Abstract
Purpose of the Study:
A comparison of longitudinal global cognitive functioning in women Veteran and non-Veteran participants in the Women’s Health Initiative (WHI).
Design and Methods:
We studied 7,330 women aged 65–79 at baseline who participated in the WHI Hormone Therapy Trial and its ancillary Memory Study (WHIMS). Global cognitive functioning (Modified Mini-Mental State Examination [3MSE]) in Veterans (n = 279) and non-Veterans (n = 7,051) was compared at baseline and annually for 8 years using generalized linear modeling methods.
Results:
Compared with non-Veterans, Veteran women were older, more likely to be Caucasian, unmarried, and had higher rates of educational and occupational attainment. Results of unadjusted baseline analyses suggest 3MSE scores were similar between groups. Longitudinal analyses, adjusted for age, education, ethnicity, and WHI trial assignment revealed differences in the rate of cognitive decline between groups over time, such that scores decreased more in Veterans relative to non-Veterans. This relative difference was more pronounced among Veterans who were older, had higher educational/occupational attainment and greater baseline prevalence of cardiovascular risk factors (e.g., smoking) and cardiovascular disease (e.g., angina, stroke).
Implications:
Veteran status was associated with higher prevalence of protective factors that may have helped initially preserve cognitive functioning. However, findings ultimately revealed more pronounced cognitive decline among Veteran relative to non-Veteran participants, likely suggesting the presence of risks that may impact neuropathology and the effects of which were initially masked by Veterans’ greater cognitive reserve.
Key Words: Women, Veterans, Cognition, Cognitive decline, Risk factors
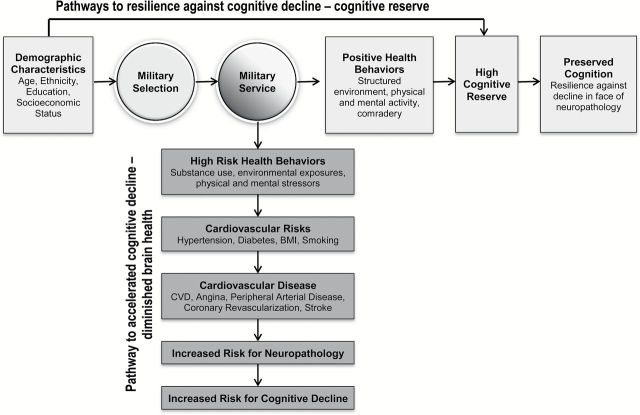
The nearly 400,000 women veterans currently aged 65 and older living in the United States today represent the oldest living cohort of women Veterans, and includes those who served in World War II (WWII), the Korean War, and the conflict in Vietnam (U.S. Department of Veterans Affairs, 2014; Weitlauf et al., 2015). This cohort of women Veterans has received limited empirical attention, particularly with respect to health and cognition in later life. As such, research that evaluates risk for cognitive decline is critically needed. Given the complex array of military-related health resilience and risk factors evident in Veterans (Figure 1; Frayne et al., 2006), important differences in the cognitive trajectories of women Veterans compared with non-Veterans might be expected. Research that illuminates these potential distinctions related to cognitive functioning could help with the development of effective early identification and intervention efforts and may guide health care policy and practices pertinent to the care of older women Veterans.
Figure 1.
Conceptual framework adapted from biopsychosocial model of healthy aging (Seeman & Crimmins, 2001).
Military Selection and Pathways to Resilience: Cognitive Reserve
Military selection, particularly for women who would now be aged 65 or older, was predicated on robust good health, educational achievement, and other markers of psychosocial adjustment. Specifically, women Veterans who served during WWII and the Korean War were more likely to be Caucasian and have higher educational attainment than non-Veteran women of the same age (Frayne et al., 2006; Weitlauf et al., 2015). In addition, military service is associated with protective factors such as highly stimulating work environment, physical and mental activity, and social comradery (Schooler, Mulatu, & Oates, 1999; Yaffe, Hoang, Byers, Barnes, & Friedl, 2014). One theoretical framework that could explain how military selection and service might impact cognitive functioning is Stern’s theory of cognitive reserve (Stern, 2009). Specifically, cognitive reserve postulates that individual differences in experiences, such as high educational and occupational attainment, increase functional brain “reserve.” This cognitive reserve can manifest as better performance on cognitive measures compared with individuals of low reserve, but more importantly cognitive reserve provides a buffer against the effects of neuropathology and subsequent cognitive decline (Potter, Helms, & Plassman, 2008; Stern, 2009; Yaffe, Hoang, Byers, Barnes, & Friedl, 2014; Yaffe, Weston, Graff-Radford, & Satterfield, 2011) by allowing the brain to functionally compensate for degrading brain health (see horizontal flow chart of conceptual framework for resilience factors in women Veterans in Figure 1). Within this context, it is critical to note that while cognitive reserve buffers against cognitive decline, it may also delay the detection of advancing neuropathology. For example, among individuals with comparable levels of neuropathology, an individual with high cognitive reserve would perform within normal limits on cognitive measures for longer than an individual with low reserve, who would show impairments more quickly. This may be problematic, as delayed detection of neuropathology or disease could prevent opportunities for early identification and intervention.
Military Service and Cardiovascular Disease Risk
Military service has been associated with greater prevalence of cardiovascular disease (CVD) risk factors including smoking, diabetes, and hypertension that may increase risk for CVD morbidity and subsequent neuropathology, although different age cohorts may also impact risk. Specifically, recent studies estimate that 25% of male and female Veterans have diabetes as opposed to 8%–10% of the U.S. population, 37% have hypertension compared with 30% in the U.S. population, and 25–36% have hyperlipidemia compared with 26% in the general population (Fryar, Hirsch, Eberhardt, Yoon, & Wright, 2010; Johnson, Pietz, Battleman, & Beyth, 2004; Richlie, Winters, & Prochazka, 1991; U.S. Department of Veterans Affairs, 2014). Moreover, recent estimates suggest that 20% of Veteran women smoke (Hoerster et al., 2012) compared with 15% of non-Veteran women (U.S. Department of Veterans Affairs, 2014), further contributing to their risk of CVD and cognitive decline associated with poor cardiovascular health (Anstey, Sanden, Salim, & O’Kearney, 2007).
