Abstract
We examined the effect of rapamycin on the life span of a mouse model of type 2 diabetes, db/db mice. At 4 months of age, male and female C57BLKSJ-lepr db/db mice (db/db) were placed on either a control diet, lacking rapamycin or a diet containing rapamycin and maintained on these diets over their life span. Rapamycin was found to reduce the life span of the db/db mice. The median survival of male db/db mice fed the control and rapamycin diets was 349 and 302 days, respectively, and the median survival of female db/db mice fed the control and rapamycin diets was 487 and 411 days, respectively. Adjusting for gender differences, rapamycin increased the mortality risk 1.7-fold in both male and female db/db mice. End-of-life pathological data showed that suppurative inflammation was the main cause of death in the db/db mice, which is enhanced slightly by rapamycin treatment.
Keywords: Life span, Obesity, mTOR Inhibition, Toxicity
Rapamycin (also known as Sirolimus) is a macrocyclic lactone produced by Streptomyces hygroscopicus, which was initially shown to inhibit to growth of fungi and other eukaryotes. Research in the early 1990s showed that rapamycin exerted its antigrowth/proliferative effects through its binding to a specific protein, Target of Rapamycin (TOR) (1–3). TOR has been shown to be a serine/threonine kinase that regulates the response of eukaryote cells to nutrients, growth factors, and cellular energy status. In mammals, TOR (mTOR) is found in two major complexes: mTORC1, which is inhibited by rapamycin (4) and mTORC2, which has been reported to be insensitive to rapamycin; however, recent data suggest that long-term rapamycin treatment might inhibit mTORC2 (5). Studies in invertebrates show that inhibition of the TOR signaling pathway increased life span of yeast, Caenorhabditis elegans, and Drosophila. In 2009, Harrison and colleagues (6) made a major discovery when they showed that feeding rapamycin to mice significantly increased the mean and maximum life span of both male and female mice.
Since the initial publication in 2009, six other reports have been published showing that rapamycin increases the life span of various strains of wild type mice (7–12). In addition, eight reports have been published showing that rapamycin increases the life span of genetically modified mouse models of various human diseases, ranging from models of cancer to mouse models of progeria and a mitochondrial disease (13–20). However, rapamycin treatment of transgenic mice overexpressing a mutant form of Cu/Zn-superoxide dismutase that mimics amyotrophic lateral sclerosis had either no effect on life span of H46R/H48Q mice (21) or decreased (~15%) the life span of G93A mice (22). Thus, although the current data show that the effect of rapamycin on life span is very robust and reproducible in a large number of strains and genetic models of mice, the studies with the amyotrophic lateral sclerosis transgenic mice suggest that rapamycin’s longevity effect may not be universal and that some genotypes might not show an increase, and perhaps even a decrease, in life span when treated with rapamycin.
The goal of our study was to determine the effect of rapamycin on the life span of a mouse model of type 2 diabetes. Type 2 diabetes is major age-related disease in the United States, and it is projected that it will increase dramatically over the next three decades because of the increase in the number of elderly adults and the changes in lifestyle and diet (23). In type 2 diabetic mice (eg, db/db mice), it is known that mTOR activation contributes to renal hypertrophy and matrix accumulation, fibrosis and renal failure (24). Our group showed that short-term treatment of young db/db mice with rapamycin ameliorates renal hypertrophy and renal matrix protein increment by inhibiting mRNA translation (24), suggesting that rapamycin treatment might be beneficial for diabetic mice. However, several studies show that mice fed rapamycin develop insulin resistance (10,25,26), and Gyurus and colleagues (27) reported that rapamycin therapy predisposes renal transplant patients to new-onset diabetes. Therefore, it was of interest to determine what effect rapamycin would have on the life span and age-related pathology of a mouse model of type 2 diabetes. We chose to study C57BLKSJ-lepr db/db mice because, similar to humans, these mice develop obesity, insulin resistance, and type 2 diabetes including complications such as diabetic nephropathy and are commonly employed as an animal model for the study of type 2 diabetes (28).
Materials and Methods
Animals
Experiments were carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio. Male and female C57BLKSJ-lepr db/db mice (stock number 000642) were obtained from Jackson Laboratory (ME) at 3 to 5 weeks of age and maintained on a standard chow diet.
