Abstract
Rapamycin, an inhibitor of the mammalian target of rapamycin pathway, has been shown to increase mammalian life span; less is known concerning its effect on healthspan. The primary aim of this study was to examine rapamycin’s role in the alteration of several physiological and behavioral outcomes compared with the healthspan-inducing effects of intermittent feeding (IF), another life-span-enhancing intervention. Male Fisher 344 × Brown Norway rats (6 and 25 months of age) were treated with rapamycin or IF for 5 weeks. IF and rapamycin reduced food consumption and body weight. Rapamycin increased relative lean mass and decreased fat mass. IF failed to alter fat mass but lowered relative lean mass. Behaviorally, rapamycin resulted in high activity levels in old animals, IF increased levels of “anxiety” for both ages, and grip strength was not significantly altered by either treatment. Rapamycin, not IF, decreased circulating leptin in older animals to the level of young animals. Glucose levels were unchanged with age or treatment. Hypothalamic AMPK and pAMPK levels decreased in both older treated groups. This pattern of results suggests that rapamycin has more selective and healthspan-inducing effects when initiated late in life.
Keywords: Life span, Healthspan, p70S6K, mTOR, Physical function, Leptin, Calorie restriction
Incredible strides have been made in the last decade regarding our understanding of the basic biology of aging, which have provided the basis for the development of preclinical models of late-life interventional strategies for combating physical decline. Pharmacological approaches may be particularly relevant late in life because not all older individuals benefit from or are capable of participating in traditional diet and/or exercise programs (eg, those that are obese, frail, or arthritic).
This notion has benefited from an evolving area of research focused on the application of calorie restriction (CR) “mimetics” (1), which target cell signaling pathways that may be involved in the basic biology of aging. The National Institute on Aging leads this initiative and has spearheaded the development of an interventions testing program (NIA ITP) to investigate CR mimetics across three different institutes in genetically diverse mice (1). Most robust to date is rapamycin, an inhibitor of the mammalian target of rapamycin (mTOR) pathway, which regulates cellular activities such as growth and survival, nutrient sensing, protein synthesis, and autophagy and has been shown to consistently increase mammalian life span (2). When rapamycin was fed to male and female mice late in life (20 months old), it led to an increase in life span of 14% for females and 9% for males (3). A subsequent study initiated at 9 months of age demonstrated increased survival in both male and female mice (4). Indeed, this life-span effect has been replicated several times, works in males and females, and most importantly, equally well when initiated either early or late in life. However less is known concerning rapamycin’s role as a modulator of healthspan. We and others have previously shown that physical function is predictive of longevity (albeit over a compressed period of time) (5) and that life-long CR improves function (6,7). Thus, a major empirical question is whether or not CR mimetics that purport to increase life span necessarily translate into mimetics of increased healthspan, including functional performance. Unfortunately, there is some indication that rapamycin treatment may actually be detrimental to health in older animals due to its aberrant effects on glucose and/or insulin signaling (8,9) as well as a potential for exacerbating age-related, down-regulated mTOR function in rat skeletal muscle (10). For these reasons, the primary aim of this study was to examine the role of rapamycin in the alteration of several physiological and behavioral outcomes, indicative of healthspan, in a rodent model of aging.
We have shown in rats and others in mice that rapamycin treatment results in a decrease in body weight (11). In mouse studies (3), this appeared to occur with no change in food intake. We favor the rat model because one can easily capture daily food intake without the social housing constraints that are inherent in mouse studies. In our rat model, we have shown that the loss in body weight is, in older animals, equivalent to that of intermittent feeding (IF) fed animals, suggesting that this loss in weight may be due to a “self-CR” on the part of the rapamycin-treated animals (11). We have previously shown an increase in physical activity, a robust marker of healthspan across a wide variety of species with CR and rapamycin treatment in two separate studies (7,11). However, it is unclear whether increases in activity observed were due to decreases in body weight or food consumption, or the direct action of rapamycin. Therefore, a secondary aim was to directly compare the healthspan effects of rapamycin treatment with an IF protocol to determine commonalities and differences between these two life-span-enhancing interventions. To these ends, we treated free-fed, young and old rats with vehicle or rapamycin for a 5-week period compared with vehicle-treated rats fed intermittently (food was removed 3 times/week). Body composition and measures of behavior physical activity were assessed along with biochemical parameters at death.
Methods
Animals
Subjects were male Fisher 344 × Brown Norway (F344BN) rats, purchased from the National Institute on Aging Colony at Harlan Laboratories (Indianapolis, IN). This rat strain was chosen because of its increased longevity and decreased cumulative lesion incidence compared with other strains (12). Furthermore, F344BN rats develop age-related body composition changes (ie, increased adiposity and decreased lean body mass) resembling those occurring during human aging (13). Animals (n = 60) were received at 6 and 25 months of age and housed individually on a 12-h light and 12-h dark cycle in a specific pathogen-free facility accredited by the American Association for Accreditation of Laboratory Animal Care. Animals were fed a standard rodent chow (18% kcal from fat, no sucrose, 3.1 kcal/g, diet 2018; Harlan Teklad, Madison, WI). Animals were allotted 4 weeks to acclimate to their housing conditions and to establish baseline rates of food intake and body weight. Health status, body weight, and food intake were monitored daily. Health assessments included checking for a sudden decline in body weight, redness around the eyes and nostrils, ruffled coat, open tail sores, and haunched posture. All experimental protocols were approved by the University of Florida’s Animal Care and Use Committee, and in accordance with the “Guide for the Care and Use of Laboratory Animals.”
Experimental Design
Animals were assessed at baseline for body weight, food intake, body composition, grip strength, and locomotor activity (Supplementary Table S1). Rats were then randomized, based on body weight, within each age, into three treatment groups: rapamycin (n = 20), IF (n = 20), and control (n = 20). Three older animals died prior to treatment, and one older, IF animal died during treatment. Data obtained from these animals were not included in any analysis. Treatment lasted for 5 weeks, and body weight and food intake were measured throughout. Baseline measurements were reassessed at the end of the fifth week. Rats were sacrificed by rapid decapitation using a guillotine and the hypothalamus and tibialis anterior muscle collected.
Behavioral and Physiological Measurements
Body weight and food intake
Body weight was obtained once per week, and average daily food intake was determined three times per week. Every Monday, Wednesday, and Friday, food was removed for 24 hours from IF animals. When not fasted, IF animals had ad-lib access to food.
Drug treatment
Drug delivery was accomplished via intraperitoneal injections, and dosages were adjusted weekly based on the animal’s weight. Every Monday, Wednesday, and Friday, rapamycin-treated animals received 1mg/kg rapamycin, whereas IF and control animals received a vehicle injection to control for handling. Rapamycin was dissolved in a vehicle of ethanol (4%), polyethylene glycol (5%), Tween 80 (5%), and sterile saline. The injection volume was 0.5ml/kg body weight.