Modifiable CVD risk factors, such as smoking, diabetes, and hypertension, together with CVD burden (e.g., CVD, peripheral arterial disease, coronary revascularization, and angina) represent critical risk factors for neuropathology and cognitive decline in older women (see vertical pathway of risk factors to cognitive decline for women Veterans in Figure 1) (American Heart Association, 1999; Battistin & Cagnin, 2010; Haring et al., 2013; Peltz, Corrada, Berlau, & Kawas, 2012; Stewart, 1999; Yaffe et al., 2004). In addition, discrete cardiovascular events like stroke (Cengic, Vuletic, Karlic, Dikanovic, & Demarin, 2011; Mulder, van Limbeek, Donders, Schoonderwaldt, & Hochstenbach, 1998) have been shown to accelerate cognitive aging in older adults, above and beyond the discrete cognitive consequences of stroke. Women have lower rates of CVD than men during perimenopause, but are equivalent to men following the menopausal transition, signaling a marked increase in the risk for hypertension, CVD, and mortality (Gorodeski, 2002; Horiuchi, 1997; Hsia et al., 2007).
Given the complex array of military-related resilience and risk factors evident in women Veterans, it is alarming to see the substantial gap in research examining late life cognition in this population. To address this gap, the present study examined the association between Veteran status and cognitive decline at baseline assessment and longitudinally in participants of the Women’s Health Initiative (WHI) Memory Study (WHIMS). WHIMS carried out annual assessments of cognitive functioning to examine the effect of hormone therapy (HT) on cognitive decline in postmenopausal women, including Veterans, aged 65 or older (Espeland, Rapp, & Shumaker, 2004; Shumaker et al., 2003; Shumaker, Reboussin, & Espeland, 1998). This provides an unprecedented opportunity to examine the complex relationships between resilience and risk factors and cognitive decline by Veterans status. We hypothesize that Veteran status will be associated with greater levels of cognitive reserve due primarily to protective factors that, despite greater underlying neuropathology, initially preserve cognitive function but eventually give way to greater cognitive decline.
Design and Methods
WHIMS participants were recruited from the WHI HT trials. These women were randomly assigned with equal probability to HT 0.625 mg·day−1 of conjugated equine estrogens (CEE-Alone) if prior hysterectomy or CEE in combination with 2.5 mg·day−1 of medroxyprogesterone acetate (CEE + MPA) if no prior hysterectomy or matching placebos. The goal of WHIMS was to test the effect of these hormonal preparations on the incidence of probable dementia and other cognitive outcomes and women 65–80 were enrolled in WHIMS 1 year after HT trial began. The study design, eligibility criteria, and recruitment procedures of the WHI and WHIMS have been reported previously (Prentice et al., 1998; Shumaker et al., 1998; WHI Group, 1998). Exclusion criteria were used to select women who were appropriate candidates for postmenopausal HT and long-term follow-up and free of dementia. The National Institutes of Health and Institutional Review Boards for all participating institutions approved protocols and consent forms. Informed written consent was obtained from all participants.
The WHI CEE + MPA trial was terminated earlier than planned in July 2002, because of increased breast cancer risk associated with HT compared with placebo and an unfavorable risk-to-benefit ratio for other specific noncognitive outcomes (Rossouw, Anderson, Prentice, & LaCroix, 2002). In 2004, the WHI CEE-Alone trial was also terminated early due to increased stroke incidence in the HT group and the absence of reduced coronary heart disease risk, the primary outcome of the HT trials (Anderson, 2004). At the conclusion of each trial, assigned study pills were stopped and participants were informed of study findings and advised to consult with their providers regarding continuation of use; however, annual follow-up was continued.
Global Cognitive Functioning
The cognitive trajectories were examined using baseline and annual assessments in WHIMS participants during clinic visits through 2007 using the Modified Mini-Mental State Exam (3MSE), with possible scores ranging from 0 to 100 (a higher score reflecting better cognitive functioning) (Espeland et al., 2004; Teng & Chui, 1987). Test items include measuring temporal and spatial orientation, immediate and delayed recall, executive function, naming, verbal fluency, abstract reasoning, praxis, writing, and visuo-constructional abilities. Trained and certified technicians administered the 3MSE.
Veteran Status
Veteran status was established by participants’ response to a single question at WHI baseline: “Have you ever served in the Armed Forces for more than 180 days?” Participants who answered affirmatively were classified as “Veterans,” all others as non-Veterans.
Covariates
The WHI collected baseline demographic, lifestyle, and clinical data via self-report and standardized assessments (Shumaker et al., 1998). Items included age, education, race/ethnicity, current CVD risk factors including current hypertension, diabetes, body mass index (BMI), and smoking and CVD burden (e.g., current or prior diagnosis of CVD, angina, peripheral artery disease, coronary revascularization, stroke). HT trial treatment assignment was another covariate, as participants in WHIMS were recruited from the WHI HT treatment trials. Demographic, lifestyle risk factors, comorbidities, and cardiovascular and cancer outcomes were updated throughout the 8-year follow-up period.