Life-span Study
The male and female C57BL/KsJ-lepr db/db mice (db/db) were randomly divided into two groups (n = 40 mice in each group) and the life span was measured as we have described previously (12). Starting at 4 months of age, one group received the laboratory chow mixed with microencapsulated rapamycin (14 ppm) ad libitum and the control group received laboratory chow containing the same amount of empty capsules ad libitum. The level of rapamycin given to the mice is identical to that used in the initial study by Harrison and colleagues (6) with UM-HET3 mice and by our group (12) with C57BL/6 mice. We found that this level of rapamycin treatment resulted in blood levels of rapamycin of ~2ng/ml for both male and female db/db mice, which is lower than the rapamycin levels observed in the UM-HET3 (6) and C57BL/6 mice (6,12). Mice were allowed free access to water and were maintained under specific pathogen-free barrier conditions with three mice per cage. The criteria for euthanasia on humane grounds were as follows: losing 20% of body weight, inability to eat or drink, debilitation, infection, and obvious pain or distress; however, only two such events occurred in this study. In addition, three mice died due to their cage being flooded. All five events occurred in rapamycin-treated males and were recorded as right-censored data points. The remaining mice were followed until they died of natural causes. However, only 144 mice were available for final pathologic analysis because of cannibalization and advanced autolysis in some of the animals.
End-of-Life Pathological Analysis
A detailed pathological evaluation was conducted on the mice used in the life-span study when they died. Upon necropsy, gross and microscopic lesions were identified. A detailed pathological assessment was performed on each mouse. The following organs and tissues were excised and preserved in a neutral 10% buffered formalin: brain, pituitary gland, heart, lung, trachea, thymus, aorta, esophagus, stomach, small intestine, colon, liver, pancreas, spleen, kidneys, urinary bladder, reproductive system (males: prostate, testes, epididymis, and seminal vesicles; females: ovaries, oviduct, uterus, and vagina), thyroid gland, adrenal glands, parathyroid glands, psoas muscle, knee joint, sternum, and vertebrae. Any other tissues with gross lesions were also excised. The fixed tissues were processed conventionally, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin–eosin. Although autolysis of varying severity occurred, it did not prevent the histopathological evaluation of lesions, with the exception of three mice. Diagnosis of each histopathological change and severity of major lesions were determined with histological classifications in aging mice previously described (29–31).
A list of pathological lesions was constructed for each mouse, which included both neoplastic and nonneoplastic diseases, and was determined independently by two pathologists. Based on these histopathological data, the tumor burden, disease burden, and severity of each lesion in each mouse were assessed. The tumor burden was calculated as the sum of different types of tumor in a mouse. For example, a mouse that had reticular sarcoma and pituitary adenoma was assigned a tumor burden score of 2. The disease burden was similarly calculated as the sum of the histopathological changes in a mouse. The severity of neoplastic and renal lesions was assessed with the grading system previously described (29–31). The percentage of tumor-bearing mice, overall and age-specific incidence of disease, was calculated for each experimental group. The percentage of tumor-bearing mice was calculated as the percentage of mice that had one or more neoplastic lesions. For this assessment, all neoplastic lesions were counted regardless of the severity of tumors, that is, both incidental (not severe enough to be the cause of death) and fatal (severe enough to be the cause of death) tumors were counted.
The probable cause of death was determined independently by two pathologists (Y.I. and G.B.H.) based on the severity of pathology found at necropsy. In cases where mice had a neoplastic lesion, mice with Grade 3 or 4 lesions were categorized as death by neoplastic disease. In more than 90% of the cases, there was agreement by the two pathologists. In cases where the two pathologists did not agree or where disease did not appear severe enough to result in death, the cause of death was categorized as unknown.
Statistical Analysis
The Cox proportional hazard model (32) was fitted to the data using rapamycin, sex, and their interaction as predictors and cohort as a stratifying variable. The proportionality assumption of each Cox model was tested as described by Grambsch and Therneau (33). Mean, median, and 90% survivorship for each group along with standard errors were obtained via Kaplan–Meier curves, and 95% confidence intervals for the latter two statistics were obtained by resampling the data from each group. For the above models, the R language was used (34) with extensive use of the survival built-in package (35,36) along with the multcomp (37) and nlme (38) add-on packages.