Determination of body composition using time-domain nuclear magnetic resonance
Body composition was assessed using time-domain nuclear magnetic resonance (TD-NMR) in restrained but fully conscious rats (TD-NMR Minispec, Bruker Optics, The Woodlands, TX). The MiniSpec identifies three components of body composition (fat, free body fluid, and lean tissue in grams) by acquiring and analyzing TD-NMR signals from all protons in the sample area. Scans were acquired by placing the rats into a cylindrical restrainer (90-mm diameter and ~250-mm length), fitted with a screw top that tightens to the length of the rat. The restrainer was then inserted into the analyzer. The total scan time was approximately 1min, and the average of two scans was used as the final measurement.
Assessment of physical performance
Forelimb grip strength was determined using an automated grip strength meter (Columbus Instruments, Columbus, OH). The rat was grasped by the base of the tail and suspended above a grip ring. After approximately 3 seconds, the rat was gently lowered toward the grip ring and allowed to grasp the ring with its forepaws. The remainder of the rat’s body was quickly lowered to a horizontal position, and the animal’s tail was pulled until grasp of the ring was broken. The mean force in grams was determined with a computerized electronic pull strain gauge that was fitted directly to the grasping ring. Maximal force obtained from three trials was used as the dependent measure.
Measurement of locomotor activity
Animals were placed in activity chambers (43×43cm, Med Associates, Inc.) for 3-minute sessions. Total distance traveled (centimeter) and spatial location (time in “center” or “margin”) were tracked with an overhead camera and Ethovision XT 7.0 software (Noldus Information Technology Inc., Wageningen, The Netherlands).
Tissue collection and Western blotting
Animals were sacrificed by rapid decapitation. Each animal received its final treatment 24 hours prior to being euthanized and had access to food during this 24-hour time frame. Trunk blood was immediately collected and processed for subsequent determination of serum leptin. The hypothalamus and tibialis anterior muscle were dissected, frozen in liquid nitrogen, and stored at −80°C until analyzed. Hypothalamus and muscle were briefly sonicated in 300 µL of 10mM Tris, pH 6.8, 2% sodium dodecyl sulfate for Western analysis. Protein was determined by DC Bradford (Bio-Rad, Hercules, CA). Protein homogenate from hypothalamus (28 µg) and muscle (160 µg) were separated on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and electro-transferred to nitrocellulose membranes (14). Immunoreactivity was assessed with antibodies specific to phosphorylated adenosine monophosphate-activated protein kinase (pAMPK) (and reprobed with antibodies specific to adenosine monophosphate-activated protein kinase (AMPK) regardless of phosphorylation state) in hypothalamus, and antibodies specific to p70S6K (Cell Signaling, Danvers, MA) in tibialis anterior muscle. Western blots for AMPK and pAMPK were reprobed with antibodies specific to GAPDH (Abcam, Cambridge, MA) and expressed as the ratio to GAPDH. Western blots for phospo-p70S6K were reprobed with antibodies specific to p70S6K regardless of phosphorylation state (Cell Signaling, Danvers, MA) and expressed as the ratio of phosphorylated to total protein.
Serum leptin and glucose
Serum was harvested by a 10-min centrifugation in serum separator tubes for the assessment of nonfasting leptin measured using rat RIA kits (Millipore, Billerica, MA). Glucose was determined using OneTouch Ultra glucose meter and test strips (Lifescan, Milpites, CA).
Data and Statistical Analyses
All data are displayed as means ± standard error of the mean. Differences in dependent measures were analyzed by analysis of variance (one-way, two-way, and repeated measures as appropriate) and post hoc comparisons were made with the Newman–Keuls or Dunnett’s tests using commercially available software (SigmaPlot 12.0). Differences were considered statistically significant when the p-value was less than .05.
Results
Baseline Measures
Supplementary Table S2 contains baseline measures for each group of rats. All animals were pseudo-randomized to groups based on body weight to ensure equality of distribution given that this outcome was fundamental to our hypothesis regarding weight loss versus the direct action of rapamycin in influencing healthspan. As expected, there were a number of measures that were different across ages. In particular, body weight (F 1,47 = 273.2; p < .001), food intake (F 1,50 = 7.56; p = .008), absolute lean (F 1,47 = 224.0; p < .001) and fat (F 1,47 = 368.6; p < .001) mass, and % fat mass (F 1,47 = 112.6; p < .001) were higher in the older, 25-month-old animals. Lean-to- fat ratio (F 1,47 = 164.1; p < .001), % lean mass (F 1,47 = 125.3; p < .001), and grip strength corrected to body weight (F 1,50 = 102.8; p < .001) were lower in the older animals. Locomotor activity was not different across groups at baseline; however, younger rats spent more time in the margin of the arena (F 1,47 = 8.0; p < .007). There was also a main effect of eventual treatment group on this measure (rapamycin > IF; F 2,47 = 3.39; p = .042). There were several other statistically significant effects: baseline food intake was higher in the eventual IF groups compared with the rapamycin groups (F 2,50 = 3.64; p = .033), and the lean-to-fat ratio was higher in the 6-month-old rapamycin animals compared with young IF and controls. Thus, in the final analyses, we present the body composition data as % of body weight in order to mitigate these initial random differences.
Rapamycin Inhibition of mTOR
One downstream effector of the mammalian mTOR pathway is p70S6K and an increase in the phosphorylation state of this kinase is a marker of mTOR activity (15). Phosphorylation level of p70S6K was examined in tibialis anterior muscle across treatments. Among rats that did not receive rapamycin, the level of mTOR activity was unchanged with age or IF. Rapamycin treatment significantly diminished p70S6K in young (74%) and aged (54%) rats indicating inhibition of mTOR activity in the periphery (F 2,21 = 6.97; p = .005; Supplementary Figure S1).