Statistical Approach
Differences between Veteran and non-Veteran women with respect to risk factors for cognitive decline (i.e., sociodemographic, CVD risk factors, and CVD morbidity) were assessed using chi-squared and t tests. Participants’ sequences of 3MSE scores were analyzed using longitudinal mixed effects modeling with adjustment for the intrasubject correlations for the repeated measures to estimate mean differences in 3MSE scores over time. To estimate relative differences in how 3MSE scores changed over time, we used an expanded model that included class terms for exams and also a fixed effect term for continuous time to compare the slopes of Veterans with non-Veterans after adjustment for these class terms. Specifically, the random intercept regression equation we fitted was as follows:
where Y ij is the cognitive function score for participant i at time j, b i is the random effect associated with participant i (i.e., the intercept), β is a vector of parameters for the fixed covariates in matrix X i, χj is the parameter for the fixed class effects marking exam E j (these capture systematic differences over time, such as learning effects), parameter δ and marker V i relate to the fixed effect for veteran status, ϕ is the parameter (fixed effect) for the (continuous) time from randomization marked by T ij (this represents slopes over time in cognitive function), γ is the parameter related to the interaction between veteran status and slopes marked by V i T ij, and εij represents random error. This is a general linear model with covariate adjustment to assess mean differences in the slope of 3MSE scores over time between Veterans and non-Veteran women (Fitzmaurice, Laird, & Ware, 2004; Littell, Milliken, Stroup, & Wolfinger, 1996). We utilized all women from WHIMS that had valid Veteran status as well as 3MSE data available to maximize power and efficiency; this provides greater statistical efficiency than using matching (Kupper, Karon, Kleinbaum, Morgenstern, & Lewis, 1981). The maximum likelihood approach we used to fit models provides a measure of robustness to any effects of differential patterns of follow-up between the longitudinal strings of 3MSE scores between Veteran and non-Veteran women, beyond what would be afforded by least squares or generalized estimating equations (GEE) approaches.
Age, education, race/ethnicity, and HT treatment assignment were included as fixed covariates in all models. Varying levels of additional covariate adjustment were used to assess the impact of differences in risk factors between groups. Tests of interaction were used to describe the consistency of findings with respect to subgroups based on age, education, occupation, smoking, hypertension, diabetes, and CVD. Multiple imputation was used to assess the sensitivity of findings with respect to missing values for cognitive risk factors (Yuan, 2011).
Exploratory Analyses
We hypothesized that Veteran women may have greater underlying CVD risk factors as well as burdens of CVD; however, the effects of this greater burden on cognitive decline may have been buffered by their cognitive reserve or the buffer provided by education and occupational attainment. To test this, we further adjusted models with the following associated risk factors of CVD: current hypertension, diabetes, and smoking; and the following conditions of CVD: CVD, angina, peripheral artery disease, coronary revascularization, and stroke. Next, we grouped women according to whether they had 0, 1, or ≥2 of the conditions related to CVD risk factors and CVD at baseline and examined whether the relative trajectory of cognitive aging was steeper among Veterans with more of these conditions.
Results
Sample Characteristics
WHIMS included 279 (3.8%) women who reported a history of military service and 7,051 (96.2%) women who reported no history. Veterans were followed an average (standard deviation) of 7.0 (3.1) years compared with 7.4 (2.8) among non-Veterans (p = .01). Including baseline visits, cognitive function was assessed an average of 7.7 (3.1) versus 8.1 (2.8) times (p = .02). Seventy-nine percent of Veterans provided at least 5 years of follow-up compared with 84% for non-Veterans. From WHIMS enrollment (beginning in 1996) through 2007, there were 62 (22.2%) deaths among the Veteran and 1,071 (15.2%) among the non-Veteran women (p = .001). Inclusion of these differences in subsequent models is described in the Statistical Approach section.
Table 1 compares the Veterans and non-Veterans at the time of enrollment into WHIMS with respect to a number of potential risk factors for cognitive decline. Compared with non-Veterans, Veterans tended to be older, were more likely to be non-Hispanic Caucasian, more highly educated, unmarried, and to have had professional or managerial occupations (p < .05). They were also more likely to have had angina or strokes and to be former smokers. Women Veterans and non-Veterans were similar with respect to other CVD risk factors (i.e., current alcohol consumption, diabetes, BMI, or WHI treatment assignment) and conditions of CVD (i.e., rates of CVD, peripheral arterial disease, coronary revascularization).
Table 1.
Distributions of Risk Factors for Cognitive Decline by Veteran Status at Enrollment in the Women’s Health Initiative Memory Study
| Non-Veteran (N = 7,051) Mean (SD) or N (%) |
Veteran (N = 279) Mean (SD) or N (%) |
t test or chi-squared test p value |
|
|---|---|---|---|
| Age | 70.9 (3.8) | 73.4 (3.8) | p < .001 |
| Age range | 65–80 | 65–80 | |
| Race/ethnicity (n = 3 missing) | |||
| Asian | 123 (1.7) | 0 (0.0) | p = .003 |
| African American | 510 (7.2) | 8 (2.9) | |
| Hispanic | 172 (2.4) | 3 (1.1) | |
| Non-Hispanic White | 6,122 (86.8) | 263 (94.3) | |
| Other | 124 (1.8) | 5 (1.8) | |
| Education (n = 11 missing) | |||
| Less than HS | 548 (7.8) | 6 (2.2) | p < .001 |
| High school, GED | 1,588 (22.6) | 33 (11.8) | |
| Some college | 2,822 (40.1) | 126 (45.2) | |
| College graduate or higher | 2,085 (29.6) | 114 (40.9) | |
| Marital status (n = 8 missing) | |||
| Never married | 227 (3.2) | 16 (5.7) | p = .03 |
| Divorced/separated | 868 (12.3) | 41 (14.7) | |
| Widowed | 2,187 (31.1) | 92 (33.0) | |
| Married/marriage-like relationship | 3,756 (53.4) | 130 (46.6) | |
| Occupation (n = 210 missing) | |||
| Managerial/professional | 2,318 (33.8) | 126 (46.2) | p < .001 |
| Technical, sales, admin support | 2,088 (30.5) | 76 (27.8) | |
| Service/laborer | 1,569 (22.9) | 53 (19.4) | |
| Homemaker only | 872 (12.7) | 18 (6.6) | |
| Employment status (n = 18 missing) | |||
| Not working | 5,783 (82.2) | 240 (86.3) | p = .08 |
| Employed | 1,251 (17.1) | 38 (13.7) | |
| Cardiovascular disease burden | |||
| Cardiovascular disease (n = 109 missing) | 1,210 (17.4) | 52 (19.0) | p = .51 |
| Angina (n = 65 missing) | 511 (7.3) | 30 (10.9) | p = .03 |
| Peripheral arterial disease (n = 58 missing) | 149 (2.1) | 9 (3.2) | p = .21 |
| Coronary revascularization (n = 132 missing) | 179 (2.6) | 9 (3.3) | p = .47 |
| Stroke | 110 (1.6) | 9 (3.2) | p = .03 |
| Cardiovascular disease risk factors | |||
| Hypertension | 2,738 (39.3) | 122 (44.5) | p = .08 |
| Diabetes | 515 (7.3) | 16 (5.7) | p = .32 |
| Smoking (miss = 107) | |||
| Never | 3,709 (53.4) | 122 (44.7) | p = .02 |
| Former | 2,740 (39.4) | 130 (47.6) | |
| Current | 501 (7.2) | 21 (7.7) | |
| Body mass index | 28.5 (5.7) | 28.0 (5.5) | p = .13 |
| Current alcohol consumption (miss = 17) | |||
| None | 3,276 (46.6) | 113 (40.8) | p = 0.15 |
| <1/day | 2,922 (41.5) | 125 (45.1) | |
| ≥1/day | 838 (11.9) | 39 (14.1) | |
| HT treatment assignment | p = .53 | ||
| CEE-Alone placebo | 1,395 (19.8) | 62 (22.2) | |
| CEE-Alone | 1,383 (19.6) | 47 (16.8) | |
| CEE + MPA placebo | 2,167 (30.7) | 90 (32.3) | |
| CEE + MPA | 2,106 (29.9) | 80 (28.7) | |
| Unadjusted 3MSE score | 95.20 (4.33) | 95.51 (3.54) | p = .23 |
Note: GED = general educational development; HT = hormone therapy; CEE = conjugated equine estrogens; MPA = medroxyprogesertone acetate; 3MSE = Modified Mini-Mental State Exam.
