Abstract
This investigation examines whether a low intermittent dose of rapamycin will avoid the hyperlipidemia and diabetes-like syndrome associated with rapamycin while still decreasing body weight and adiposity in aged obese rats. Furthermore, we examined if the rapamycin-mediated decrease in serum leptin was a reflection of decreased adiposity, diminished leptin synthesis, or both. To these ends, rapamycin (1mg/kg) was administered three times a week to 3 and 24-month old rats. Body weight, food intake, body composition, mTORC1 signaling, markers of metabolism, as well as serum leptin levels and leptin synthesis in adipose tissue were examined and compared to that following a central infusion of rapamycin. Our data suggest that the dosing schedule of rapamycin acts on peripheral targets to inhibit mTORC1 signaling, preferentially reducing adiposity and sparing lean mass in an aged model of obesity resulting in favorable outcomes on blood triglycerides, increasing lean/fat ratio, and normalizing elevated serum leptin with age. The initial mechanism underlying the rapamycin responses appears to have a peripheral action and not central. The peripheral rapamycin responses may communicate an excessive nutrients signal to the hypothalamus that triggers an anorexic response to reduce food consumption. This coupled with potential peripheral mechanism serves to decrease adiposity and synthesis of leptin.
Keywords: Leptin synthesis, mTORC1, Rapamycin
The prevalence of obesity among mature adults has risen dramatically over the last two decades and is projected to continue to increase (1). Obesity significantly increases the risk for a number of conditions including insulin resistance, diabetes, heart disease, atherosclerosis, and stroke, which ultimately lead to impaired physical performance, sarcopenia, disability, and premature death (2). The Fischer 344 × Brown Norway (F344BN) rat demonstrates a steady increase in body weight through early senescence that is accompanied by increased adiposity and diminished lean mass (3,4). Our companion article demonstrates that peripheral rapamycin treatment reduces this age-related body weight gain (5). More importantly, rapamycin targets adiposity, reversing the age-related fat content to that of a 6-month old rat, while mostly sparing lean mass. This sparing of lean mass is apparently specific because an intermittent fed (IF) group that lost a similar amount of weight demonstrated a greater reduction in lean mass (5). Rapamycin is an inhibitor of the mammalian target of rapamycin (mTOR) pathway, which regulates cellular activities such as growth, nutrient sensing, protein synthesis, and autophagy, and has been shown to consistently increase mammalian lifespan (6). These findings suggest that rapamycin, aside from the potential benefits of lifespan extension, may alleviate some of the consequences of age-related obesity and muscle wasting. However, rapamycin treatment also causes glucose intolerance, hyperlipidemia, increased hepatic gluconeogenesis, and impairs lipid deposition in fat tissue (7). Thus, the potential benefits of reduced adiposity with preservation of lean mass may be offset by worsening hyperlipidemia and diabetes-like syndrome.
Rapamycin inhibits both complexes of mTOR, complex 1 (mTORC1) and complex 2 (mTORC2). mTORC1 signals in part by phosphorylation of p70 S6 kinase and its downstream protein target, S6, whereas mTORC2 has a regulatory role in the insulin signaling pathway including PKC and AKt (8). It has been suggested that disruption of mTORC2 primarily affects metabolism (8), thus many of the detrimental consequences of rapamycin may be the result of mTORC2 inhibition. Ideally, selective inhibition of mTORC1 may reduce adiposity and preserve lean mass in aged rodents without the detrimental consequences on metabolism. Glucose levels were not elevated following rapamycin treatment in our companion article (5), suggesting that our dose and dosing schedule may selectively inhibit mTORC1 over mTORC2. This study aims to evaluate mTORC1 signaling coupled with an assessment of several metabolic factors to further evaluate this postulate.
In rodents, mTORC1 signaling is known to decrease with age in male F344BN rat skeletal muscle (although it is increased in females (9)), suggesting inhibition of mTORC1 may not be as effective in aged rodents. Paradoxically, in our companion paper, we demonstrated that rapamycin is more efficacious in aged male rats (5). In contrast to skeletal muscle, mTORC1 signaling is augmented in the hypothalamus of 12-month old mice and this elevation was normalized by rapamycin treatment (10). Although the latter study did not assess mTORC1 signaling in senescent mice, the results from 12-month old mice hint that our observations with rapamycin may be the result of a central action of rapamycin on the energy homeostatic centers in the hypothalamus. Our second aim is to compare peripheral administration of rapamycin to central infusion of rapamycin along with mTORC1 signaling in young and senescent rats.
One peripherally produced metabolic factor that targets the energy homeostatic centers in the hypothalamus is leptin, and this protein has been causally linked to age-related obesity (11). Leptin was reduced with rapamycin treatment, especially in the aged rat (5), Circulating leptin levels increase with age apparently due to both increased adiposity with age and increased leptin synthesis per unit of adipose tissue with age (3). Adipose tissue is a major target of rapamycin, and we suggest that rapamycin directly decreases leptin synthesis in adipose tissue. The present report also examines this postulate.