Food Consumption
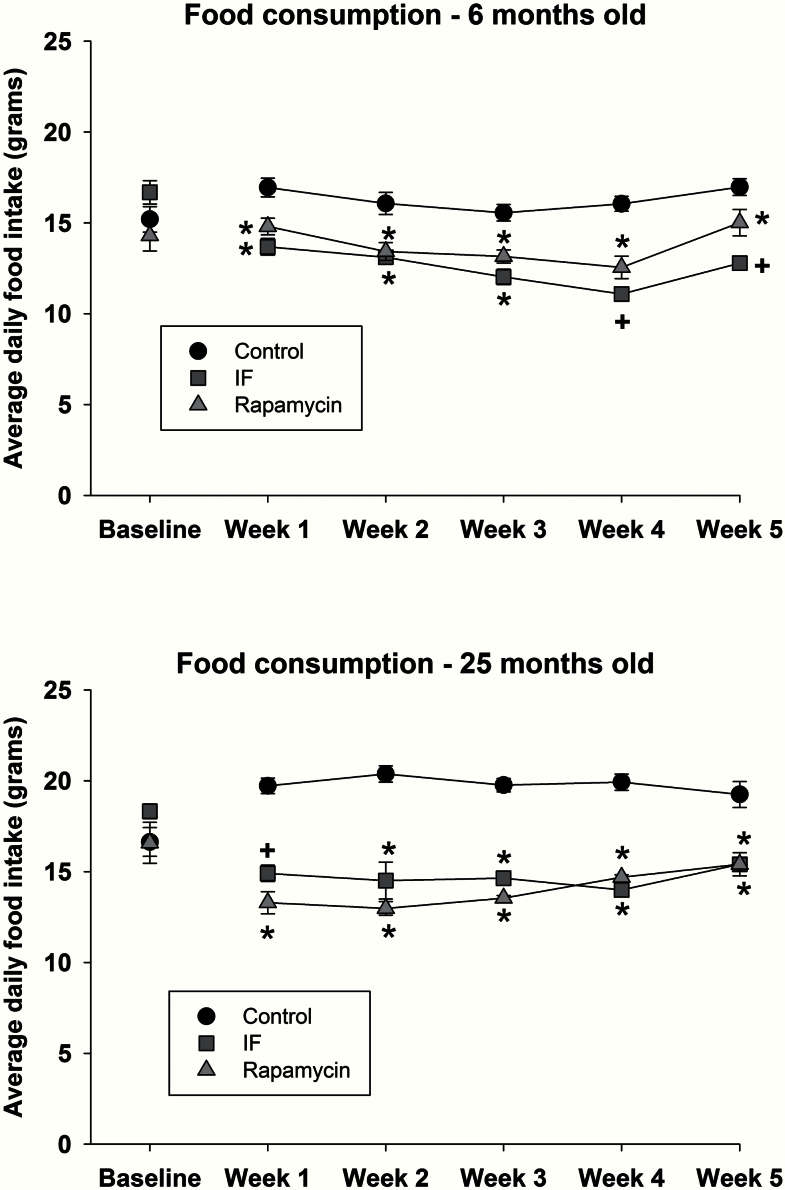
Food consumption (displayed as average daily food intake in grams over a 1-week period) assessed at baseline and during the 5 weeks of treatment is shown for 6-month-old (Figure 1, top) and 25-month-old (Figure 1, bottom) rats. At all time points during treatment with either rapamycin or IF, food consumption was significantly lower relative to young (F 10,162 = 5.05; p < .001) and old (F 10,138 = 8.6; p < .001) control animals.
Figure 1.
Food consumption in 6-month (top) and 25-month old rats (bottom) at baseline and during 5 weeks of rapamycin, intermittent feeding (IF), or control treatment. Mean data are shown, and error bars represent standard error of the mean. * indicates a significant decrease relative to control, and + indicates a significant difference from control and rapamycin.
Although IF treatment resulted in an overall decreased level of food intake, on the days that animals had access to food, they consumed significantly more than their original baseline levels (F 1,16 = 8.08; p < .012). That is, prior to IF treatment, young and old animals consumed 16.7 ± 0.6 and 18.3 ± 0.4g of food, respectively (p < .05). However, during IF treatment, the daily average food intake on the days that food was available increased to 22.0 ± 0.5 and 26.2 ± 0.5g for the young and old rats (p < .05), respectively.
Body Weight
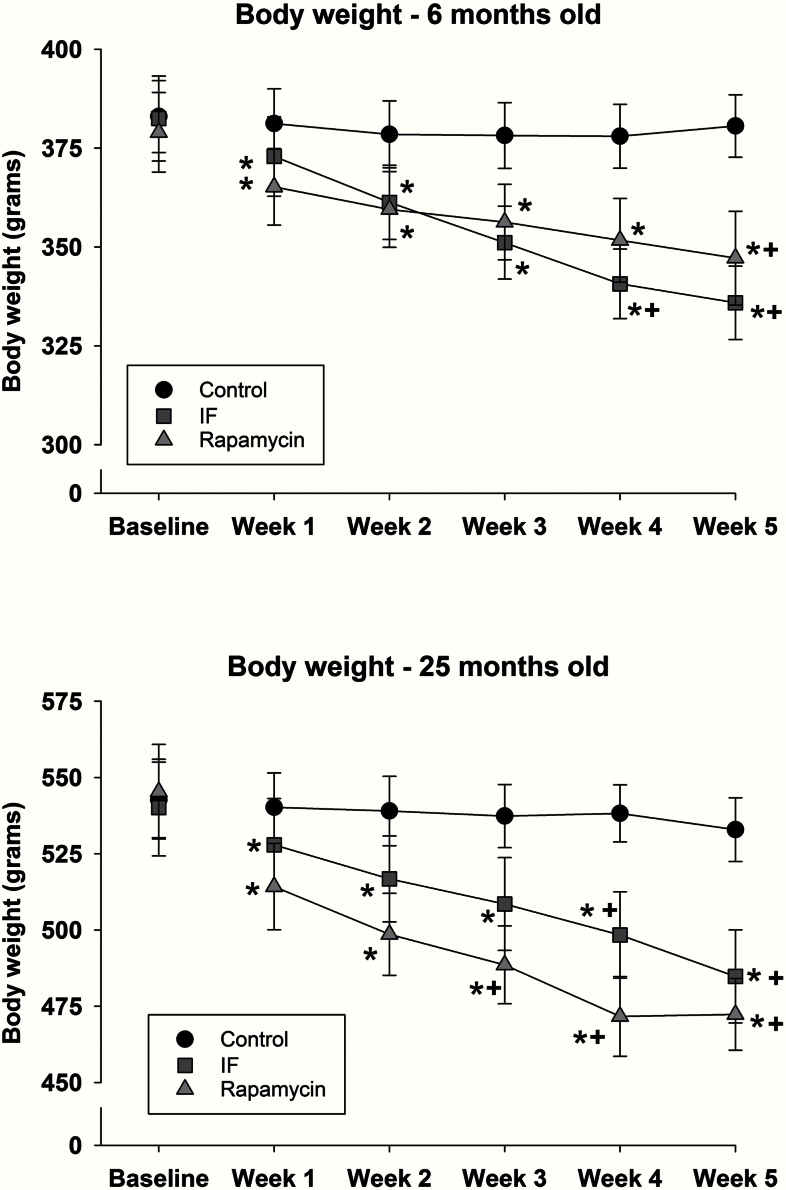
At baseline there was, as expected, a significant difference in body weight between young and old rats (F 1,50 = 273.2; p < .001; Supplementary Table S2). Decreased food consumption with the rapamycin and IF treatment resulted in progressive decreases in body weight in both young (Figure 2, top) and old (Figure 2, bottom) rats. Body weights for both young (F 10,135 = 28.9; p < .001) and old (F 10,115 = 13.2; p < .001) animals treated with rapamycin or IF were decreased relative to baseline at each assessment time (indicated by “*”). However, compared with control animals, young rapamycin-treated rats had significantly lower body weights at Week 5, but in the old animals, the decrease was apparent from Weeks 3 to 5 (indicated by “+”). IF treatment resulted in decreased body weight during Weeks 4 and 5 relative to control animals (indicated by “+”).
Figure 2.
Body weight in 6- (top) and 25-month-old rats (bottom) at baseline and during 5 weeks of rapamycin, intermittent feeding (IF), or control treatment. Mean data are shown, and error bars represent standard error of the mean. * indicates a significant decrease relative to baseline, and + indicates a significant difference from control.