Baseline Global Cognitive Functioning
Table 2 compares the mean 3MSE scores of Veterans and non-Veterans at WHIMS enrollment with limited and more broad-based covariate adjustment (unadjusted means appear in Table 1). There was little difference in mean 3MSE scores between groups at baseline, regardless of the level of covariate adjustment.
Table 2.
Modified Mini-Mental State Examination (3MSE) Scores by Veteran Status at Enrollment in the Women’s Health Initiative Memory Study
| Covariate adjustment | Baseline mean (SE) (p value) |
|---|---|
| Age, education, race/ethnicity | |
| Non-Veterans (n = 7,051) | 95.21 (.05) |
| Veterans (N = 279) | 95.13 (.23) |
| Difference | −0.08 (.24) |
| p value | p =.72 |
| Additional factors (identified in Table 1 with p ≤ 0.05a | |
| Non-Veterans | 95.41 (.05) |
| Veterans | 95.30 (.23) |
| Difference | −0.11 (.24) |
| p value | p =.64 |
| Factors expected to be related to cardiovascular disease and associated risk factorsb
|
|
| Non-Veterans | 95.19 (0.05) |
| Veterans | 95.84 (0.26) |
| Difference | −0.66 (0.26) |
| p value | p =.01 |
aAge, race/ethnicity, education, marital status, occupation, angina, stroke, smoking.
bCardiovascular disease, angina, peripheral artery disease, coronary revascularization, stroke, hypertension, and diabetes.
Cognitive Trajectories
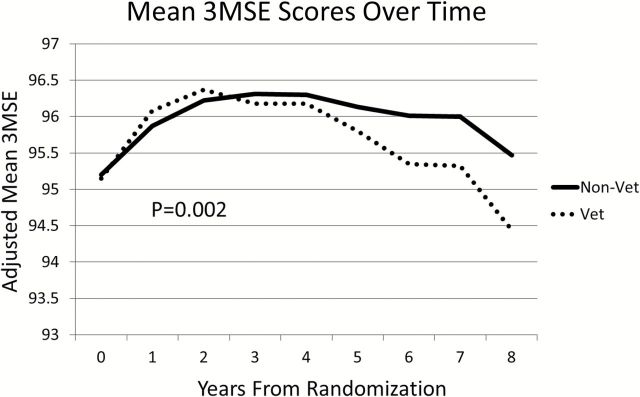
Figure 2 portrays mean 3MSE scores from the mixed effects models without the statistical terms used to remove curvature. Relative deficits in 3MSE scores among Veterans began to emerge 5–6 years into follow-up, so that by year 8, Veteran women’s 3MSE scores averaged approximately 1 point lower compared with non-Veteran women. Specifically, Figure 2 depicts mixed effects model mean with adjustment for age, education, race/ethnicity, and HT trial treatment assessment, which are generated from 3MSE by HT interaction. The maximum likelihood approach we used to fit models provided a measure of robustness to any effects of differential patterns of follow-up between groups. With covariate adjustment for age, education, race/ethnicity, and HT trial treatment assignment, the average differences over time remained significant (p = .002).
Figure 2.
Trajectory of mean Modified Mini-Mental State Examination (3MSE) scores over time for Women’s Health Initiative Memory Study participants grouped by Veteran status with adjustment for age, education, race/ethnicity, and Hormone Therapy treatment assignment.
We used year from WHIMS enrollment as a class variable to account for the systematic curvature (related to learning effects) across all women and fit a model in which differences between Veterans and non-Veterans expanded linearly with time. This approach allowed us to compare the slopes in 3MSE scores over time after statistically removing the curvature seen in Figure 2. From this model, the difference (SE) between slopes over time for Veterans and non-Veterans was 0.16 (0.05) units per year (p = .002). Table 3 presents the consistency of this difference among subgroups of women based on age, education, and occupation, using tests of interaction. The relative greater rates of decline in 3MSE scores seen among Veterans were more pronounced among older women (p = .008), those with college educations (p = .001), and those with professional/managerial occupations (p = .03). Removing women with a history of stroke from the models did not appreciably change our findings.
Table 3.
