stone et al., 2013). In one study it was reported that the depression scores increased in a group of chronic ketamine users over a 12 month period (Morgan et al., 2010). But the assessments were done in a relatively small sample size and they did not measure the psychosis or anxiety symptoms which were observed in chronic ketamine users. Tang et al. (2013) reported depressive symptoms were more frequently found in current ketamine users than ex-ketamine users and control. Morgan and Curran (2012) reviewed recent literature and noted that ketamine users sometimes reported psychotic symptoms but concluded that there was little evidence of any link between chronic heavy use of ketamine and a subsequent diagnosis of a psychotic disorder. Since experimental studies have linked ketamine to both psychotic and depressive symptoms, a more systematic study of the symptoms associated with chronic ketamine abuse may be informative.
In the present study we recruited chronic ketamine users hospitalized at 2 hospitals in Guangzhou, China for detoxification and/or treatment of symptoms related to chronic ketamine use. All subjects were assessed with standard and widely used measures of schizophrenia, depression and anxiety symptomatology: the Positive and Negative Syndrome Scale (PANSS) for psychotic symptoms (Kay et al., 1988), the Beck Depression Inventory (BDI) (Beck et al., 1961) and the Beck Anxiety Inventory (BAI) (Beck et al., 1988) for depressive and anxiety symptoms, respectively. The severity of these symptoms was identified by standard published severity cutoffs on the PANSS, BDI and BAI. Using these measures we sought to systematically profiling the symptoms associated with chronic ketamine use. We thus seek to better understand the problems faced by chronic ketamine users.
2. Methods
2.1. Sample and data collection
From January 2012 to December 2013, 187 ketamine users who were voluntarily hospitalized for detoxification and/or for treatment of symptoms related to long term ketamine use and who were willing to join the study were recruited at the substance-abuse department of the Guangzhou Brain Hospital and the voluntary drug rehabilitation ward of Guangzhou Baiyun mental hospital.
All the participants underwent a 2-h semi-structured interview to assess sociodemographic characteristics, psychopathological status, and substance use during the first two weeks of their current hospitalization. Current illicit drug use of all participants was validated through urine toxicology as well through self-report data. The interviews were conducted by clinicians with 3 or more years of clinical experience. Inclusion criteria required that participants: 1) be chronic ketamine users admitted to Guangzhou Brain Hospital or Guangzhou Baiyun Mental Health Hospital for detoxification or treatment of ketamine-related symptoms; 2) no other substance dependence other than ketamine and tobacco according to DSM-IV-TR; 3) subjects with ketamine as a drug of choice for longer than 6 months; 4) being capable of providing written informed consent form. Exclusion criteria included: (1) any known organic diseases or (2) history of head trauma with loss of consciousness, (3) any unstable physical illnesses, or (4) impairment of color vision or hearing. The study was approved by the Institutional Ethics Committee and written informed consents were signed prior to enrollment.
2.2. Measures
2.2.1. Socio-demographic characteristics
The subjects' age, gender, marital status, years of education, employment, medical history and family relationships were documented.
2.2.2. Drug use
Information on drug use included age of first ketamine use, lifetime duration of ketamine use, frequency of ketamine use in the past month, recent polydrug use, smoking patterns and history of alcohol use.
2.2.3. Standardized scales
The Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1988), a structured rating scale for the symptoms of schizophrenia was assessed by trained raters. The cutoffs used for PANSS were <58 less than mildly ill, 58–75 mildly ill, 75–95 moderately ill, >95 markedly ill, >116 severely ill (Leucht et al., 2005).
Ketamine users were asked to complete Beck Depression Inventory (BDI,13-item; Beck and Beamesderfer, 1974) and the Beck Anxiety Inventory (BAI; Beck et al., 1988) which assessed their depressive and anxiety symptoms during the two weeks before they were hospitalized., The cutoffs used for BDI, a widely used structured rating scale for depression,were 0–4 for no depression, 5–7 for mild depression, 8–15 for moderate depression, and ≥16 severe depression(Beck, Epstein et al. 1974). The cutoffs used for BAI, a structured rating scale for anxiety disorder were 0–7 minimal level of anxiety, 8–15 mild anxiety, 16–25 moderate anxiety, 26–63 severe anxiety (Beck et al., 1988).
Assessment of inter-rater reliability for raters in this study was in the excellent to good range on the PANSS, with intra-class correlations ranging from 0.90 to 0.96.
2.3. Analyses
Symptom levels on the PANSS, BDI and BAI were classified using published, standard severity levels (Beck and Beamesderfer, 1974; Beck et al., 1988; Leucht et al., 2005) to determine whether symptom patterns were more reflective of schizophrenia, depression or anxiety disorder.