Body Composition
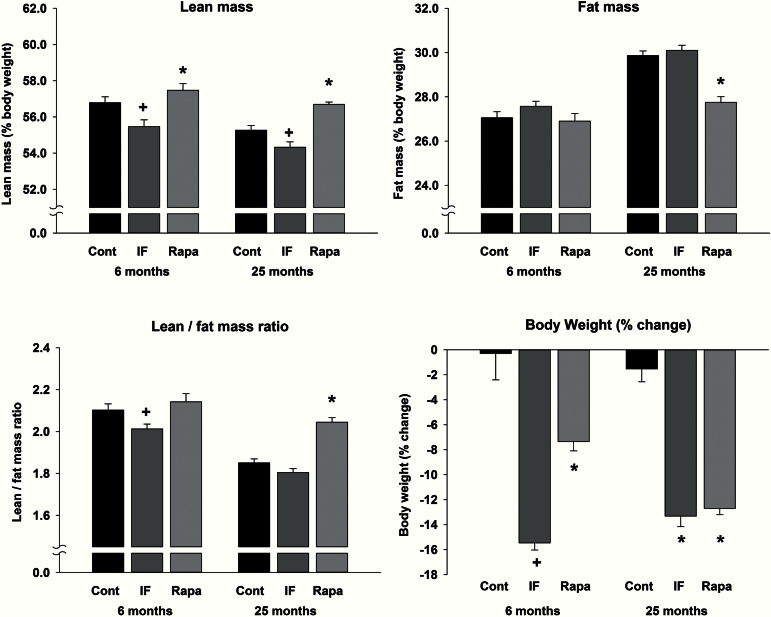
Figure 3 (bottom right) shows the net change in body weight over the course of the experiment. There was a significant interaction (F 2,50=5.3; p < .008) such that in the old animals both IF and rapamycin produced a decrease of approximately 13%, whereas in the young animals, rapamycin produced a 7% decrease and IF resulted in a 15% decrease. The decreases in body weight in the IF and rapamycin groups were reflected differentially in body composition (ie, changes in lean and fat mass). Lean mass (represented as % body weight; Figure 3, top left) was significantly different between ages (F 1,50 = 20.2; p < .001) and was altered differentially by the two treatments such that levels of lean mass were highest in rapamycin-treated rats and lowest in the IF group (F 2,50 = 24.3; p < .001). Regarding fat mass (Figure 3, top right), there were the expected increases in fat mass with age (significant interaction; F 2,50 = 8.1; p < .001). In the young rats, there were no differences due to treatment conditions; however, in the old rats, rapamycin treatment resulted in significantly lower levels of fat mass. Another measure of body composition, lean-to-fat mass ratio, was significantly different as a result of treatment (Figure 3, bottom left; F 2,50 = 4.3; p < .019). This ratio was decreased in the young, IF animals relative to the control and rapamycin groups, whereas there was an increase in the old, rapamycin animals such that they were not different from young control animals. Taken together, the changes among the measures of body composition suggest that rapamycin treatment in old animals results in a body composition profile of a young rat.
Figure 3.
Measures of % lean mass (top left), % fat mass (top right), and lean-to-fat mass ratio (bottom left). * indicates a significant change relative to control and intermittent feeding (IF) groups, and + indicates a significant difference from control and rapamycin groups. Change in body weight over the course of the experiment (bottom right). Both treatment conditions decreased body weight, with a similar magnitude of effect in the old animals, and a greater effect in IF young animals compared with rapamycin-treated animals. * indicates a significant decrease relative to control, and + indicates a significant difference from control and rapamycin.
Locomotor Activity, Anxiety-Like Responses, and Grip Strength
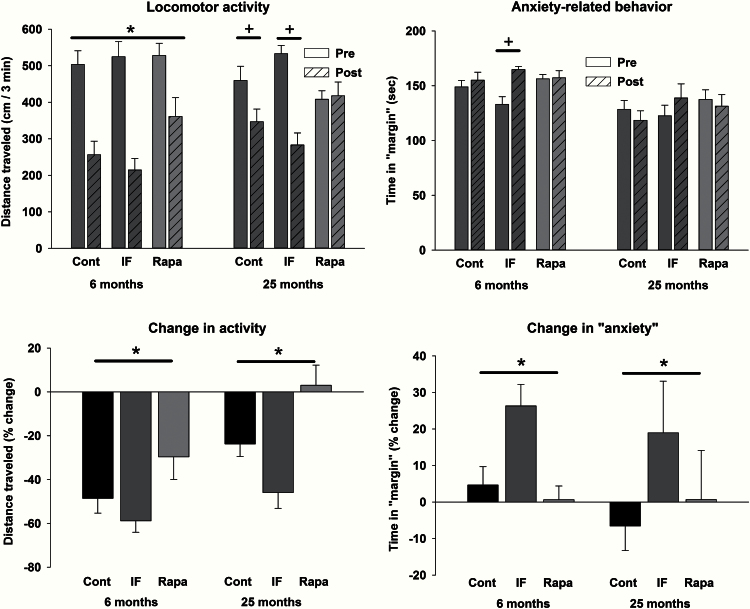
Locomotor activity was assessed at baseline and at the end of the treatment period. The absolute levels of activity (top left) and % change in distance traveled (top right) are shown in Figure 4. There was a significant main effect of treatment phase in the young rats (F 1,26 = 81.1; p <.001), and a significant interaction in the old rats (F 2,21=14.4; p <.001), with decreases in activity in the control and IF rats and no change in the rapamycin rats (Figure 4, top left). Examination of the % change measure revealed that younger animals showed a greater decrease in activity across the two testing time points compared with older animals (Figure 4, top right; main effect of age: F 1,47 = 15.2; p <.001). For both ages, the rank order of decreases in activity was IF > control > rapamycin (F 2,47 = 13.8; p < .001). During these sessions, the amount of time spent in the “center” and in the “margin” of the open field was calculated, as time in the margin is oftentimes taken as a measure of anxiety. In the young rats, there was a significant interaction (F 2,26 = 7.6; p = .003) with IF treatment resulting in an increase in anxiety-like behavior (Figure 4, bottom left). Examination of the % change revealed a main effect of treatment such that the IF treatment resulted in a significant increase in this behavior at both ages (Figure 4, bottom right; F 2,47 = 4.8; p < .012). Grip strength was assessed before and at the end of treatment. Across ages and groups there was a change in grip strength that ranged from a decrease of 8% to an increase of 34%; however, there were no statistically significant differences.
Figure 4.
Locomotor activity (left panels) and a measure of anxiety (right panels) in control, intermittent feeding (IF), and rapamycin treatment groups of both ages. Regarding absolute levels of locomotor activity (top panel), there was a significant main effect of phase in the young animals (activity decreased in all groups with repeated testing), and a significant interaction in the old animals (decreases in control and IF rats, with no change in rapamycin-treated animals). Regarding the % change in activity (bottom panel), there were significant main effects of age (larger decrease in young rats) and treatment condition (rapamycin> control > IF). For the anxiety absolute measures (top panel), there was a significant interaction with increases in anxiety due to treatment in the young animals. Regarding the % change in anxiety (bottom panel), IF animals spent more time in the margin following treatment compared with control and rapamycin groups at both ages. For the absolute measures, * indicates a significant main effect of phase, and + indicates a difference following a significant interaction. For the relative measures, * indicates a significant main effect of treatment group.