Consistency of Relative Differences in the Rate of Modified Mini-Mental State Examination (3MSE) Changes in Women Veteran and Non-Veteran Participants in the Women’s Health Initiative Memory Study over Time across Subgroups of Baseline Risk Factors for Cognitive Declinea
| Subgroup | Relative difference (SE) in the rate of change in 3MSE scores between veterans and non-veterans: units/year | Consistency of differences across subgroups (p value) |
|---|---|---|
| Overall | −0.16 (0.03) | |
| Women with no stroke history | −0.16 (0.03) | |
| Age | ||
| 60–69 | 0.03 (0.06) | p = .008 |
| 70–80 | −0.15 (0.03) | |
| Education | ||
| College graduate | −0.25 (0.04) | p = .001 |
| Other | −0.08 (0.04) | |
| Occupation | ||
| Professional/Manager | −0.21 (0.04) | p = .03 |
| Other | −0.10 (0.04) | |
| Number of conditions related to cardiovascular disease and risk factors at baseline | ||
| 0 | −0.10 (0.08) | p = .02 |
| 1 | −0.14 (0.09) | |
| 2 | −0.36 (0.12) |
aFrom models controlling for systematic learning effects across subgroups, HT treatment assignment, age race/ethnicity and education; examined by linear regression models of risk factors for predicting cognitive decline.
Exploratory Analyses
At baseline, the average (SD) number of the following conditions and risk factors known to be associated with greater CVD was 0.61 (0.72) among Veteran women compared with 0.51 (0.67) among non-Veterans (p = .01): history of CVD, angina, peripheral artery disease, coronary revascularization, stroke, hypertension, diabetes, and smoking. Among Veteran women, 42.3% had no conditions, 33.3% had one condition, and 24.4% had two or more conditions; among non-Veteran women, these rates were 46.5%, 33.9%, and 19.6%, respectively. With covariate adjustment for these conditions and age, the baseline 3MSE scores of Veteran women averaged (SE), 0.66 (0.26) units higher than for non-Veterans (p = .01).
As seen in Table 3, among women with no or one condition, the rate of cognitive decline associated with military service was only slightly greater among Veterans by 0.10 (0.08) and 0.14 (0.09) 3MSE units/year, respectively. Among women with two or more of these conditions, relative rates of change in 3MSE scores were 0.36 (0.12) MSE units/year among Veterans compared with non-Veterans. This interaction between markers of vascular burden and Veteran status on rates of decline was statistically significant (p = .003).
Because follow-up times vary between Veterans and non-Veterans, we divided women into whether they provided at least 5 years of follow-up and used logistic regression to examine whether relationships that follow-up time had with the risk factors in Table 1 varied between Veterans and non-Veterans. We found evidence (p <.05) for an interaction for three of these factors. For history of CVD, among non-Veterans 85% of those with no history and 80% of those with a history provided 5 years of follow-up data; for Veterans, 84% with no history and 58% with a history of CVD provided 5 years of data. For BMI among non-Veterans, 85%, 84%, and 85% provided 5 years of data for BMI < 25, 25–29, and 30+, respectively; for Veterans, these percentages were 88%, 76%, and 71%. Lastly, for alcohol intake, of non-Veterans who reported no drinking, <1 drink per day, and more than one drink per day, 83%, 85%, and 84% provided 5 years of data; for Veterans, these percentages were 69%, 86%, and 87%. From these analyses, relative to non-Veterans, veteran women with prior CVD, higher BMI, and no current alcohol intake provided less follow-up years.
In supporting analyses, missing covariates were imputed to create five complete data sets and the analyses described in Table 2 and Figure 2 were repeated. Missing data are quantified per variable in Table 1. These sensitivity analyses produced results that did not differ from those described above.
Discussion
This is the first study to examine the association between Veteran status and longitudinal cognitive decline in a large, well-characterized sample of older, postmenopausal women. At baseline, Veteran women had similar 3MSE scores to non-Veterans; however, during the next 8 years, their scores evidenced a greater relative difference in the rate of decline. This pattern was more pronounced among Veterans who were older, more highly educated, and in managerial or professional occupations as compared with non-Veterans. In addition, Veteran women had baseline 3MSE scores that were higher than non-Veterans with comparable CVD risk factors and CVD profiles. The greater rate of change in cognitive decline in Veterans compared with non-Veterans also occurred, to a greater degree, among women with two or more conditions related to CVD risk factors and CVD. All findings held after adjustment for age, education, ethnicity, and HT trial treatment assignment, and in sensitivity analyses featuring more inclusive cohorts of women and when accounting for differential rates of follow-up. Taken together, these results suggest that the latent, but steeper trajectory of cognitive decline in women Veterans may reflect both the effects of cognitive reserve and CVD risk factors and CVD burden, which may make detection of incipient cognitive decline more difficult.
Two possible explanatory mechanisms were explored (see conceptual framework Figure 1). First, Veterans showed a pattern of decline consistent with the buffering effect or cognitive reserve theory in which cognitive decline occurs at a later age but more precipitously in older adults with higher educational and occupational attainment (Stern, 2009). In support of this theory, cognitive decline was more marked in Veteran women who had higher education and higher occupational attainment. Second, Veterans demonstrated a greater relative difference (decline) in cognitive functioning over time—which is consistent with increased prevalence of CVD risk and CVD that overwhelms the protective effects of higher educational and occupational attainment and leading, eventually, to a greater relative decline in cognitive functioning (Anstey et al., 2007; Cengic, Vuletic, Karlic, Dikanovic, & Demarin, 2011; Haring et al., 2013; Mulder et al., 1998). To test this hypothesis, baseline and longitudinal analyses were adjusted for markers of CVD risk factors and CVD. After covariate adjustment to align groups according to the markers of CVD risk factors and CVD profiles, baseline 3MSE scores for Veterans were significantly higher than for non-Veterans, perhaps due to higher educational and occupational attainment. During follow-up, Veterans with the greatest number of conditions related to CVD risk and CVD had the greatest relative difference in rates of change in cognitive function.