To these ends, we administered a low dose of rapamycin, three times a week to 3 and 24-month old rats and examined body weight, food intake, body composition, mTORC1 signaling, and markers of metabolism, as well as serum leptin levels and leptin synthesis in adipose tissue, and compared these outcomes to those following a central infusion of rapamycin.
Materials and Methods
Experimental Animals
Three and 24 month-old male F344 × Brown Norway (F344xBN) rats were obtained from Harlan Sprague–Dawley (Indianapolis, IN). Upon arrival, rats were examined and remained in quarantine for 1 week. Animals were cared for in accordance with the principles of the Guide to the Care and Use of Experimental Animals and protocols were approved by the University of Florida Institutional Animal Care and Use Committee. Rats were housed individually with a 12:12h light–dark cycle (07:00 to 19:00 hours) and were fed a standard rodent chow (18% kcal from fat, no sucrose, 3.1 kcal/g, diet 2018, Harlan Teklad; Madison, WI).
Experimental Design
Animals were assessed at baseline for body weight, food intake, and body composition. Rats were then randomized, based on body weight, within each age, into two treatment groups: rapamycin (n = 12/age) and control (n = 12/age). Treatment lasted for 5 weeks, and body weight and food intake were measured throughout. Body composition was reassessed at the end of the third and fifth week. Body composition was assessed using time-domain nuclear magnetic resonance (TD-NMR) in restrained but fully conscious rats (TD-NMR Minispec, Bruker Optics, The Woodlands, TX, USA).
Rapamycin Administration
Rapamycin was dissolved in 100% ethanol (20mg/ 0.4ml) and diluted to a final concentration of 2.0mg/ml in a final solution of 5% PEG, 5% Tween 80, 4% ethanol in saline. Rapamycin (1mg/kg body weight) or vehicle was administered by i.p. injection Monday, Wednesday, and Friday for 5 weeks. Dosages were adjusted weekly based on the animal’s weight.
In a second experiment, rats were infused with rapamycin (30 μg/day) for 28 days into the lateral ventricle by osmotic mini pump. The brain infusion cannula (Durect Corporation) was stereotaxically placed into the lateral ventricle using the following coordinates, 1.3mm posterior to bregma, 1.9mm lateral to the midsaggital suture and to a depth of 3.5mm. The brain infusion cannula was anchored to the skull using acrylic dental cement. A catheter tube was connected from the brain infusion cannula to the osmotic mini pump flow moderator. A subcutaneous pocket on the dorsal surface was created using blunt dissection and the osmotic mini pump was inserted. The incision was closed with sutures, and rats were kept warm until fully recovered. The initial 14 day pumps were filled with artificial cerebral spinal fluid to allow rats to fully recover from the effects of the surgery prior to the delivery of rapamycin. After the rats reached a stable postsurgical level of food consumption, the initial osmotic mini pump was replaced with a new mini pump containing either vehicle (10% dimethyl sulfoxide, 90% PEG400) or rapamycin (5 μg/μl in 10% dimethyl sulfoxide, 90% PEG400) for an additional 28-day infusion.
Tissue Harvest and Preparation
Rats were killed by thoracotomy under 5% isoflurane anesthetic 24 hours after the last rapamycin dose. Whole blood was taken by cardiac puncture and serum collected following centrifugation in serum separator tubes. Subsequently, 40ml of cold saline were perfused through the circulatory system. The perirenal, retroperitoneal, and epididymal white adipose tissues (PWAT, RTWAT, and EWAT, respectively) along with interscapular brown adipose tissue (BAT), livers, and hypothalamus were excised and their individual weights recorded.
Hypothalamus, liver (20 mg), and BAT (30 mg) were briefly sonicated in 10mM Tris, pH 6.8, 2% SDS for Western analysis (300, 600, and 300 µl, respectively). Protein was determined by DC Bradford (Bio-Rad, Hercules, CA).
Western Analysis
Protein homogenate from hypothalamus (SirT1, 25 µg; phospho-S6, 10 µg) and liver (p70S6K, 100 µg; p70S6, 10 µg) were separated on a SDS-PAGE gels and electrotransferred to nitrocellulose membranes (12). Immunoreactivity was assessed with antibodies specific to SirT1, phospho-p70S6K, and S6 (Cell Signaling, Danvers, MA). Western blots for phospho-S6 were reprobed with antibodies specific to S6 regardless of phosphorylation state and blots for phospho-p70S6K were reprobed with antibodies specific to p70S6K regardless of phosphorylation state (Cell Signaling, Danvers, MA) and data expressed as ratio of phosphorylated to total protein. Blots for SIRT-1, and acetyl p53 were reprobed and normalized to GAPDH (Abcam, Cambridge, MA). BAT homogenates (5 µg) were probed for UCP1 (Abcam, Cambridge, MA). Immunoreactivity was detected with ECL prime (GE Healthcare, Piscataway, NJ), scanned with a ChemiDoc XRS+ (BioRad, Hercules, CA) and quantified using ImageQuant software (Molecular Dynamics).