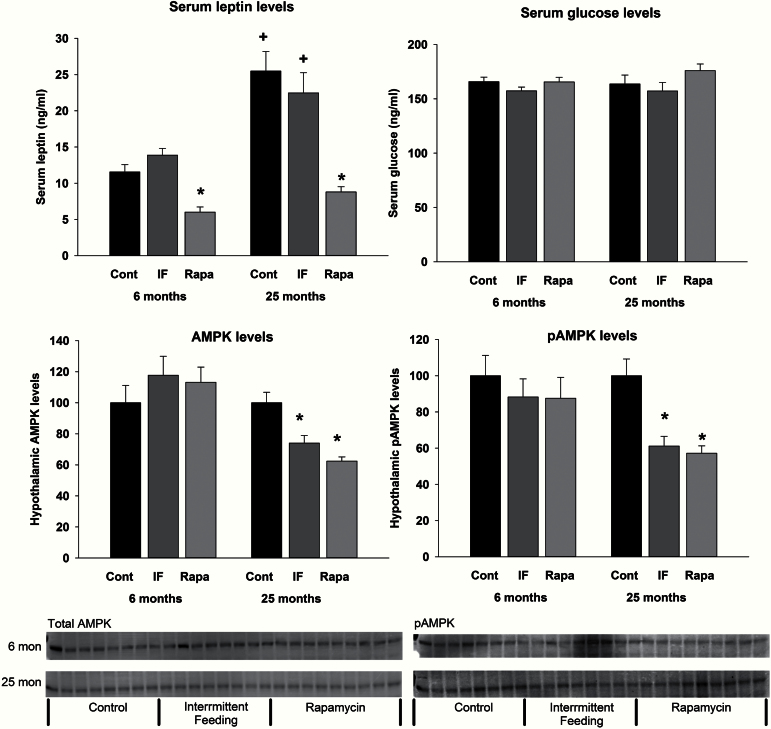
Serum Leptin and Glucose
Serum leptin levels increased with age in both the control and IF groups (Figure 5, top left; F 2,49 = 5.21, p = .009 for the interaction). In both the young and old rapamycin-treated animals, leptin levels were significantly lower than control animals. Supplementary Figure S2 shows the relationship between absolute fat mass and serum leptin levels. There was a significant correlation between these variables (r = 0.589, p < .00001). In general, older animals (squares) had higher levels of leptin relative to younger animals (circles). Of particular interest, however, was that both young and old rapamycin-treated animals (blue circles and blue squares, respectively) had the lowest levels of leptin regardless of fat mass. Notably, there were no differences in serum glucose levels among the groups (Figure 5, top right).
Figure 5.
Serum leptin, serum glucose, hypothalamus AMPK (total), and hypothalamus pAMPK levels at the end of the treatment. Leptin levels increased as a function of age in the control and IF groups, but not in the rapamycin-treated animals. At both ages, leptin levels were significantly lower in the rapamycin-treated animals relative to control and IF groups. Total AMPK and pAMPK levels were not altered by treatment in young animals. Levels of both total AMPK and pAMPK were significantly decreased following IF and rapamycin treatment. AMPK and pAMPK are expressed as the ratio to GAPDH. * indicates a decrease relative to control animals, and + indicates an increase relative to young animals.
Hypothalamic AMPK
The nutrient-sensing marker pAMPK was examined in the hypothalamus at death. pAMPK was unchanged with age and with treatment in the young rats. In contrast, pAMPK was diminished with both IF and rapamycin treatment in the old rats (F 2,20 = 13.286, p < .001; Figure 5, bottom right). pAMPK was expressed relative to GAPDH levels because both IF and rapamycin treatment in the aged rats decreased levels of total AMPK (F 2,21 = 15.861, p < .001; Figure 5, bottom left).
Discussion
Rapamycin is the most robust pharmacological intervention for extending life span in mammalian models, namely mice. This compound extends longevity whether administered early or late in life, does so in males and females, and at multiple laboratories around the country that serve as sites for the NIA ITP (3,4). These mice studies focused primarily on longevity but also included limited measures of physical function such as rotorod performance, and reported lowered body weight without a reduction in food intake. Therefore, a more extensive evaluation of the impact on physical function is warranted to evaluate whether or not rapamycin should be recommended for improving healthspan when administered late in life.
There is reason to think that increased life span may be related to increased healthspan effects given the relationship, in both humans and rodents, between function and mortality (5,16). We have used the F344BN rat as our model given the extensive body of published studies reported in this strain, regarding age-related changes in both behavioral function and skeletal muscle biology. Furthermore, there are many conceptual similarities between age-related changes observed in this strain and what is observed in humans. For example, several studies have shown that this strain proceeds from 80% to 50% survival between 24 and 30 months of age (17). In humans, this same pattern of survival mirrors an exponential increase in disability (16). In fact, we have demonstrated that assessment of functional limitations in the vicinity of the 50% survival range is highly predictive of mortality in both rats and humans. This rat strain is an excellent model to explore the question of the efficacy of late-life interventions for improving healthspan and physical function.
However, there are several reasons to question whether or not rapamycin administered late in life will actually improve physical function and hence healthspan given that (a) in almost every case, rapamycin reduces body weight making dissociation of the direct action of rapamycin from the effects of changes in body weight and/or food consumption muddled; (b) rapamycin blocks mTOR, a protein that is already reduced in aged muscle and is essential for cell maintenance and nutrient sensing, calling into question the impact of further reducing signaling in this system; and (c) rapamycin has been shown to impair glucose functioning, which in older organisms is universally disrupted, directly associated with declining physical performance, and could potentially become exacerbated with rapamycin treatment (8). We attempt to address in this article and in our companion article (18), these reservations by examining rapamycin’s role in the alteration of several physiological and behavioral outcomes, indicative of healthspan, in a rodent model of aging and to directly compare rapamycin treatment with an IF protocol to determine commonalities and differences between these two life-span-enhancing interventions on healthspan. Table 1 provides a summary of the effects of 5 weeks of rapamycin or IF treatment and the specifics are discussed below.
Table 1.
Summary of 5-Week Treatment Effects in Young and Old Rats
| Intermittent Feeding | Rapamycin | |||
|---|---|---|---|---|
| 6 | 25 | 6 | 25 | |
| Food consumption*,† | ↓ | ↓ | ↓ | ↓ |
| Body weight*,† | ↓ | ↓ | ↓ | ↓ |
| Lean mass*,† | ↓ | ↓ | ↑ | ↑ |
| Fat mass*,† | — | — | — | ↓ |
| Lean-to-fat ratio*,† | ↓ | — | — | ↑ |
| Locomotor activity‡ | ↓ | ↓ | ↓ | — |
| Anxiety‡ | ↑ | ↑ | — | — |
| Grip strength | — | — | — | — |
| Leptin*,† | — | — | ↓ | ↓ |
| AMPK*,† | — | ↓ | — | ↓ |
| pAMPK*,† | — | ↓ | — | ↓ |
| Glucose | — | — | — | — |
Notes:*Age-related effects described in text.