Our results are on par with and add to the literature on CVD risk factors and CVD burden that are known to accelerate cognitive decline in older adults (Cengic, Vuletic, Karlic, Dikanovic, & Demarin, 2011; Haring et al., 2013; Mulder et al., 1998). In our study, Veteran women with two or more CVD risk factors or CVD diagnoses evidenced the steepest relative trajectory of cognitive decline. The profile of cognitive decline is consistent with prior literature on the Veteran/non-Veteran health “cross-over” effect in which Veterans, despite many years of robust good health, evidence rapid decline in health and well being relative to non-Veterans in late life, typically after age 70 (Waller & McGuire, 2011; Weitlauf et al., 2015). Similarly, it has been proposed that the time following active duty may be an important transition stage and an opportunity to address some important and higher rates of key risk factors (e.g., smoking) in Veterans (Waller & McGuire, 2011; Yaffe et al., 2014). The current study was limited in this regard, by its observational timeframe, which occurred during the participants’ postmenopausal years. The importance of targeting this transition period (from active duty military personnel to Veterans) for identification and intervention of modifiable health risk factors (e.g., smoking) should be more carefully examined in future work.
Limitations to our study include the relatively low number of women Veterans included in the WHIMS study, which may have limited the power needed to fully detect all possible three-way interactions among Veteran status, education/occupational attainment, and CVD risk factors and CVD on cognitive decline. The WHI did not collect data on the nature of women’s service, which limited the contextual nature of military service. The cohort also contained a limited number of women from traditionally underrepresented ethnic/racial minority groups. Second, while the 3MSE measure of global cognitive functioning has been used extensively and reliably in WHIMS, (Espeland et al., 2005; Espeland, Shumaker, & Hogan, 2009; Rapp et al., 2003; Shumaker et al., 1998), it is a screening instrument designed to offer a quick, global assessment of cognitive status. The 3MSE is limited by its potential for ceiling effects, particularly in highly educated individuals, like the Veteran subgroup evaluated in the present work. Additionally, a relative Veteran to non-Veteran difference of 1 unit on the 3MSE by the 8th year of follow-up may not be clinically meaningful as a standalone measure of cognition. However, principal findings from the WHIMS trials included that HT was associated with a mean relative decrement in 3MSE slopes of <0.10 unit per year, which was associated with a 75% increased risk for cognitive impairment (Espeland et al., 2004), which is clinically meaningful. Future studies are needed to more fully examine clinically useful units of change in cognition of women Veterans. On par, the 3MSE is likely insufficiently sensitive to detect subtle changes in cognitive functioning and the total score does not provide information on specific cognitive domains. The women Veterans in WHIMS had fewer assessment visits across the 8-year follow-up period and also had higher rates of mortality, which is consistent with the larger WHI Veteran group (Weitlauf et al., 2015). This may have impacted the current findings in that women Veterans in the current study are those who survived throughout the follow-up period and the expectation would be that reduced follow-up rates would be higher among women with poor cardiovascular health and declining cognition. In fact, there were fewer follow-up years in Veteran women, relative to non-Veterans, with history of CVD, higher BMI, and no alcohol intake, all of which are risks for accelerated cognitive decline (Espeland et al., 2005; Haring et al., 2013; Yaffe et al., 2014). Thus, the impact of such differential follow-up would be to decrease observed differences, and therefore the current results are likely an underestimate of relative cognitive declines among Veteran women. Indeed, WHIMS has demonstrated lower follow-up rates among women who had poorer health and lower cognitive function (Espeland et al., 2013). In addition, the generalizability of our findings may be limited as Veteran participants in WHIMS represent women whose military service likely occurred predominantly before the conflict in Vietnam (e.g., WWII and Korea) with a small number who would have been eligible to serve during the Vietnam War. As such, this Veteran cohort is likely distinct from more contemporary groups of women Veterans both with respect to their socio-demographics and the nature and length of their military service (Frayne et al., 2006). This too would be expected to impact the generalizability of our findings. Lack of more specific information in the WHI regarding women’s participation in military service prevents the examination of cognitive decline due to military-related exposures or the physical or mental health correlates (e.g., posttraumatic stress disorder) of military service (Weiner, Friedl, Pacifico, & Chapman, 2013). Finally, as volunteers and suitable candidates for the WHI HT trial, the women in our analyses may not reflect more general populations; in particular, the enrollment process differentially lead to underrepresentation of women with cognitive deficits, as this was exclusionary for participation in WHIMS.
Study strengths include the use of a large national sample of older women; a high rate of follow-up retention; and inclusion of a multitude of variables associated with cognition. WHIMS provides an unprecedented opportunity to examine long-term cognitive trajectories in postmenopausal women Veterans, and provides a foundation for future research to examine the risk and resiliency factors that lead to cognitive decline in these unique women.
Taken together, the findings of the present study yield valuable information about the risks for cognitive decline among women Veteran participants in WHI. This cohort of Veteran women likely demonstrates a phenomenon in which, early in the process of cognitive decline, protective factors such as high educational and occupational attainment initially mask or delay a decline process, which may accelerate in its rate of decline over time. This underscores the importance of prevention, early identification of modifiable risk factors, and knowledge of contextual resilience factors in these women. Of note, the average decline observed is subtle over this time frame and continued study of this population will be important. Additional research is needed to increase our understanding of the interplay of between CVD risk factors, CVD, socio-demographic protective factors such as education and occupational attainment, and cognitive decline in older women Veterans (see Figure 1 for conceptual framework). Future work on this topic should prioritize the use of more sensitive measures of cognitive decline to fully understand the relationship with risk and resilience. For example, as is typically emphasized during structured neuropsychological assessments, the examination of cognitive status in the context of critical demographic information (e.g., education/occupational attainment) should be highlighted for Veteran women. Testing the more nuanced mechanisms underlying the cognitive trajectories of women Veterans will be possible in future work with the subset of WHIMs participants who received a more comprehensive neuropsychological assessment (Goveas, Espeland, & Hogan, 2014) and on whom neuroimaging data are available (Resnick et al., 2009).
Funding
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health; and the U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Acknowledgments
The views expressed are those of the authors and do not necessarily reflect the views of the Department of Veterans Affairs. This research is supported, in part, by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment and the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), the Women’s Mental Health and Aging Core of the Sierra Pacific MIRECC, the Office of Women’s Health and VA Health Services Research and Development FOP 14–439.