NADPH Oxidase Activity
NADPH oxidase activity was measured with a lucigenin-enhanced chemiluminescence assay using hypothalamus homogenates. The microplate containing approximately 20 µg of hypothalamic homogenates was maintained at 37 °C while luminescence was recorded using a micro-plate reader. Relative light units were obtained for 30 minutes in the presence of NADH (474 μm) and lucigenin (218 μM), and background-corrected values were normalized to protein content.
Serum Leptin and Triglycerides
Serum leptin (Millipore, Billerica, MA) levels were determined by ELISA. Serum triglycerides were measured using a CardioChek meter and triglyceride test strips. (Polymer Technology Systems, Indianapolis, IN.)
Adipose Tissue Leptin Content
Retroperitoneal adipose tissue (50 mg) was homogenized using a motor driven pestle in 400 ul TES buffer (10 mM Tris, 1 mM EDTA and, 250 mM sucrose, pH 7.4) containing a protease inhibitor cocktail. (Thermo Scientific). Homogenates were centrifuged 20,800×g for 15 minutes, at 4 °C. Infranatant was carefully transferred to a clean tube and assayed by ELISA for leptin peptide (Millipore, Billerica, MA).
Adipose Tissue mRNA
Total RNA from RTWAT (200 mg) was extracted using TRI reagent (Sigma, St Louis, MO). Total RNA was quantified and (2 µg) were treated with RNase-free DNase using a DNA free kit. (Ambion, Life Technologies, Grand Island, NY). First strand DNA was synthesized using 0.5 µg of DNase treated total RNA and random primers with M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA). Relative quantitative RT-PCR was performed using the QuantumRNA 18s internal standard kit (Life Technologies, Grand Island, NY). First strand cDNA was multiplexed with specific primers for leptin (fwd 5′-TGACACCAAAACCCTCATCA, rev 5′-TGAGCTATCTGCAGCACGTT) or hormone sensitive lipase (HSL) (fwd 5′-ATTAGAGCCCAAAGGGAGGT, rev 5′-CTGTCCCCACGTGTTCATCT) and coamplified with 18s primers using TAQ polymerase (Invitrogen, Carlsbad, CA). Amplification was performed under the following conditions; Leptin-19 cycles, 94 °C 20 seconds, 61 °C 20 seconds, 72 °C 20 seconds; HSL-26 cycles, 94 °C 20 seconds, 58 °C 20 seconds, and 72 °C 20 seconds. The PCR product was electrophoresed on a 5% acrylamide gel and stained with SYBR green (Lonza, Rockland, ME) for visualization. Gels were scanned using STORM fluorescent scanner and digital data analyzed with Imagequant (Molecular Dynamics).
Statistical Analysis
Data were analyzed by two-way analysis of variance. If a significant interaction was present, Bonferroni post hoc tests were applied to determine differences between experimental groups. A p value of less than .05 was considered statistically significant.
Results
Body Weight and Food Consumption
Prior to rapamycin treatment, body weight was different across age (p < .001), but not within age groups (308 ± 6g, 3-month control; 312 ± 6g, 3-month rapamycin; 575 ± 17g, 24-month control; 586 ± 15g, 24-month rapamycin). As expected, the young control rats demonstrated a steady increase in delta body weight whereas the aged control rats remained unchanged throughout the 5-week period. With rapamycin treatment, there was a decrease in delta body weight that was evident by Day 2 in both the young and aged rats (Figure 1, top). In the young rats, this resulted in a slowing of the growth rate, such that body weight was 11% less in the rapamycin rats at 5 weeks (360 ± 7g, control; 319 ± 6g, rapamycin, p < .001), whereas in the aged rats, there was a 13% loss of body weight (564 ± 16g, control; 491 ± 10g, rapamycin, p < .001; Figure 1, top inset).
Figure 1.
Top: Change in body weight in young (squares) or aged (circles) rats following administration of vehicle (open symbols) or rapamycin (closed symbols). Initial body weight was not different within age groups, but greater in aged compared with young (see text). Rapamycin (1mg/kg body weight) was administered three times a week starting at Day 0. Delta body weight was significantly different with rapamycin treatment. (p < .001) beginning at Day 2 in both young and aged rats. Inset: Body weight at end of experiment. *p < .001 for difference with age or rapamycin by two-way analysis of variance (ANOVA). Values represent the mean ± SEM of 12 rats/group. Bottom: Daily food consumption following administration of vehicle (open symbols) or rapamycin (closed symbols) to young (squares) or aged (circles) rats. Inset: Cumulative food intake over the 5-wk period was significantly different with rapamycin treatment. *p < .001, two-way ANOVA with Bonferroni post hoc analysis for difference from vehicle.