Bivariate spearman correlation analysis was used to identify correlations between socio-demographic, substance use characteristics and symptom measures. Further multiple linear regression analysis were done on variables which were significant on bivariate spearman correlation analysis.
All analyses were conducted under SPSS version 13.0.
3. Results
3.1. Participant characteristics
Socio-demographic characteristics, drug use and PANSS, BDI, BAI scores are presented in Table 1. Altogether there were 187 chronic ketamine users enrolled in the study of which 173 were male (92.5%), 14 were female (7.5%). The average age was 26.2±5.0 (Mean± SD) (range 15–44 years). The average time from first ketamine use to the present was 6.3±3.1 years (Mean±SD) and the time from becoming dependent to the present was 3.1±2.0 years (Mean±SD). The average dose of ketamine on a typical day of use in the past 30 days was 3.4±2.7 gram/day (Mean± SD). 141 (75.4%) patients reported using ketamine daily, 14 patients (7.3%) used ketamine more than 4 times per week, and 32 patients (17.1%) used ketamine less than 4 times per week. There were 158 subjects (84.5%) who had used psychoactive drugs other than ketamine, although all the subjects were with ketamine as a drug of choice for longer than 6 months.
Table 1.
Sample characteristics (mean±S.D.).
| Ketamine abuser | |
|---|---|
| Gender (males/females) | 173(92.5%)/14(7.5%) |
| Age | 26.2±5.0 |
| Years of education | 10.5±2.7 |
| Marital status (single/married) | 101/86 |
| Employment (employed/unemployed) | 135/52 |
| Family history of mental illness(yes/no) | 12/175 |
| Family history of drug abuse(yes/no) | 59/128 |
| Past hospitalization for ketamine use(yes/no) | 77/110 |
| Age of first ketamine use | 20.0±5.0 |
| Duration of ketamine use(year)a | 6.3±3.1 |
| Duration of dependence(year)b | 3.1±2.0 |
| Current frequency of usec | |
| daily | 141(75.4%) |
| ≧4/week | 14(7.5%) |
| <4/week | 32(17.1%) |
| Current average dose/day (g) | 3.4±2.7 |
| Current maximum dose (g) | 6.9±6.0 |
| Polydrug use d(yes/no) | 158/29 |
| Smoking (yes/no) | 180/7 |
| Alcohol drinking (yes/no) | 124/63 |
| PANSS total scoree | 45.3±8.4 |
| Positive symptom subscore | 8.0±1.7 |
| Negative symptom subscore | 13.2±3.9 |
| General psychopathology subscore | 24.2±4.9 |
| Beck Depression Inventoryscore | 13.1±6.5 |
| Beck Anxiety Inventory score | 15.7±9.6 |
Data are the mean±standard deviation. Data about use of other psychoactive compounds, alcohol consumption and tobacco smoking are the amount of cases (percentage).
Total months of ketamine use from the first time till now.
Total months of ketamine use from dependent till now.
Frequency of ketamine use in recent one month.
Use of other psychoactive compounds in one's life span, alcohol and tobacco excluded.
Positive and negative symptom scale.
3.2. Evaluation by standardized scales
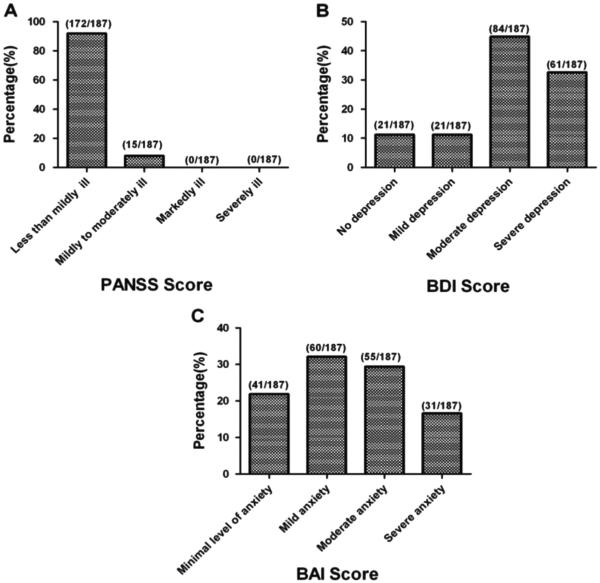
The average PANSS total score, positive symptom subscore, negative symptom subscore and general psychopathology subscore were 45.3±8.4, 8.0±1.7, 13.2± 3.9 and 24.2± 4.9 respectively. BDI score was 13.1±6.5. BAI score was 15.7±9.6.The percentages of subjects clarified by the cutoffs on standardized scales are presented in Fig. 1. On the PANSS, 172 (92.0%) had PANSS total scores less than 58, indicating less than mild or minimal illness; 15 (8.0%) had PANSS total scores between 58 and 75, indicating for mild illness. No PANSS total scores were in the moderate or severe range. On the BDI, 21 subjects (11.2%) scored 0–4 (no depression), 21 (11.2%) scored 5–7 (mild depression), 84 (44.9%) scored 8–15 (moderate depression), the most common response range, and 61 (32.6%) scored ≥16 (severe depression), the second most common response range with a resulting total of 77.5% in the moderate to severe range. For the measure of anxiety, 41 (21.9%) subjects showed minimal level of anxiety (score= 0–7), 60 (32.1%) showed mild anxiety (score 8–15), 55 (29.4%) moderate anxiety (score 16–25), and 31 (16.6%) severe anxiety (score 26–63), for a total of 46.0% in the moderate-severe range. Thus schizophrenia symptoms were predominantly less than mild; anxiety symptoms, mostly mild or less but with almost one-third in the moderate range; and three fourths of patients showing depressive symptoms in the moderate to severe range.