The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute, U.S. Department of Health and Human Services. Wyeth Pharmaceuticals provided the study drug and the placebo to the WHI trial. The Women’s Health Initiative Memory Study was funded by Wyeth Pharmaceuticals, Inc; St. Davids, PA; Wake Forest University; and the National Heart, Lung, and Blood Institute.
References
- American Heart Association. (1999). Diabetes mellitus: a major risk factor for cardiovascular disease. A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation, 100, 1132–1133. doi:10.1161/01.CIR.100.10.1132 [DOI] [PubMed] [Google Scholar]
- Anderson G. L. (2004). Effects of estrogen-only treatment in postmenopausal women—reply. JAMA, 292, 683. doi:org/10.1001/jama.292.6.683-b [Google Scholar]
- Anstey K. J. von Sanden C. Salim A., & O’Kearney R (2007). Smoking as a risk factor for dementia and cognitive decline: A meta-analysis of prospective studies. American Journal of Epidemiology, 166, 367–378. doi:10.1093/aje/kwm116 [DOI] [PubMed] [Google Scholar]
- Battistin L., & Cagnin A (2010). Vascular cognitive disorder. A biological and clinical overview. Neurochemical Research, 35, 1933–1938. doi:10.1007/s11064-010-0346-5 [DOI] [PubMed] [Google Scholar]
- Cengic, L., Vuletic, V., Karlic, M., Dikanovic, M., & Demarin, V. (2011). Motor and cognitive impairment after stroke. Acta Clinica Croatica, 50, 463-467. [PubMed] [Google Scholar]
- Espeland M. A. Gu L. Masaki K. H. Langer R. D. Coker L. H. Stefanick M. L.,…Rapp S. R (2005). Association between reported alcohol intake and cognition: results from the Women’s Health Initiative Memory Study. American Journal of Epidemiology, 161, 228–238. doi:10.1093/aje/kwi043 [DOI] [PubMed] [Google Scholar]
- Espeland M. A. Pettinger M. Falkner K. L. Shumaker S. A. Limacher M. Thomas F.,…Johnson K. C (2013). Demographic and health factors associated with enrollment in post-trial studies: The Women’s Health Initiative Hormone Therapy Trial. Clinical Trials, 10, 459–468. doi:10.1177/1740774513477931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeland M. A. Rapp S. R., & Shumaker S. A (2004). Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA, 291, 2959–2968. doi:10.1001/jama.291.24.2959 [DOI] [PubMed] [Google Scholar]
- Espeland M. A. Shumaker S. A. Hogan P. E., & Resnick S. M (2009). Women’s Health Initiative Memory Study (WHIMS) program: emerging findings. Hormones, cognition and dementia: State of the art and therapeutic strategies. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Fitzmaurice G. M. Laird N. M., & Ware J. H (2004). Applied longitudinal analysis. Hoboken, NJ: John Wiley and Sons. [Google Scholar]
- Frayne S. M. Parker V. A. Christiansen C. L. Loveland S. Seaver M. R. Kazis L. E., & Skinner K. M (2006). Health status among 28,000 women veterans. Journal of General Internal Medicine, 21, S40–S46. doi:10.1111/j.1525-1497.2006.00373.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryar C. D. Hirsch R. Eberhardt M. S. Yoon S. S., & Wright J. D (2010). Hypertension, high serum total cholesterol, and diabetes: Racial and ethnic prevalence difference in U.S. adults, 1999–2006. NCHS Data Brief, 36, 1–8. [PubMed] [Google Scholar]
- Gorodeski G. I. (2002). Update on cardiovascular disease in post-menopausal women. Best Practice & Research. Clinical Obstetrics & Gynaecology, 16, 329–355. [DOI] [PubMed] [Google Scholar]
- Goveas J. S. Espeland M. A., & Hogan P. E (2014). Depressive symptoms and longitudinal changes in cognition Women’s Health Initiative Study of cognitive aging. Journal of Geriatric Psychiatry and Neurology, 27, 94–102. doi:10.1177/0891988714522697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring B. Leng X. Robinson J. Johnson K. C. Jackson R. D. Beyth R.,…Wassertheil-Smoller S (2013). Cardiovascular disease and cognitive decline in postmenopausal women: Results From the Women’s Health Initiative Memory Study. Journal of the American Heart Association, 2, e000369–e000369. doi:10.1161/JAHA.113.000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerster K. D. Lehavot K. Simpson T. McFall M. Reiber G., & Nelson K. M (2012). Health and health behavior differences: U.S. Military, veteran, and civilian men. American Journal of Preventive Medicine, 43, 483–489. doi:10.1016/j.amepre.2012.07.029 [DOI] [PubMed] [Google Scholar]
- Horiuchi S. (1997). Postmenopausal acceleration of age-related mortality increase. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 52, B78–B92. [DOI] [PubMed] [Google Scholar]
- Hsia J. Margolis K. L. Eaton C. B. Wenger N. K. Alluson M. Wu L.,…Black H. R., for the Women’s Health Initiative Investigators. (2007). Prehypertension and cardiovascular disease risk in the Women’s Health initiative. Circulation, 115, 855–886. doi:10.1161/CIRCULATIONAHA.106.656850 [DOI] [PubMed] [Google Scholar]
- Johnson M. L. Pietz K. Battleman D. S., & Beyth R. J (2004). Prevalence of comorbid hypertension and dyslipidemia and associated cardiovascular disease. Am J Manag Care, 10, 926–932. [PubMed] [Google Scholar]
- Kupper, L. L., Karon, J. M., Kleinbaum, D. G., Morgenstern, H., & Lewis, D. K. (1981). Matching in epidemiological studies: Validity and efficiency considerations. Biometrics, 37, 271–291. [PubMed] [Google Scholar]
- Littell R. C. Milliken G. A. Stroup W. W., Wolfinger R. D, & Schabenberger, O. (1996. ). SAS system for linear mixed models. Cary, NC: SAS Institute. [Google Scholar]
- Mulder T. van Limbeek J. Donders R. Schoonderwaldt H., & Hochstenbach J (1998). Cognitive decline following stroke: A Comprehensive Study of Cognitive Decline Following Stroke. Journal of Clinical and Experimental Neuropsychology, 20, 503–517. doi:10.1076/jcen.20.4.503.1471 [DOI] [PubMed] [Google Scholar]