For the longitudinal body weight experiments, a mixed-effect model was used. The response variable was the first derivative of body weight with respect to day (ie, per-day change in body weight). This was necessary in order to make the data stationary and linear. Most of the rapamycin-treated mice were dead after 400 days of treatment. Therefore after 400 days, the sample would be biased in favor of control mice. To avoid an unbalanced sample, only the data up to the 400th day of the diet were used for each mouse. When the full data set was fit, there were a number of interactions involving sex that could mask a sex-specific rapamycin response. Therefore, the data were split into male and female subsets and analyzed separately. Diet, time on diet, current body weight (centered on the mean body weight at the first measurement for which a first derivative could be calculated), the diet-by-time interaction, and the body-weight-by-time interaction were used as the fixed-effect terms. Centered body weight, time on diet, and their interaction were used as the random-effect terms (ie, within-individual variation). For the purposes of visualizing the data, the fitted values and their 95% confidence intervals were back transformed to the original scale.
Results
Rapamycin Shortens Life Span of db/db Mice
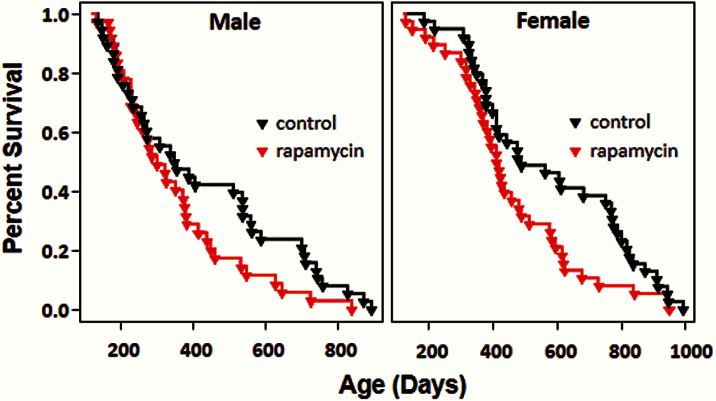
Figure 1 shows the survival curves for male and female db/db mice after 4 months of age at which time they were put on the two diets. Visually, the survival curves of the db/db mice on the control diet appear to exhibit an exponential decrease with age rather than a Gompertz curve. Using a likelihood ratio test to compare exponential and Gompertz models fitted to these data, we found that the Gompertz model fits these data far better than the exponential model (p = .00000001 for females and p < .00000001 for males). On the control diet, the median survival of male and female db/db mice was 349 and 487 days, respectively, and 90% of the male and female mice died by 758 and 913 days of age, respectively (Figure 1, Table 1). We found that the hazard ratio (the daily probability of dying relative to the control group) as estimated by the Cox model was lower (~60%) for females than for males (p = .0242) fed the control diet (Table 2). As shown in Table 1, the mean, median, and maximum survival (defined as age at which 90% of the group are dead) were all decreased in the mice fed rapamycin. After adjusting for the sex difference, rapamycin was found to significantly increase (p = .0496, adjusted) the mortality risk relative to the respective control diet-fed groups by 1.7-fold for both male and female db/db mice (Table 1). As shown by the survival curves in Figure 1, the negative effect of rapamycin on life span occurred in the latter half of the life span, that is, after 400 days of age. Thus, our data show that rapamycin shortens the life span of the short-lived db/db, diabetic mice.
Figure 1.
Survival of diabetic mice fed control diet or rapamycin-containing diet. Male and female db/db mice were randomized to control diet (black) or diet containing 14 ppm of rapamycin (red) at the age of 4 months and followed until natural death (n = 35 mice for rapamycin-treated males and 40 mice in the three other groups). After adjusting for gender difference, rapamycin was associated with higher rate of death by 1.7-fold (p = .0496) in male and female db/db mice.
Table 1.