Fig. 1.
Ketamine users' evaluation by PANSS, BDI and BAI A: the percentages of subjects clarified by the cutoffs on PANSS. B: the percentages of subjects clarified by the cutoffs on BDI. C: the percentages of subjects clarified by the cutoffs on BAI.
3.3. Correlation between symptom measures and substance use characteristics
Bivariate correlation analysis (Table 2) showed that the BDI score was positively correlated with ketamine use frequency, duration of ketamine dependence, average and maximum ketamine dose as well as history of past hospitalization. The BAI score was positively correlated with frequency of ketamine use. The PANSS total score was positively correlated with duration of ketamine dependence and past hospitalization. The PANSS positive subscale score was positively correlated with past hospitalization and negatively correlated with current average ketamine dosage. The PANSS general psychopathology subscale score was positively correlated with duration of ketamine dependence and past hospitalization. Further multiple linear regression analysis (Table 3) on variables which were significant on bivariate correlation analysis showed that the BDI score was significantly positively correlated with ketamine use frequency. The BAI score was positively correlated with frequency of ketamine use. The PANSS total score was positively correlated with past hospitalization. The PANSS positive subscale score was positively correlated with past hospitalization and negatively correlated with average ketamine dosage.
Table 2.
The correlation between symptom measures and substance use characteristics.
| BDI score | BAI score | PANSS total scorea | P Subscoreb | N Subscorec | G subscored | |
|---|---|---|---|---|---|---|
| Ketamine use frequency | 0.244** | 0.233** | 0.061 | −0.009 | −0.007 | 0.102 |
| Age of first ketamine use | −0.099 | −0.090 | 0.004 | 0.015 | −0.082 | 0.043 |
| Duration of ketamine use | 0.046 | −0.038 | −0.014 | −0.006 | −0.022 | 0.009 |
| Duration of ketamine dependence | 0.158* | 0.141 | 0.161* | 0.120 | 0.093 | 0.182* |
| Average ketamine dose | 0.204** | 0.104 | −0.075 | −0.156* | −0.033 | −0.061 |
| Maximum ketamine dose | 0.180* | 0.107 | −0.097 | −0.132 | −0.060 | −0.094 |
| Poly drug use | 0.054 | 0.046 | −0.02 | 0.083 | −0.020 | −0.004 |
| Past hospitalization | 0.169* | 0.108 | 0.164* | 0.172* | 0.140 | 0.152* |
Values listed in the table were R2 for spearman correlation
P<0.05.
P<0.01.
Positive and negative symptom scale.
PANSS positive symptom subscale.
PANSS negative symptom subscale.
PANSS general psychopathology subscale.
Table 3.
The standardized coefficient between symptom measures and substance use characteristics using multiple linear regressions.
| B | Beta | P | R2 | |
|---|---|---|---|---|
| BDI | 0.108 | |||
| Ketamine use frequency | 1.241 | .283 | <.001** | |
| Duration of ketamine dependence | .008 | .030 | .692 | |
| Maximum ketamine dose | .208 | .089 | .414 | |
| Average ketamine dose | −.076 | −.070 | .528 | |
| Past hospitalization | 1.551 | .118 | .130 | |
| BAI | .063 | |||
| Ketamine use frequency | 1.048 | .251 | .001** | |
| PANSS total score | 0.044 | |||
| Duration of ketamine dependence | .036 | .100 | .181 | |
| Past hospitalization | 2.751 | .162 | .031* | |
| P Subscore | 0.045 | |||
| Average ketamine dose | −.104 | −.164 | .027* | |
| Past hospitalization | .578 | .166 | .026* | |
| G Subscore | 0.045 | |||
| Duration of ketamine dependence | .028 | .136 | .068 | |
| Past hospitalization | 1.314 | .133 | .075 |
P<0.05.