†Change relative to control.
‡Change relative to baseline.
First, rapamycin in almost every case induces weight loss in old and young animals. In the clinical literature, controversy exists regarding the circumstances under which older adults should be advised to lose weight and in some cases, weight loss is associated with increased mortality and acceleration of declining function (19–22). This most likely is associated within which body compartment (eg, muscle vs adipose tissue) the weight loss occurs. Thus, elucidating how rapamycin affects weight loss is critical. As stated previously, it is also critical to dissociate the body weight and/or food consumption effects of rapamycin from the direct pharmacological action of the compound itself.
To address both of these questions, we opted to compare rapamycin treatment with a group of young and old rats undergoing an IF protocol. Our IF protocol mimics the rapamycin treatment protocol in so far as animals are receiving “treatment” approximately every other day, and more importantly, results in a food consumption and body weight pattern that is similar to that following rapamycin treatment. Furthermore, rats exposed to such an IF regimen when given the opportunity on feeding days do not completely compensate for their lack of food on nonfeeding days thereby resulting in approximately a 25%–30% reduction in food intake (this study) (23). This is in contrast to some strains of mice that actually make up for the majority of the lost opportunity to eat and only demonstrate a 10% reduction in body weight (24). Interestingly, although dependent on strain and age of initiation, both rats and mice experience increased maximal life span (23,24). Thus, IF is an appropriate life-span-enhancing control to compare with rapamycin for dissociating weight loss versus target effects on healthspan.
In this study, both IF and rapamycin reduced food consumption, and thus body weight although the effects across age were different. The young IF animals lost more weight than their rapamycin-fed counterparts. This was correlated with food intake. Young and old IF as well as old rapamycin-treated animals reduced their food intake by 20%–30%, whereas the young rapamycin-treated animals reduced intake by less than 10%. Interestingly, in the IF fed animals, although they binged on those days were they had access to food, they did not completely double their intake to adjust for the deficit.
This is in agreement with a previously published data set (11); however, at that time we were unclear whether or not the loss of weight would be selective for lean or fat mass. In this study, the effects on body composition were dramatically different depending on treatment condition as measured by TD-NMR. In older animals, rapamycin treatment resulted in a relative increase in lean mass and a decrease in fat mass, resulting in a lean-to-fat mass ratio similar to young control rats. IF treatment, however, failed to alter fat mass and resulted in relative lower levels of lean mass across both ages.
Data from the National Institute on Aging ITP indicate that rapamycin-treated mice experience a reduction in body weight without a concomitant decrease in food intake (3). There are several reasons for this disparity: (a) food intake is difficult to measure in a group housing situation as with the ITP; (b) there is a relatively small level of food intake per day, so it is perhaps not easily measured, and (c) there is a need to measure several times during the week to get an accurate account as there is variability, even intra-animal, in food intake across the week. This again highlights the advantage of the rat model in understanding the impact on food intake on body weight using late-life CR mimetics such as rapamycin. This is a critical piece given that unless proper food intake measures are assessed; dissociating the impact of the hypophagia versus the direct action of the pharmacological intervention is impossible.
Finally, we did not experience any increased mortality or morbidity in the older animals in either treatment group although weight loss onset was quite rapid. This was initially a concern given the data in human studies (20) demonstrating that weight loss, whether intentional or unintentional, over the age of 55 is associated with increased risk for mortality. This has also been demonstrated in rats (25) showing that traditional daily 40% CR initiated late in life accelerates mortality. To be sure, our study was truncated in nature (5 weeks). However, the two studies from Goodrick and colleagues (23,24) demonstrate that IF, even when initiated late in life, may have profound effects on longevity; as such, IF and traditional CR are explicitly different flavors of calorie reduction. Moreover, as discussed below, IF and rapamycin treatment, although resulting in similar calorie reduction and concomitant weight loss, differentially impacted various physiological and behavioral measures in young and old animals. As an aside, one clue to what these differences may be has been described in a article by Miller and colleagues (26), albeit in mice and only in the context of differential gene expression. It is the opinion of these authors that an investigation of the physiology and behavior is critical and represents strength of this study for clinical translation of functional outcomes.
A major concern with administering rapamycin to aged animals, and translation to humans, lies in the mechanism of action of the compound itself. “Chronic” rapamycin treatment in mice leads to insulin resistance, severe glucose intolerance, and increased gluconeogenesis, although treatment lasted only for 15 days (8). Similarly, obese high-fat-fed KK and HlJ mice treated with rapamycin for 42 days, lost body weight and adiposity but had higher serum insulin levels and food intake (9). This resulted in a marked decline in glucose tolerance, paralleled by increased generation of plasma reactive oxygen species. Lamming and colleagues (27) have confirmed that the selectivity of rapamycin for TORC1 versus TORC2 is negated with extended treatment (14 days), thus leading to dysregulation in glucose and/or insulin signaling.
However, length and regimen of dosing may be key for achieving healthspan-inducing effects with rapamycin treatment. For example, Fang and colleagues (28) observe impaired glucose tolerance in mice, early in dosing that is resolved over time (beginning at ~6 weeks and continuing through 20 weeks). Interestingly, Liu and colleagues (29) demonstrated a reversal of aberrant glucose signaling, in two strains of mice, fed either chow or high-fat diets, after rapamycin treatment was removed, and suggest that regulating dosing in an intermittent fashion may be optimal. In this study, chronic rapamycin treatment (5 weeks) in a well-described rat model of aging did not alter glucose levels and our companion article reports that triglyceride levels were actually reduced. Furthermore, confirming the observation of Liu and colleagues (29), our 3× per week, low-dose (1mg/kg), intraperitoneal injection regimen may have been responsible for these positive results. Future studies optimizing dose, route, and regimen, in multiple rodent species, will ensure proper translation for the use of rapamycin treatment in older individuals to maximize healthspan.
Furthermore, mTOR is central to the regulation of cellular activities such as growth and survival, nutrient sensing, protein synthesis, and autophagy (30). However, in the F344BN rat, mTOR protein levels are known to decrease with age in skeletal muscle an effect that is highly correlated with atrophy (10). As expected, in this study, we observed a nearly 70% reduction of p70S6K in the tibialis anterior muscle only in the rapamycin-treated animals of both ages. Thus, rapamycin treatment could potentially be causative for accelerated sarcopenia or loss of muscle quality and declining physical function even in the face of a potential for increased life span. This has been the heart of many arguments within the biology of aging community, which suggest that any life span-enhancing compound is bound to also increase healthspan, often times with no assessment of healthspan to bolster this stance.