Life-span Data for db/db Mice Fed Rapamycin
| Days Survived Since Diet Start, Males | Days Survived Since Birth, Males | |||||||
|---|---|---|---|---|---|---|---|---|
| Control | Rapamycin | ∆ Days | % ∆ | Control | Rapamycin | ∆ Days | % ∆ | |
| Mean | 309.1 (37.8) | 232.3 (28.2) | −76.8 | −24.90% | 420 (37.7) | 351.3 (28.2) | −68.7 | −16.40% |
| 50% Mortality | 237 (146, 442) | 183 (128, 264) | −54 | −22.80% | 349 (265, 539) | 302 (247, 383) | −47 | −13.50% |
| 90% Mortality | 639 (590, —) | 510 (342, —) | −129 | −20.20% | 758 (709, —) | 629 (461, —) | −129 | −17.00% |
| Days Survived Since Diet Start, Females | Days Survived Since Birth, Females | |||||||
| Control | Rapamycin | ∆ Days | % ∆ | Control | Rapamycin | ∆ Days | % ∆ | |
| Mean | 459.8 (37.7) | 338.1 (31.3) | −121.7 | −26.50% | 578.8 (37.7) | 439.5 (32.1) | −139.3 | −24.10% |
| 50% Mortality | 368 (292, 652) | 301 (251, 394) | −67 | −18.20% | 487 (411, 771) | 411 (365, 488) | −76 | −15.60% |
| 90% Mortality | 794 (705, —) | 610 (498, —) | −184 | −23.20% | 913 (824, —) | 704 (617, —) | −209 | −22.90% |
| Cox Proportional Hazard Analysis | ||||||||
| Hazard Ratio | p Value (adjusted) | |||||||
| Rapamycin effect in males | 1.72 | .0496 | ||||||
| Females | 1.6972 | .0496 | ||||||
Note: Standard errors around the means are shown in parentheses; 95% confidence intervals are shown for the quantiles.
Table 2.
Effect of Rapamycin and Sex on Survivorship of db/db Mice—Cox Proportional Hazard Analysis
| Effect (Hazard Ratio) | Beta | SE | Z | p value | |
|---|---|---|---|---|---|
| Female vs Male | 0.5873 | −0.5323 | 0.2361 | −2.2547 | .0242 |
| Rapamycin vs Control | 1.72 | 0.5423 | 0.2439 | 2.2235 | .0262 |
| Diet × Sex | 0.9868 | −0.0133 | 0.331 | −0.0403 | .9679 |
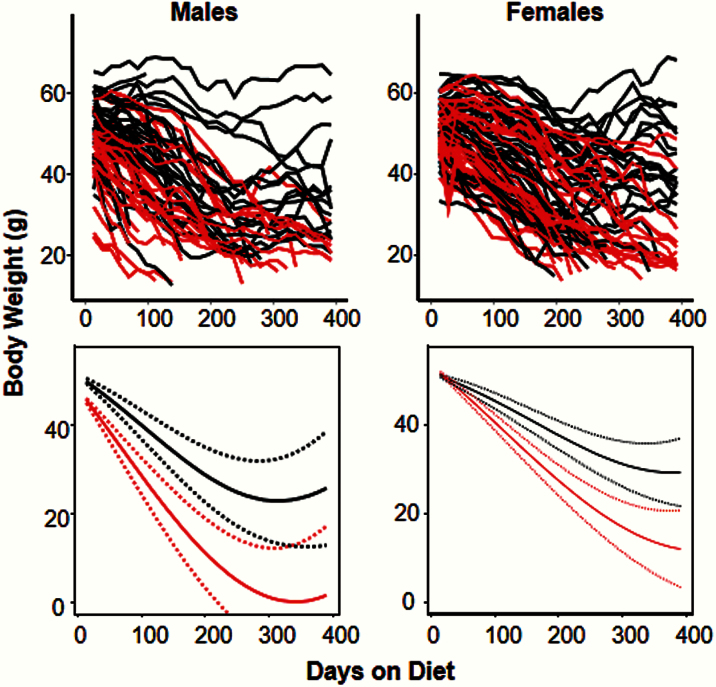
We also followed the body weights of all mice in the survival study over their life span as shown in Figure 2. For the mice fed the rapamycin-containing diet, we observed a significantly greater decrease in body weight compared with the mice consuming the control diet. However, the decrease in body weight became less with age. In female mice, the gap between rapamycin and control mice narrowed with age. The body weight of the rapamycin-treated mice mice lagged behind mice fed the control diet in all cases.
Figure 2.