Daily food consumption, initially, paralleled the change in body weight. In young rats, food intake was slightly diminished with rapamycin treatment with a 13% decrease in cumulative intake for the 5-week period (Figure 1, bottom and insert). In the aged rats, initially there was a rapid decrease in food intake reaching a nadir (50% decrease, p < .001) at Day 5 followed by a gradual recovery such that by Day 28, there was no longer a difference in food intake (Figure 1, bottom). Overall, cumulative food consumption over the 5-week period was diminished by 32% (Figure 1, bottom insert). These findings are similar to those in our companion article examining 24-month and 6-month old rats (5).
Body Composition, Serum Leptin Levels, and Leptin Synthesis in WAT
Adiposity level and lean mass were determined by time-domain NMR on conscious rats prior to initiating treatment, at Week 3, and prior to the terminal phase of the experiment. In addition, the weights of three adipose depots, PWAT, RTWAT, and EWAT were determined at the time of death (Supplementary Table 1). In young animals, fat and lean mass increased steadily over time, however these rates of increase were considerably attenuated by rapamycin compared with control (Figure 2). However, because body weight gain was also attenuated, percent body fat was unchanged at the end of the study (25.19 ± 0.20, control vs 25.60±0.13, rapamycin). In aged control rats, lean mass, but not fat mass diminished slightly over the course of the experiment, whereas with rapamycin treatment, there was a significant decline in both fat and lean mass over time (Figure 2). As such, the percent body fat was diminished with rapamycin treatment (28.95 ± 0.39, control vs 27.85±0.19, rapamycin; p = .02). In addition, the lean/fat ratio was diminished with age and significantly diminished with rapamycin only in the young rats (Figure 2). In contrast, in aged rats, rapamycin treatment increased the lean/fat ratio, such that, overall, the age-related decline was reversed by rapamycin, suggestive of preferential adiposity loss (Figure 2).
Figure 2.
Fat and lean mass (top row), percent fat and lean mass (middle row), lean/fat mass ratio (bottom left), and body weight (bottom right) over time as determined by TD-NMR in the experiment described in Figure 1. Body composition was determined prior to the start of treatment and during Weeks 3 and 5 of the experiment. In young rats, fat and lean mass increased over time however this was attenuated with rapamycin treatment (significant interactions: p < .001). In aged rats, there was a time-dependent decrease in fat mass only in the rats treated with rapamycin (significant interaction: p < .001), whereas lean mass decreased in both treatment groups (significant interaction: p < .001). Values represent the mean ± SEM of 12 rats/ group. Body weight represents body weight at time of TD-NMR. Brackets represent a significant main effect of time and treatment condition (no interaction). *represents a significant difference from the pre-experimental time point within that age/treatment group. +represents a significant difference from the Control group within that age/treatment group.
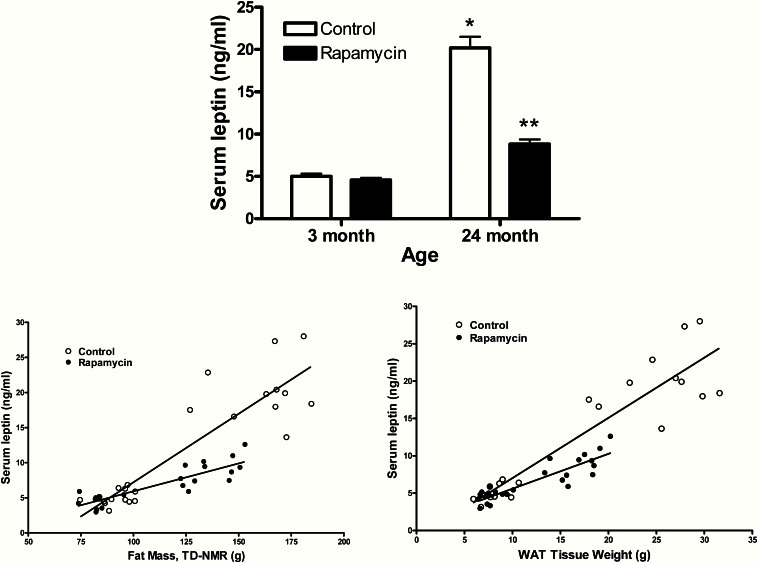
Serum leptin levels, another marker of adiposity, paralleled the changes in adiposity with a fourfold increase with age and a 56% decrease (p < .001) with rapamycin treatment in the aged rats but no change in young (Figure 3, top). Serum leptin was correlated with total adiposity (by TDNMR) and with the sum of the fat mass (Figure 3, bottom). In both cases the slope of the line was diminished with rapamycin treatment, suggesting the decrease in serum leptin is greater than would be predicted by the loss of fat mass. Therefore, we examined whether the decreases in serum leptin were the result of reduced fat mass, decreased leptin synthesis in WAT, or a combination of both by examining the leptin content and leptin mRNA levels of RTWAT.
Figure 3.