- Peltz C. B. Corrada M. M. Berlau D. J., & Kawas C. H (2012). Cognitive impairment in nondemented oldest-old: Prevalence and relationship to cardiovascular risk factors. Alzheimer’s & Dementia, 8, 87–94. doi:10.1016/j.jalz.2011.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter G. G. Helms M. J., & Plassman B. L (2008). Associations of job demands and intelligence with cognitive performance among men in late life. Neurology, 70(19 Pt 2), 1803–1808. doi:10.1212/01.wnl.0000295506.58497.7e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice R. Rossouw J. Furberg C. Johnson S. Henderson M. Cummings S., & Anderson G (1998). Design of the WHI Clinical Trial and Observational Study. Controlled Clinical Trials, 19, 61–109. doi:10.1016/S0197-2456(97)00078-0 [DOI] [PubMed] [Google Scholar]
- Rapp S. R. Espeland M. A. Shumaker S. A. Henderson V. W. Brunner R. L. Manson J. E.,…Bowen D (2003). Effect of estrogen plus progestin on global cognitive function in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA, 289, 2663–2672. doi:10.1001/jama.289.20.2663 [DOI] [PubMed] [Google Scholar]
- Resnick S. M. Espeland M. A. Jaramillo S. A. Hirsch C. Stefanick M. L. Murray A. M.,…Davatzikos C (2009). Postmenopausal hormone therapy and regional brain volume: The WHIMS-MRI Study. Neurology, 72, 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richlie D. G. Winters S., & Prochazka A. V (1991). Dyslipidemia in veterans. Multiple risk factors may break the bank. Archives of Internal Medicine, 151, 1433–1436. doi:10.1001/archinte.1991.00400070181025 [DOI] [PubMed] [Google Scholar]
- Rossouw J. E. Anderson G. L. Prentice R. L., & LaCroix A. Z (2002). Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal. JAMA, 288, 321–333. doi:10.1001/jama.288.3.321 [DOI] [PubMed] [Google Scholar]
- Schooler C. Mulatu M. S., & Oates G (1999). The continuing effects of substantively complex work on the intellectual functioning of older workers. Psychology and Aging, 14, 483–506. doi:10.1037/0882-7974.14.3.483 [DOI] [PubMed] [Google Scholar]
- Seeman T. E., & Crimmins E (2001). Social environment effects on health and aging: Integrating epidemiologic and demographic approaches and perspectives. Annals of the New York Academy of Sciences, 954, 88–117. doi:10.1111/j.1749–6632.2001.tb02749.x [DOI] [PubMed] [Google Scholar]
- Shumaker S. A. Legault C. Rapp S. R. Thal L. Wallace R. B. Ockene J. K.,…Wactawski-Wende J (2003). Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: The Women’s Health Initiative Memory Study: A randomized controlled trial. JAMA, 289, 2651–2662. doi:10.1001/jama.289.20.2651 [DOI] [PubMed] [Google Scholar]
- Shumaker S. A. Reboussin B. A., & Espeland M. A (1998). The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Controlled Clinical Trials, 19, 604–621. doi:10.1016/S0197-2456(98)00038-5 [DOI] [PubMed] [Google Scholar]
- Stern Y. (2009). Cognitive reserve. Neuropsychologia, 47, 2015–2028. doi:10.1016/j.neuropsychologia.2009.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R. (1999). Hypertension and cognitive decline. The British Journal of Psychiatry, 174, 286–287. doi:10.1192/bjp.174.4.286 [DOI] [PubMed] [Google Scholar]
- Teng E. L., & Chui H. C (1987). The modified mini-mental state examination (3MS). Can J Psychiatry, 41, 114–121. [PubMed] [Google Scholar]
- The Women's Health Initiative Study Group. (1998). Design of the Women’s Health Initiative Clinical Trial and Observational Study-examples from the Women’s Health Initiative. Controlled Clinical Trials, 19, 61–109. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. (2014). Veteran population. The Veteran population projection model 2014 (VetPop2014) Retrieved November 7, 2014, from http://www.va.gov/vetdata/veteran_population.asp
- Waller M., & McGuire A (2011). Changes over time in the healthy soldier effect. Population Health Metrics, 9, 7. doi:10.1186/1478-7954-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner M. W. Friedl K. E. Pacifico A., & Chapman J. C (2013). Military risk factors for Alzheimer’s disease. Alzheimer’s & Dementia, 9, 445–451. doi:10.1016/j.jalz.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitlauf J. C. LaCroix A. Z. Bird C. E. Woods N. F. Washington D. L. Katon J. G.,…Stephanick M. L (2015, in press). Prospective Analysis of Health and Mortality Risk in Veteran and non-Veteran Participants in the Women’s Health Initiative. Women’sHealthIssues. doi:10.1016/j.whi.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Group for the Women’s Health Initiative Investigators. (2002). Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA, 288, 321–333. doi:10.1001/jama.288.3.321 [DOI] [PubMed] [Google Scholar]
- Yaffe K. Blackwell T. Kanaya A. M. Davidowitz N. Barrett-Connor E., & Krueger K (2004). Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology, 63, 658–663. doi:10.1212/01.WNL.0000134666.64593.BA [DOI] [PubMed] [Google Scholar]
- Yaffe K. Hoang T. D. Byers A. L. Barnes D. E., & Friedl K. E (2014). Lifestyle and health-related risk factors and risk of cognitive aging among older veterans. Alzheimer’s & Dementia, 10, S111–S121. doi:10.1016/j.jalz.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Yaffe K. Weston A. Graff-Radford N. R., & Satterfield S (2011). Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA, 305, 261–266. doi.10.1001/jama.2010.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y. (2011). Multiple imputation using SAS software. Journal of Statistical Software, 45, 1–25. [Google Scholar]