Body weight of diabetic mice fed control diet or rapamycin-containing diet. The body weights of the male and female db/db mice in Figure 1 were followed over their life spans. The graphs on the top show the raw body weights with a separate line for each mouse maintained on either the control (black) or rapamycin-containing (red) diet. The graphs on the bottom show the fitted body weights with 95% confidence intervals (dotted lines) for the male and female mice fed the two diets. In both genders, the rate of body weight loss for mice fed the rapamycin-containing diet was significantly (p < .0001) greater than that of the mice fed the control diet. In female mice, the gap between the two groups diminished significantly over time but nevertheless persisted throughout the 400-day follow-up period.
Probable Causes of Death
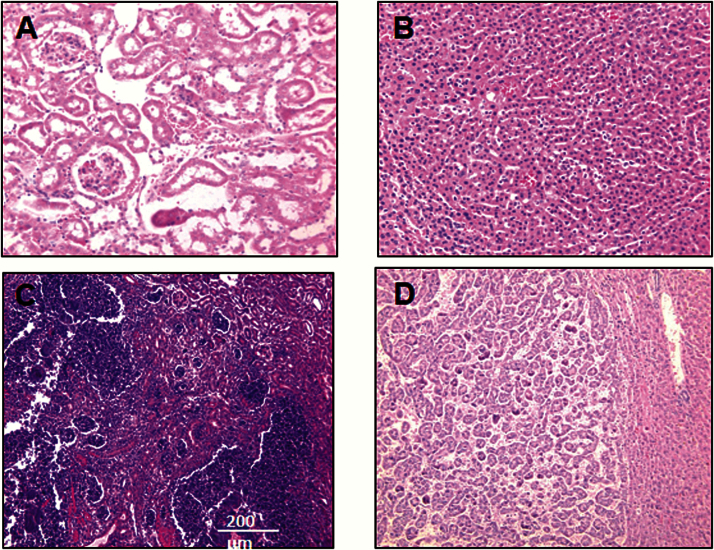
Table 3 shows the probable causes of death in male and female db/db mice fed control or rapamycin diet. Nonneoplastic diseases accounted for the majority of deaths in male and female db/db mice fed the control and rapamycin-containing diet. The major fatal nonneoplastic disease observed in the control diet-fed db/db mice was suppurative inflammation (61% in males and 29% in females), which was observed in the kidney (Figure 3C), urinary tract, peritoneum, liver, lung, and spleen. Both male and female db/db mice fed rapamycin showed a slightly higher incidence of nonneoplastic diseases and a higher incidence of fatal suppurative inflammation compared with db/db mice fed the control diet, although this difference did not reach statistical significance. Approximately 25% of the control diet-fed db/db mice died of neoplastic diseases; hepatocellular carcinoma (Figure 2D) accounted for the majority of cancers. Other presumptively fatal neoplastic diseases observed in the control diet-fed db/db mice were lymphoma and sarcoma. The incidence of fatal neoplasia was reduced in both female (p < .05) and male (p < .001) db/db mice fed the rapamycin-containing diet compared with db/db mice fed the control diet (Table 3).
Table 3.
End-of-life Pathology in Diabetic db/db Mice
| Male | Female | |||
|---|---|---|---|---|
| Control Diet | Rapamycin Diet | Control Diet | Rapamycin Diet | |
| Nonneoplasm | 25 | 35 | 25 | 30 |
| Suppurative inflammation | 22 (61%) | 26 (74%) | 11 (29%) | 18 (51%) |
| Glomerulonephritis | 2 | 0 | 1 | 0 |
| Other | 1 | 9 | 13 | 12 |
| Neoplasm | 9 | 0*** | 9 | 4* |
| Neoplasm and nonneoplasm | 2 | 0 | 4 | 1 |
| Total | 36 | 35 | 38 | 35 |
Note: *p < .05, ***p < .001 compared with control diet.
Figure 3.
Major Pathological Lesions in db/db mice. (A, C) Slides of sections (H.E.) from the kidneys of db/db mice on the control diet: (A) an example of normal kidney tissue (×200) and (C) an example of suppurative inflammation in kidney (×100). (B, D) Slides of sections (×100) from the livers of db/db mice on the control diet: (B) an example of normal liver tissue and (D) an example of hepatocellular carcinoma in liver.