Serum leptin (top) and correlations between serum leptin and fat mass by TD-NMR (bottom left) and between serum leptin and adipose tissue weight (bottom right). Top: Values represent the mean ± SE of 11–12 rats per group. *p < .003 for difference with age by two-way analysis of variance (ANOVA); **p < .0001 by two-way ANOVA with Bonferroni post hoc analysis for difference with rapamycin from age-matched control. Bottom left: Control; r 2 =.803, p < .0001, slope =.195; Rapamycin; r 2 =.768, p < .0001, slope = .079. Bottom right: Control; r 2 =.856, p < .0001, slope =.806; Rapamycin; r 2 =.784, p < .0001, slope = .486.
The leptin protein content per mg of RTWAT roughly mirrored the serum leptin with an increase in the old rats that was nearly reversed by rapamycin (Figure 4, left). As opposed to the unchanged serum leptin, the leptin content of RTWAT was also diminished in the young rats by rapamycin (Figure 4, left). Leptin mRNA, a marker of leptin synthesis, was increased with age in RTWAT, but to lesser extent than leptin content or serum leptin (Figure 4, right). Remarkably, leptin mRNA was decreased with rapamycin by proportionally greater extent than the decrease in RTWAT leptin content or serum leptin values (Figure 4, right).
Figure 4.
Leptin content of RTWAT expressed per mg RTWAT weight (left) and leptin mRNA expressed per unit of 18s rRNA (right). Values represent the mean ± SE of 11–12 rats per group. The values of 3-mo control for leptin mRNA/18s RNA are arbitrarily set to 100 with SEM adjusted proportionally with remaining groups normalized to the level in 3-mo control. *p < .003 for difference with age by two-way ANOVA; **p < .0001 (24 mo) or p < .02 (3 mo) by two-way analysis of variance (ANOVA) with Bonferroni post hoc analysis for difference with rapamycin from respective age-matched control. ***p = .03 for difference with rapamycin by two-way ANOVA.
We also examined HSL, an enzyme involved in the mobilization of the stored fats that is also expressed in adipose tissue. In contrast to the decrease in leptin synthesis, HSL mRNA was not diminished by rapamycin in either young or old rats in RTWAT (Table 1), suggesting the effects of rapamycin on leptin synthesis are specific.
Table 1.
HSL, Triglycerides, BAT, UCP1, NADPH Oxidase Activity, Acetyl p53, and SIRT-1 with Age and Rapamycin Treatment.
| Young | Aged | |||
|---|---|---|---|---|
| Control | Rapamycin | Control | Rapamycin | |
| HSL mRNA (arbitrary units) | 100±15.2 | 117±14.9 | 118±16.5 | 109±12.2 |
| Triglycerides (mg/dl) | 97.75±6.67* | 74.18±3.35c | 150.64±20.47† | 77.5±3.38‡ |
| BAT, mg | 290±9* | 371±13 | 638±50† | 552±42 |
| UCP1/total BAT (arbitrary units) | 100±10.3 | 73.7±5.2c | 83.4±10.1 | 49.3±2.5‡ |
| NADPH oxidase (units/ug protein) | 100±6.0 | 97.5±6.4 | 128.3±4.9§ | 124.3±8.1§ |
| SIRT-1 (arbitrary units) | 100±5.9 | 114.4±9.4 | 71.1±7.1§ | 76.7±6.6§ |
| Acetyl p53 (arbitrary units) | 100±22.9 | 80.7±24.2 | 238.1±71.4§ | 172.1±11.9 |
Notes: Data represent the mean ± SE of 11–12 rats per group. HSL represents ratio of mRNA to 18s rRNA from EWAT. NADPH oxidase activity, SIRT-1, and acetyl p53 levels were determined in the hypothalamus and the latter two were normalized to GAPDH levels in each individual sample. The value of 3-mo control for each graph is arbitrarily set to 100 with SEM adjusted proportionally with remaining groups normalized to the level in 3-mo control (except for BAT and triglycerides, which represent actual indicated units). BAT = brown adipose tissue; HSL = hormone sensitive lipase; mRNA = messenger RNA.
*p < .001 (p < .03, triglycerides) for interaction by two-way ANOVA.
†p < .001 (p < .02, triglycerides) for difference with age by post hoc analysis.
‡p < .05 (p < .001, triglycerides) for difference with rapamycin by post hoc analysis.
§ p < .001 for difference with age by two-way ANOVA.
Serum triglycerides were, as expected, elevated with age by 50% (Table 1). Rapamycin treatment decreased triglycerides in the young, and completely reversed the augmented triglycerides with age (Table 1).
BAT UCP1 Levels
BAT weight was elevated with age by greater than twofold, whereas with rapamycin treatment there were no significant changes (Table 1). In contrast, when UCP1 levels were examined and total UCP1 per BAT tissue calculated, total UCP1/BAT was unchanged with age, but decreased with rapamycin treatment, such that the level of UCP1/BAT in rapamycin treated aged rats was less than one half that in young controls (Table 1).