Luckily, investigators are backpedalling somewhat in this regard and more recently several studies have been published to investigate the impact of rapamycin on physical function and physiology in muscle. Ye and colleagues (31) have recently shown that in two strains of mice, rapamycin given at doses that increase life span (2mg/kg injected daily), decreases mitochondrial transcripts in the absence of an effect on mitochondrial DNA integrity, protein levels, or treadmill endurance. However, it should be noted that although there was no detrimental impact on endurance, there was certainly no improvement either. This is in agreement with other studies. For example, Neff and colleagues (32) reported an increase in locomotor activity in C57/Bl6 mice treated with rapamycin for 12 months beginning at either 4 or 20–22 months of age but no effect on either rotorod performance or grip strength. This is in contrast to Zhang and colleagues (33) who reported preserved function on a rotorod test; however, this difference is perhaps due to different methodologies in conducting these behavioral assays, which makes the argument for establishing a standardized set of physical performance measures in mice and rats as we have argued for several years (5,34).
In this study, we demonstrated that rapamycin treatment resulted in the high levels of activity (ie, there was no decrease in activity from baseline to the post-treatment assessment) in the old animals. This effect was absent in IF-treated animals and is actually in direct conflict with traditional 40% CR data we have published previously (7). However, this may be explained in terms of a behavioral mechanism given that food-deprived animals experience an increase in physical activity or what may also be interpreted as foraging behavior. In fact, food deprivation is a common tool used in a variety of behavioral assays for increasing the motivational level of the animal to perform. However, in this study, all animals had ad-lib access to food and water 24 hours prior to final behavioral assessments and euthanasia. Thus, the IF animals, and all other animals for that matter with the exception of the older rapamycin-treated animals which were approximately 25% hypophagic, were actually sated during the locomotor test. In agreement with Neff and colleagues (32), we found no effect of rapamycin or IF on grip strength. Again, the negative finding with grip strength was not expected in the IF animals and may also be explained given their motivational level. A final contributing factor to motivational state in the IF group was that they demonstrated increased levels of “anxiety” at both ages, as measured in the open field test, spending more time in the margins of the arena rather than crossing the center. As a whole, these data suggest, and for the most part are in agreement with the mouse literature, that rapamycin has limited effects on physical performance (strength and endurance) and that any effect on physical activity may simply be indicative of their motivational state (hunger). Future studies should employ more extensive batteries before conclusions firmly be drawn regarding rapamycin’s impact on this aspect of healthspan.
We further investigated the possibility that the “state” of central satiety contributes to the reduction of food intake in IF- and rapamycin-treated animals through the AMPK nutrient-sensing pathway and found very different results in young versus old animals. Feeding centers within the brain are the primary regulators of food intake via sensing neural cellular energy status through the (AMP) to (ATP) ratio. During periods of fasting, ATP is depleted, and the high ratio of AMP to ATP leads to phosphorylation of AMPK. Conversely, excessive nutrients increase available ATP, lower the (AMP)-to-(ATP) ratio, and decrease pAMPK. Elevated pAMPK signals through decreasing malonyl-CoA levels promoting food consumption, whereas diminished pAMPK leads to diminished food consumption (35,36).
The status of pAMPK at death in this study may reflect either the consequences of chronic inhibition of mTOR pathway or the more immediate nutrient status due to the feeding or fasting state of the animals at the time of death. The acute action of rapamycin through inhibition of the anabolic action of mTORC-1 may result in an initial increase in available nutrients. Supporting this, in this study, we observed age-dependent effects with hypothalamic levels of total AMPK and pAMPK, such that, IF and rapamycin treatment resulted in significant decreases only in the old animals with no change in the young animals.
Firstly, it is puzzling that the young rats did not display similar decreases in pAMPK with IF or rapamycin. We know that in young rats, any disturbance in energy homeostasis is rapidly reversed, but in old rats, the responses are slower and incomplete (37,38). This is true with both recovery from caloric restriction (37) and normalization with high-fat feeding (38). It is possible that the chronic nature of the experiment has enabled the young rats to compensate to a new equilibrium in which, although food consumption persists at a slightly reduced level, the nutrient-sensing system is apparently unchanged or only minimally changed, for the IF and rapamycin-treated young animals, respectively. This is supported by the fact that the younger animals, relative to the older animals, had an attenuated response to the food intake-reducing impact of rapamycin treatment.
Secondly, we observed a decrease in pAMPK in both IF- and rapamycin-treated animals. At the time of death, the aged IF rats were in a state of excess nutrients (ie, they had access to food the previous 24 hours), which was reflected by low levels of pAMPK. Thus, the reduction in pAMPK in the older animals may on the one hand reflect a state of actual satiation in the IF animals binging before euthanasia, and on the other hand may reflect an mTOR-dependent central mechanism that creates a “state” satiation in the rapamycin-treated animals. More extensive studies, beyond the present scope comparing fed and sated motivational states in IF versus rapamycin versus paired-fed animals, both young and old, would contribute to our understanding of modulation of nutrient-sensing pathways with these various food intake-reducing interventions, initiated early or late in life.
However, more relevant to this study and our companion article (18) is the contribution of leptin to the effects of reduction in food intake and fat loss. Leptin, synthesized in adipose tissue, is one of the most important peptides involved in energy homeostasis and this hormone communicates to the hypothalamus and other important reward centers the nutritional and/or satiety state. In this study, circulating leptin levels were reduced in the rapamycin-treated animals compared with the control and IF groups. Normally, low leptin levels would evoke an increase in food consumption. In this case, these low leptin levels were associated with diminished food consumption. Moreover, leptin levels are one marker of adiposity, and normally characterized by a tight relationship between circulating leptin and adiposity. Although, both the IF- and rapamycin-treated animals lost the same amount of weight, the rapamycin groups selectively lost a greater amount of weight in their fat mass. Even more interesting, the older rapamycin-treated rats, even though they still had more adipose tissue than young controls, had circulating leptin levels no different from young controls. Even more puzzling, there was no relationship between absolute levels of fat mass and leptin levels in the rapamycin groups. These data suggest that rapamycin disrupts the normal balance between adiposity, leptin, and feeding. Our companion article further explores this relationship by examining the role of rapamycin on synthesis of leptin and other fat-specific factors following both peripheral and central administration of rapamycin.
In conclusion, we have found that there are differential effects of IF and rapamycin treatment on a variety of physiological and behavioral outcomes when initiated both early and late in life in a rodent model of aging. A general pattern of results suggests that rapamycin has more selective and protective effects in old animals. Overall, there were limited findings of improvement with either intervention in measures relating to strength in older animals, suggesting that a more thorough behavioral assessment of a variety of physical function measures is warranted before a clearly articulated recommendation for clinical application. Examination of route, dose, regimen, and species differences will more clearly define the translational usefulness of rapamycin to enhance human healthspan. Finally, whether the beneficial effects of rapamycin we observed on healthspan are mediated via a central or peripheral mechanism is an empirical question that is further addressed in our companion article (18).
Supplementary material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This study was supported by National Institutes of Health (P30 AG028740 and DK091710) and the Medical Research Service of the Department of Veterans Affairs.