Severity of Pathological Lesions
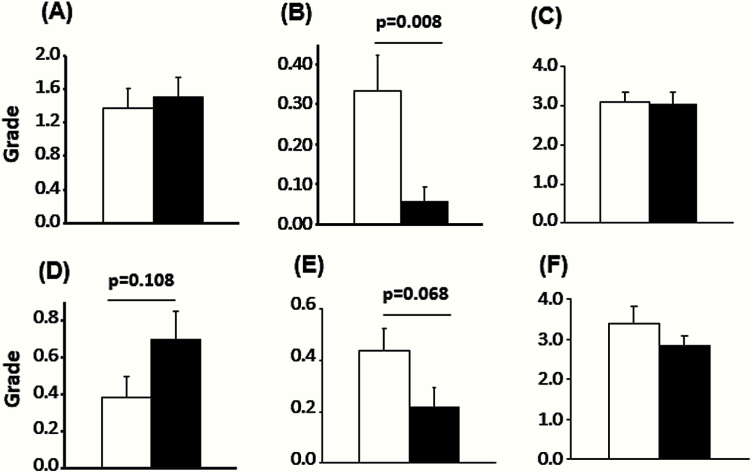
The effect of rapamycin feeding on the severity of suppurative inflammation and pyelonephritis/glomerulonephritis, two of the most common diseases observed in the db/db mice, was examined using a grading system. The severity of suppurative inflammation was similar between rapamycin-fed and control groups. The severity of pyelonephritis/glomerulonephritis was not significantly different for male and female mice fed the two diets even though the female mice fed rapamycin showed more severe renal lesions (Figure 4). The tumor burden, which is defined as the number of different types of neoplastic lesions in a mouse, was also compared because aging mice develop multiple tumors in several organs (39). The tumor burden was significantly lower in rapamycin-fed male and female db/db mice compared with mice fed the control diet (p = .008 and p = .068, respectively, Figure 4). The disease burden, defined as the total number of histopathological changes in a body, has been used as an overall index of pathological status of an animal, for example, disease burden has been shown to increase with age and to be reduced by dietary restriction and in dwarf mice and therefore is a general indication of the overall health status of the animal (29–31). As shown in Figure 3, rapamycin had no significant effect on the disease burden, which may occur because reduction in tumor incidence was countered by a slightly higher incidence of suppurative inflammation.
Figure 4.
Severity of pathological lesions. The severity of renal lesions (A, D), tumor burden (B, E), and disease burden (C, F) of male (A–C) and female (D–F) db/db mice fed a control diet (white bars) or rapamycin-containing diet (black bars) are shown. These data were obtained from the mice listed in Table 3. The severity of the renal lesions was scored using the criteria as follows: Grade 0: no lesions; Grade 1: minimal change in glomeruli (minimal glomerulosclerosis); Grade 2: Grade 1 with a few (less than 10) casts in renal tubules; Grade 3: Grade 1 with more than 10 casts in renal tubules; and Grade 4: Grade 3 with interstitial fibrosis. Tumor burden was calculated as the average number of different types of tumors in the group. The disease burden is the average of total number of pathological changes of any type found in individual mice from each group. The data were analyzed using ANOVA, and tumor burden was shown to be significantly different in the mice fed the control diet and the rapamycin-containing diet.
Discussion
Our study clearly shows that rapamycin reduces the life span of male and female db/db mice. The db/db mice we studied have been used extensively to study type 2 diabetes; they are hyperphagic and develop significant obesity, fasting hyperglycemia, and hyperinsulinemia by 6 weeks of age (40). The course of the disease is markedly influenced by genetic background with the most severe diabetic phenotype shown on the C57BL/KsJ background, for example, an uncontrolled rise in blood sugar, severe depletion of the insulin-producing beta cells of the pancreatic islets, and early deaths (41). To our knowledge, our study is the first to characterize the life span (mean, medium, and maximum) and end-of-life pathology of C57BL/KsJ-lepr db/db mice. As can be seen from the survival curves in Figure 1, the db/db mice die continuously after 4 months of age, resulting in a mean life span of approximately 14 and 19 months for male and female mice, respectively, with the female mice living significantly longer than the male mice. The life span of the db/db mice is much shorter than that of male and female C57BL/6 mice, which both have a mean life span of ~30 months in our facilities; few C57BL/6 die before 20 months of age (12,42).