Rapamycin Inhibits the mTORC1 Pathway
One downstream effector of the mammalian mTORC1 signaling pathway is p70 S6 kinase and increases in the phosphorylation state of this kinase and its protein target, S6 are endogenous reporters of mTORC1 activity (13). Phosphorylation level of p70 S6 kinase and S6 were examined in liver from control and rapamycin-treated animals. Among control rats, the level of mTORC1 activity was diminished with age as evidenced by a nearly 70% decrease in phospho-p70 S6 kinase although phospho-S6, was not significantly diminished with age (Figure 5 top). Rapamycin treatment nearly abolished mTORC1 signaling in liver of both young and aged rats with phospho-p70 S6 kinase reduced to a level equal to only 20% of the amount in young control (Figure 5, top left). To determine if the peripheral administered rapamycin also inhibited mTORC1 activity in the brain, phosphorylation of S6 was examined in the hypothalamus. Protein levels of phospho-S6 were similar in the hypothalamus from young and aged control rats. Rapamycin treatment diminished mTORC1 signaling in the hypothalamus, as evidenced by a nearly 30% reduction in S6 in young and a 45% decrease in aged rats (Figure 5, bottom). Notably, the extent of the decrease in the young rats was not nearly the magnitude of the decrease in the liver (Figure 5). Levels of phospho-p70 S6 kinase were below detection in the hypothalamus (data not shown).
Figure 5.
Liver phospho-P70 S6 kinase (top left), liver phospho-S6 (P-S6, top right), and hypothalamic phospho-S6 (bottom) protein levels with age and rapamycin treatment. The value of 3-mo control for each graph is arbitrarily set to 100 with SEM. adjusted proportionally with remaining groups normalized to the level in 3-mo control. Values represent the mean ± SE of 4–5 rats per group. Phospho-P70 S6 kinase: p = 0.025 for interaction by two-way analysis of variance (ANOVA). *p < .01 for difference with age and **p < .01 for difference with rapamycin by Bonferroni post hoc analysis. ***p = .0004 for significant main effect with rapamycin by two-way ANOVA. Brackets indicate significant main effect with age for hypothalamic phospho-S6 (p < .01).
NADPH Oxidase Activity
NADPH oxidase activity, one marker of oxidative stress was determined in the hypothalamus. NADPH oxidase activity was unchanged with rapamycin treatment, but was significantly increased with age (Table 1).
SIRT-1 and Acetyl p53 Levels in Hypothalamus
As a control, we also examined the SIRT-1 pathway in the hypothalamus, another pathway involved in energy metabolism and aging. Both SIRT-1 levels and the downstream signaling component, acetyl p53 (Table 1) levels were unchanged by rapamycin treatment. However, SIRT-1 levels were significantly diminished with age, whereas acetyl p53 levels were increased with age (Table 1).
Central Administration of Rapamycin
To examine whether peripherally administered rapamycin is acting centrally to reduce body weight and food consumption, rapamycin was infused into the third cerebral ventricle for 28 days in young rats. The change in body weight was similar between groups over the first 16 days, after which body weight diverged (Supplementary Figure 1, top). Delta body weight was significantly less between Days 17 and 28 with an approximate 25% difference by Day 28 (Supplementary Figure 1, top). In contrast, cumulative food consumption was unchanged over the course of the experiment between control (614 ± 15g) and rapamycin treated rats (593 ± 8g). In addition, serum leptin was unchanged with central rapamycin infusion (3.03 ± 0.26ng/ml, control and 2.87±27ng/ml, rapamycin).
Following rapamycin infusion, phosphorylation of S6 was examined in two brain regions, hypothalamus and hindbrain, as well as the liver. Similar to the outcome with peripheral treatment with rapamycin, phospho-S6 was greatly diminished in the liver to a level equal to only 66% of the control (Supplementary Figure 1, bottom left). Notably, there was no effect of rapamycin on phospho-S6 in the hypothalamus (Supplementary Figure 1, bottom right) or hindbrain (data not shown).
Discussion
The phenotype of the F344BN older rat is characterized by increased body weight and adiposity, diminished lean body mass, and reduced physical activity and performance (3,4). Our companion paper demonstrated that peripheral rapamycin treatment reduces this age-related body weight gain. In the clinical literature, controversy exists regarding the circumstances under which older adults should be advised to lose weight and in some cases, weight loss is associated with increased mortality and acceleration of declining function, and this issue is discussed in length in the companion article (5). More important than weight loss, is the body compartment (eg muscle vs adipose tissue) from which the weight loss occurs. In our companion article, rapamycin targeted adiposity, reversing age-related fat content to that of a 6-month old rat, while sparing lean mass, especially as compared with an IF group that lost a similar amount of weight (5). These data suggest that intermittent feeding alters behavior and physiology in the same manner regardless of age; however rapamycin has more selective and healthspan-inducing effects when initiated late in life (5). The present study replicates and extends those findings by demonstrating two salient points. First, rapamycin nearly normalizes the age-related elevation in serum leptin through both decreasing total adiposity and an inhibition of leptin synthesis per unit of adiposity. Second, despite decreased food consumption, the primary site of rapamycin action appears to be peripheral and not central.