P<0.001.
4. Discussion
The present study used standard scales to characterize the psychotic, depressive and anxiety symptoms of chronic ketamine users who were hospitalized for detoxification or treatment of ketamine-related symptoms. The sample included a relatively large group of ketamine users who frequently used ketamine at relatively high doses over an average of more than six years.
In this group of chronic ketamine users standardized scales showed a predominance of depressive symptoms followed by anxiety symptoms with 77.5% having moderate to severe depressive symptoms, 46% having moderate to severe anxiety symptoms, and scores on a measure of psychotic symptoms (the PANSS) all in the minimal to mild range.
In 2000 and 2004 Curran & Morgan reported slightly higher scores on depression among ketamine users as compared to non-users measured with the Hospital Anxiety and Depression scale (HADS) on the night of drug use and three days later (Curran and Morgan, 2000; Morgan et al., 2004a). They again reported higher scores of ketamine users on depression when measured at a 3 year follow-up assessments as compared to a control group who did not use ketamine, although the ketamine users were not within a clinical range of depression. (Curran and Morgan, 2000; Morgan et al., 2004a). Another study in community ketamine users found that 35% of current ketamine users suffered from a mood or anxiety or psychiatric disorder (Tang et al., 2014). Our findings reveal somewhat similar features in chronic ketamine users with predominantly depressive symptoms. In Curran & Morgan's study the frequency of ketamine use and dosage of the subjets decreased by over 80% after 3 years which suggest a non–addictive use pattern. In contrast, most subjects in present study had developed dependence on ketamine and the dosage and frequency had increased over time suggesting that the pronounced more severe depressive symptoms may be specifically associated with prolonged use.
Bivariate correlation analysis showed that the higher frequency and dosage of ketamine use were associated with higher BDI score. But the correlation coefficient of 0.2 is of low correlation even significant. Acute ketamine has been shown to have a rapid-acting antidepressant effect (Berman et al., 2000). Acute and chronic ketamine use may have different effects. We must, nevertheless be cautious in imputing causality to ketamine because we do not have data on the depressive status of the subjects before they began to use ketamine. It is possible that they had depressive symptoms before initiating ketamine use and turned to ketamine to relieve their underlying depression. The evidence presented here reports that of both more frequent and higher dose of ketamine was associated with more severe symptoms on BDI. Alternatively their depressive symptoms may reflect a withdrawal effect or rebound, or the development of tolerance to the long-term antidepressant effects of ketamine. In a study which investigated different drug type users reported that ketamine-only group scored higher on the subscales of Anxiety-Depression of the Brief Psychiatric Rating Scale (BPRS) than amphetamine type stimulant (ATS)-only group (Zhang et al., 2014). In present study we found polydrug use history has no significant correlation with depressive symptoms. It is possible that depressive symptoms were characteristically more predominant and common in ketamine users. Further studies are needed to clarify the relationship between chronic ketamine use and depressive mood and the comorbidity of depressive with ketamine users.
Although the present study employed a relatively large sample of chronic ketamine users, several limitations should be acknowledged. First, this group of ketamine users was composed of inpatients that sought voluntary hospitalization and would like to join the study. Their presentation may not thus be generalizable to other chronic ketamine users. Second, the measurement of depression and anxiety were based on self-report scales in Chinese translation, albeit widely used and well-validated scales in both China and elsewhere in the world, evaluations by physicians may also be necessary. Further assessment from clinically experienced physicians using standard rating scales would have helped confirm the study findings. Third, even though the Chinese version of the scales were widely used and validated, the cut-offs of the scales were the ones for the English version even the same cut-offs were also used in other study on Chinese population cautiousness is needed (Wang et al.1999; Wang et al. 2012). Finally, we cannot make causal conclusions that the observed symptoms are due to chronic ketamine use as this is an cross-sectional non-controlled study and there may have been other pharmacologic or psychosocial etiologies of the observed symptoms. In spite of these potential limitations this study clearly revealed a predominance of depressive symptoms in chronic ketamine users, and observation which deserves further study.
5. Conclusion
Using multiple measurements and analytic strategies, this study revealed mild psychotic symptoms and moderate to severe depression and anxiety symptoms in chronic ketamine users. Patterns of heavier reported ketamine use were associated with more severe symptoms.