Supplementary Material
References
- 1. Ingram DK, Anson RM, de Cabo R, et al. Development of calorie restriction mimetics as a prolongevity strategy. Ann N Y Acad Sci. 2004;1019:412–423. doi:10.1196/annals.1297.074 [DOI] [PubMed] [Google Scholar]
- 2. Lamming DW, Ye L, Astle CM, Baur JA, Sabatini DM, Harrison DE. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell. 2013;12:712–718. doi:10.1111/acel.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi:10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi:10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carter CS, Sonntag WE, Onder G, Pahor M. Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci. 2002;57:B193–B197. doi:10.1093/gerona/57.5.B193 [DOI] [PubMed] [Google Scholar]
- 6. Carter CS, Hofer T, Seo AY, Leeuwenburgh C. Molecular mechanisms of life- and health-span extension: role of calorie restriction and exercise intervention. Appl Physiol Nutr Metab. 2007;32:954–966. doi:10.1139/H07-085 [DOI] [PubMed] [Google Scholar]
- 7. Carter CS, Leeuwenburgh C, Daniels M, Foster TC. Influence of calorie restriction on measures of age-related cognitive decline: role of increased physical activity. J Gerontol A Biol Sci Med Sci. 2009;64:850–859. doi:10.1093/gerona/glp060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Houde VP, Brûlé S, Festuccia WT, et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi:10.2337/db09-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang GR, Wu YY, Chiu YS, et al. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol. 2009;105:188–198. doi:10.1111/j.1742-7843.2009.00427.x [DOI] [PubMed] [Google Scholar]
- 10. Paturi S, Gutta AK, Katta A, et al. Effects of aging and gender on muscle mass and regulation of Akt-mTOR-p70s6k related signaling in the F344BN rat model. Mech Ageing Dev. 2010;131:202–209. doi:10.1016/j.mad.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 11. Carter CS, Marzetti E, Leeuwenburgh C, et al. Usefulness of preclinical models for assessing the efficacy of late-life interventions for sarcopenia. J Gerontol A Biol Sci Med Sci. 2012;67:17–27. doi:10.1093/gerona/glr042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lipman RD, Chrisp CE, Hazzard DG, Bronson RT. Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age. J Gerontol A Biol Sci Med Sci. 1996;51:B54–B59. doi:10.1093/gerona/51A.1.B54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rice KM, Linderman JK, Kinnard RS, Blough ER. The Fischer 344/NNiaHSd X Brown Norway/BiNia is a better model of sarcopenia than the Fischer 344/NNiaHSd: a comparative analysis of muscle mass and contractile properties in aging male rat models. Biogerontology. 2005;6:335–343. doi:10.1007/s10522-005-4808-0 [DOI] [PubMed] [Google Scholar]
- 14. Scarpace PJ, Matheny M, Tümer N. Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience. 2001;104:1111–1117. doi:10.1016/S0306-4522(01)00142-7 [DOI] [PubMed] [Google Scholar]
- 15. Blouet C, Ono H, Schwartz GJ. Mediobasal hypothalamic p70 S6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008;8:459–467. doi:10.1016/j.cmet.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi:10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi:10.1093/gerona/54.11.B492 [DOI] [PubMed] [Google Scholar]
- 18. Scarpace PJ, Matheny M, Strehler KYE, et al. Rapamycin normalizes serum leptin by alleviating obesity and reducing leptin synthesis in aged rats. J Gerontol A Biol Sci Med Sci. 2014. doi:10.1093/gerona/glu230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49:1309–1318. doi:10.1046/j.1532-5415.2001.49258.x [DOI] [PubMed] [Google Scholar]
- 20. Morley JE. Pathophysiology of weight loss in older persons. Nestle Nutr Workshop Ser Clin Perform Programme. 2005;10:167–172. doi:10.1159/000083304 [DOI] [PubMed] [Google Scholar]
- 21. Wannamethee SG, Shaper AG, Lennon L. Reasons for intentional weight loss, unintentional weight loss, and mortality in older men. Arch Intern Med. 2005;165:1035–1040. doi:10.1001/archinte.165.9.1035 [DOI] [PubMed] [Google Scholar]
- 22. Dirks AJ, Leeuwenburgh C. Caloric restriction in humans: potential pitfalls and health concerns. Mech Ageing Dev. 2006;127:1–7. doi:10.1016/j.mad.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 23. Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Differential effects of intermittent feeding and voluntary exercise on body weight and lifespan in adult rats. J Gerontol. 1983;38:36–45. doi:10.1093/geronj/38.1.36 [DOI] [PubMed] [Google Scholar]
- 24. Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider N. Effects of intermittent feeding upon body weight and lifespan in inbred mice: interaction of genotype and age. Mech Ageing Dev. 1990;55:69–87. doi:10.1016/0047-6374(90)90107-Q [DOI] [PubMed] [Google Scholar]
- 25. Lipman RD, Smith DE, Bronson RT, Blumberg J. Is late-life caloric restriction beneficial? Aging (Milano). 1995;7:136–139. doi:10.1007/BF03324303 [DOI] [PubMed] [Google Scholar]
- 26. Miller RA, Harrison DE, Astle CM, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi:10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi:10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fang Y, Westbrook R, Hill C, et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. doi:10.1016/j.cmet.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Y, Diaz V, Fernandez E, et al. Rapamycin-induced metabolic defects are reversible in both lean and obese mice. Aging (Albany NY). 2014;6:742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest. 2013;123:980–989. doi:10.1172/JCI64099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ye L, Widlund AL, Sims CA, et al. Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging (Albany NY). 2013;5:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neff F, Flores-Dominguez D, Ryan DP, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi:10.1172/JCI67674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Y, Bokov A, Gelfond J, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69:119–130. doi:10.1093/gerona/glt056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Justice JN, Carter CS, Beck HJ, et al. Battery of behavioral tests in mice that models age-associated changes in human motor function. Age (Dordr). 2014;36:583–592. doi:10.1007/s11357-013-9589-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolfgang MJ, Cha SH, Sidhaye A, et al. Regulation of hypothalamic malonyl-CoA by central glucose and leptin. Proc Natl Acad Sci USA. 2007;104:19285–19290. doi:10.1073/pnas.0709778104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cha SH, Wolfgang M, Tokutake Y, Chohnan S, Lane MD. Differential effects of central fructose and glucose on hypothalamic malonyl-CoA and food intake. Proc Natl Acad Sci USA. 2008;105:16871–16875. doi:10.1073/pnas.0809255105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gruenewald DA, Marck BT, Matsumoto AM. Fasting-induced increases in food intake and neuropeptide Y gene expression are attenuated in aging male brown Norway rats. Endocrinology. 1996;137:4460–4467. doi:10.1210/endo.137.10.8828508 [DOI] [PubMed] [Google Scholar]
- 38. Judge MK, Zhang J, Tümer N, Carter C, Daniels MJ, Scarpace PJ. Prolonged hyperphagia with high-fat feeding contributes to exacerbated weight gain in rats with adult-onset obesity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R773–R780. doi:10.1152/ajpregu.00727.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.