This study included 3- and 24-month old groups compared with the companion paper that examined rats at 6 and 25 months of age. By 6 months of age, there is already an increase in total adiposity and serum leptin compared with a fully mature, 3-month old rat (3). Whereas, rapamycin decreased body weight in the 6-month old rats (5), there was no change in body weight in the 3-month old rats, although rapamycin reduced the weight gain observed in 3-month control rats. Both of these responses were less than the body weight reductions in the aged rats. In young rats, rapamycin appeared to temper the rate of weight gain, adiposity gain, and lean mass gain to similar extents, such that by the end of the experiment, percent body mass, percent lean mass, and lean/fat ratio were preserved by rapamycin. In contrast, in the senescent rats, there was a preferential loss of adiposity compared with lean mass resulting in an increase in lean to fat ratio. The observation that rapamycin is most effective in rats with greater adiposity is consistent with previous findings that suggests rapamycin targets adipose tissue (7). In that study, rapamycin downregulated genes involved with lipid deposition and promoted hyperlipidemia and gluconeogenesis. As a result, glucose and triglycerides were elevated with an associated increased insulin resistance, and glucose intolerance (7). Rapamycin also inhibited MTORC1/P70 S6 signaling and well as the mTORC2 pathway (7). In contrast, in these studies, triglycerides were not elevated; triglycerides were decreased in the young rats and notably, the elevated triglycerides with age were reversed with rapamycin. In addition, glucose levels were not elevated in our companion article (5). The differences may be due to the higher dose of rapamycin (2mg/kg) and a more frequent dosing interval (once/day) employed in the Houde and colleagues (7) study. This higher dose likely contributed to the observed inhibition of the mTORC2 pathway and the resulting adverse blood values. In contrast, the lower dose and less frequent dosing schedule employed in our studies appears to inhibit only the mTORC1 pathway, thus minimizing the adverse consequences resulting in more favorable glucose and triglycerides, reduced adiposity, normalized serum leptin, increased lean/fat ratio, and preserved activity levels (present study and 5). Another study employed a higher dose of rapamycin (4 mg/kg) that apparently inhibited both the mTORC1 and mTORC2 pathways, but observed that long-term treatment of 20 weeks reversed the adverse effect on glucose metabolism observed at 2 and 6 weeks of treatment (14). Thus, both dose and length of treatment appear to be important in the rapamycin regulation of glucose metabolism.
Leptin, synthesized in adipose tissue, is one important peptide involved in energy homeostasis. Leptin communicates to the brain the nutritional state of the animals and is one marker of total body adiposity. In some conditions, especially in lean animals, leptin is a potent anorexic and adiposity reducing agent. With adipose tissue a major target of rapamycin, it is not surprising that serum leptin is diminished, presumably as a consequence of decreasing adipose tissue. We previously reported that with aging, serum leptin levels are augmented and increase to a greater extent than the elevation in adiposity with age would predict, suggesting an increase in leptin synthesis with age (3). Herein, we demonstrate that leptin synthesis in RTWAT (leptin mRNA/18s rRNA) is not significantly increased with age among control rats, but that leptin content per unit of RTWAT is elevated indicating increased leptin storage in RTWAT with age. Moreover, rapamycin inhibits leptin synthesis in RTWAT in both young and aged rats with greater inhibition in the 24-month old rats. This effect on leptin appear to be specific, because rapamycin does not alter expression levels of HSL, a enzyme produced in adipose tissue and involved in mobilization of stored fat. In the absence of rapamycin, there is correlation between serum leptin and adiposity, measured either by TDNMR or adipose tissue weight. In the presence of rapamycin, the correlations remain, but the slope of the line is diminished, indicating the leptin contribution from adipose tissue is diminished independent of the size of the WAT, consistent with an inhibition of leptin synthesis per unit of adipose tissue. We suggest that this inhibition of leptin synthesis in conjunction with a decrease in total adiposity with rapamycin treatment accounts for the normalization of serum leptin in rapamycin-treated aged rats.