Acknowledgment
This study was funded by grants to N.F. from Chinese National Natural Science Funds (No: 81571304) Guangzhou Municipal Key Discipline in Medicine to Guangzhou Brain Hospital (No. GBH2014-ZD03), and by grants to H.H. from Chinese National Natural Science Funds (No: 81371506) and grant from Guangdong Natural Science Funds (No: S2013040012414).
We would like to thank all the staffs of the ward 1 and 2 in Guangzhou Baiyun voluntary drug rehabilitation hospital for their invaluable assistance.
Reference
- Aalto S, Ihalainen J, Hirvonen J, Kajander J, Scheinin H, Tanila H, Någren K, Vilkman H, Gustafsson LL, Syvälahti E, Hietala J. Cortical glutamate-dopamine interaction and ketamine-induced psychotic symptoms in man. Psychopharmacology. 2005;182:375–383. doi: 10.1007/s00213-005-0092-6. [DOI] [PubMed] [Google Scholar]
- Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. Mod. Probl. Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Eebaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Becker A, Peters B, Schroeder H, Mann T, Huether G, Grecksch G. Ketamine-induced changes in rat behaviour: a possible animal model of schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:687–700. doi: 10.1016/S0278-5846(03)00080-0. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Curran HV, Morgan C. Cognitive, dissociative and psychotogenic effects of ketamine in recreational users on the night of drug use and 3 days later. Addiction. 2000;95:575–590. doi: 10.1046/j.1360-0443.2000.9545759.x. [DOI] [PubMed] [Google Scholar]
- Frohlich J, Van Horn JD. Reviewing the ketamine model for schizophrenia. J. Psychopharmacol. 2014;28:287–302. doi: 10.1177/0269881113512909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry. Res. 1988;23:99–110. doi: 10.1016/0165-1781(88)90038-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr., Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophr. Res. 2005;79:231–238. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Curran HV. Ketamine use: a review. Addiction. 2012;107:27–38. doi: 10.1111/j.1360-0443.2011.03576.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Muetzelfeldt L, Curran HV. Consequences of chronic ketamine self-administration upon neurocognitive function and psychological wellbeing: a 1-year longitudinal study. Addiction. 2010;105:121–133. doi: 10.1111/j.1360-0443.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Monaghan L, Curran HV. Beyond the K-hole: a 3-year longitudinal investigation of the cognitive and subjective effects of ketamine in recreational users who have substantially reduced their use of the drug. Addiction. 2004a;99:1450–1461. doi: 10.1111/j.1360-0443.2004.00879.x. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Riccelli M, Maitland CH, Curran HV. Long-term effects of ketamine: evidence for a persisting impairment of source memory in recreational users. Drug. Alcohol. Depend. 2004b;75:301–308. doi: 10.1016/j.drugalcdep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Stone JM, Pepper F, Fam J, Furby H, Hughes E, Morgan C, Howes OD. Glutamate,N-acetylaspartateand psychotic symptoms in chronic ketamine users. Psychopharmacology. 2013;231(10):2107–2116. doi: 10.1007/s00213-013-3354-8. [DOI] [PubMed] [Google Scholar]
- Tang WK, Liang HJ, Lau GC, Tang A, Ungvari GS. Relationship between cognitive impairment and depressive symptoms in current ketamine users. J. Stud. Alcohol Drugs. 2013;74(3):460–468. doi: 10.15288/jsad.2013.74.460. [DOI] [PubMed] [Google Scholar]
- Tang WK, Morgan CJ, Lau GC, Liang HJ, Tang A, Ungvari GS. Psychiatric morbidity in ketamine users attending counseling and youth outreach services. Subst. Abus. 2014;36:67–74. doi: 10.1080/08897077.2014.935560. [DOI] [PubMed] [Google Scholar]
- Wang XD, Wang XL, Ma H. Rating scales for mental health. Chinese Mental Health Journal, Beijing. 1999 [Google Scholar]
- Wang XQ, Liang CM, Li XD. Comparing BDI-13, social support questionnaire and VAS in 30 heroine dependents and 30 ketamine dependents. Chin. J. Drug. Abus. Prev. Treat. 2012;18(3):138–140. [Google Scholar]
- Zhang Y, Xu Z, Zhang S, Desrosiers A, Schottenfeld RS, Chawarski MC. Profiles of psychiatric symptoms among amphetamine type stimulant and ketamine using inpatients in Wuhan, China. J. Psychiatr. Res. 2014;53:99–102. doi: 10.1016/j.jpsychires.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]