Peripheral rapamycin treatment inhibited the mTORC1 pathway in the liver as well as the hypothalamus. Moreover, the latter was associated with a decrease in hypothalamic phospho-AMP kinase in our companion article (5). AMP kinase is involved with the nutrient sensing pathway and neural cellular energy status is monitored through the [AMP]/[ATP] ratio (15). When ATP is depleted, the high ratio of AMP to ATP leads to phosphorylation of AMPK, thus decreasing malonyl-CoA levels leading to increased food consumption (16,17). This, diminished phospho-AMP kinase is consistent with the observed reduced feeding with peripheral rapamycin treatment. These data, coupled with inhibition of hypothalamic mTORC1, are suggestive of a central action of rapamycin, at least with respect to the decrease in food consumption. However, the observation that direct central infusion of rapamycin fails to alter food intake, serum leptin, or mTORC1 signaling suggests against a direct central action of rapamycin on feeding behavior in this study. It is possible that central infused rapamycin levels or length of treatment were insufficient to act on hypothalamic targets, even though phospho-S6 was diminished in the liver, indicating inhibition of peripheral mTORC1 signaling. The latter may be the result of leakage of rapamycin into the circulation or a direct neural inhibition of hepatic mTORC1 activity. Alternatively, the peripheral effects of rapamycin on body weight and adiposity may be mediated centrally, secondary to a peripheral mechanism. It is also possible that our observations from central infusion of rapamycin in young rats do not reflect the action of rapamycin in aged rats. In particular, older animals appear to be more sensitive to the adiposity-reducing action of rapamycin with both a larger reduction in food intake and body weight, and these augmented responses may be the result of a central action of rapamycin that is specific to older animals, thus not observed in young. Thus, the actions of rapamycin in aged rats could potentially be mediated by a central mechanism or by synergistic action between peripheral and central. Ongoing studies will address this possibility.
Collectively, these data suggest that the present dosing schedule of rapamycin acts on peripheral targets to inhibit mTORC1 signaling, preferentially reducing adiposity and sparing lean mass in an aged-model of obesity resulting in favorable outcomes on blood triglycerides, increasing lean/fat ratio, and normalizing elevated serum leptin with age. This decrease in serum leptin is due to both, a reduction in adipose tissue and a diminished leptin synthesis. The initial mechanism underlying the rapamycin responses appears to have a peripheral and not a central action. The peripheral rapamycin responses may communicate an excessive nutrients signal to the hypothalamus that triggers an anorexic response to reduce food consumption. The normalization of obesity-associated elevated serum leptin may have implications for the treatment obesity-associated leptin resistance.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
Supported by the NIH DK091710, P30 AG028740, and the Medical Research Service of the Department of Veterans Affairs.
Supplementary Material
References
- 1. Keith SW, Redden DT, Katzmarzyk PT, et al. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond). 2006;30:1585–1594. doi:10.1038/sj.ijo.0803326 [DOI] [PubMed] [Google Scholar]
- 2. Kaur J. A comprehensive review on metabolic syndrome. Cardiol Res Pract. 2014;2014:943162. doi:10.1155/2014/943162 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Li H, Matheny M, Nicolson M, Tümer N, Scarpace PJ. Leptin gene expression increases with age independent of increasing adiposity in rats. Diabetes. 1997;46:2035–2039. doi:10.2337/diabetes.46.12.2035 [DOI] [PubMed] [Google Scholar]
- 4. Carter CS, Cesari M, Ambrosius WT, et al. Angiotensin-converting enzyme inhibition, body composition, and physical performance in aged rats. J Gerontol A Biol Sci Med Sci. 2004;59:416–423. doi:10.1093/gerona/59.5.B416 [DOI] [PubMed] [Google Scholar]
- 5. Carter CS, Khamiss D, Matheny M, et al. Rapamycin vs. intermittent feeding: dissociable effects on physiological and behavioral outcomes when initiated early and late in life. J Gerontol A Biol Sci Med Sci. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi:10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Houde VP, Brûlé S, Festuccia WT, et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi:10.2337/db09-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi:10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paturi S, Gutta AK, Katta A, et al. Effects of aging and gender on muscle mass and regulation of Akt-mTOR-p70s6k related signaling in the F344BN rat model. Mech Ageing Dev. 2010;131:202–209. doi:10.1016/j.mad.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 10. Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron. 2012;75:425–436. doi:10.1016/j.neuron.2012.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scarpace PJ, Zhang Y. Leptin resistance: a prediposing factor for diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2009;296:R493–R500. doi:10.1152/ajpregu.90669.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Scarpace PJ, Matheny M, Tümer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience. 2001;104:1111–1117. doi:10.1016/S0306-4522(01)00142-7 [DOI] [PubMed] [Google Scholar]
- 13. Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459–467. doi:10.1016/j.cmet.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fang Y, Westbrook R, Hill C, et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. doi:10.1016/j.cmet.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wynne K, Stanley S, McGowan B, Bloom S. Appetite control. J Endocrinol. 2005;184:291–318. doi:10.1677/joe.1.05866 [DOI] [PubMed] [Google Scholar]
- 16. Wolfgang MJ, Cha SH, Sidhaye A, et al. Regulation of hypothalamic malonyl-CoA by central glucose and leptin. Proc Natl Acad Sci USA. 2007;104:19285–19290. doi:10.1073/pnas.0709778104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cha SH, Wolfgang M, Tokutake Y, Chohnan S, Lane MD. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proc Natl Acad Sci USA. 2008;105:16871–16875. doi:10.1073/pnas.0809255105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.