The major end-of-life pathological lesion observed in both male and female C57BL/KsJ-lepr db/db mice was suppurative inflammation, which are acute inflammatory changes in various tissues characterized by massive leukocyte infiltration. The leptin receptor mutation may be a contributing factor for increased occurrence of suppurative lesions because it has been implicated in a delayed immune response (43). In C57BL/6 mice, suppurative inflammation is relatively rare under specific pathogen-free conditions with the major cause of death due to neoplasia (11,30,42). In db/db mice, the incidence of neoplasia was relatively low, most likely because of the shortened life span. Interestingly, the predominant form of cancer in the db/db mice was hepatocellular carcinoma rather than lymphoma, which is common in other mouse strains (11,30,42), suggesting that diabetes may alter the tumor phenotype. We observed a similar profile of tumors in Sod1 -/- mice (44); therefore, it is possible that diabetes-induced oxidative stress predisposes the db/db mice to develop hepatocellular carcinoma rather than lymphoma. It is also important to note that the tumors occur earlier in the db/db mice compared with C57BL6 mice.
Our observation that rapamycin reduces the life span of male and female db/db mice was unexpected because 14 ppm rapamycin, the dose of rapamycin used in this study, had been shown to increase the life span of both genetically heterozygous [UM-HET3 (6,7,10)] and an inbred [C57BL/6 (9,12)] strains of mice. In fact, the study by our group showing that rapamycin increased the life span of male and female C57BL/6 mice from 10% to 16% (12) was conducted at the same time and in the same facilities as this study. Therefore, differences in husbandry cannot account for the different effects of rapamycin on these two mouse models. In addition, the C57BL/6 and db/db mice were given the same level of rapamycin (14 ppm) starting at the same age (4 months). However, blood levels of rapamycin achieved by 14 ppm in the db/db mice were lower (~ 2ng/ml) than we observed in the C57BL/6 mice (3 to 5ng/ml) (12). Thus, the reduced life span of the db/db mice cannot be attributed to increased circulating levels of rapamycin. Rather, relatively low circulating levels of rapamycin are toxic to db/db mice. It should be noted that rapamycin levels as high as 42 ppm have been shown to increase the life span of UM-HET3 mice (10).
One of the questions our study raises is why is rapamycin toxic for C57BL/KsJ-lepr db/db mice whereas it increases the life span of 12 different genotypes of mice. Dietary restriction, the manipulation most widely shown to increase life span and delay aging, has been shown to shorten the life of some genotypes (45). Therefore, it is possible that the toxicity of rapamycin is due to the genetic background of the mice, that is, the C57BL/KsJ background. While BKS was originally presumed to be a substrain of C57BL/6J, Davis and colleagues (46) showed that approximately 70% of the C57BL/KsJ mouse genome is derived from C57BL/6, with approximately 20% from DBA and another 9% from an unidentified donor, which appears to be a less common inbred strain or an outbred or wild strain. While we cannot rule out that rapamycin is toxic to the C57BL/KsJ strain, we feel it is highly unlikely because C57BL/6 and DBA make up most of the background of this strain, and mice containing these backgrounds have been shown to live longer when fed rapamycin.
We believe that the toxic effect of rapamycin on the C57BL/KsJ-lepr db/db mice is most likely due to the leptin receptor mutation, which leads to obesity, diabetes, and a variety of other pathologies (41), and as we have shown, a dramatically shortened life span. For example, we found that rapamycin increased the major cause of death in the db/db mice. Although not statistically significant, the incidence of suppurative inflammation was increased from 20% to 75% in the male and female db/db mice, respectively. Rapamycin has been shown to be proinflammatory when given to mice with insulin resistance and obesity (47). Whether a similar effect is exerted by rapamycin in diabetic db/db mice needs to be directly studied. In addition, because the leptin receptor mutation has been implicated in delayed immune response (43) and because rapamycin was initially used in a cocktail with other compounds to prevent rejection in transplant patients, it is possible that rapamycin may further enhance the susceptibility of db/db mice to infections. Although rapamycin suppresses some immune functions, others are enhanced (19,48), that is, rapamycin is not globally immunosuppressive. In fact, a recent study in humans showed that elderly patients given a derivative of rapamycin (RAD001) actually showed increased response to influenza vaccine (49). On the other hand, we observed that rapamycin significantly reduced both the incidence and severity of neoplasia in the male and female db/db mice, which is consistent with numerous studies showing an anticancer phenotype for mice fed rapamycin, for example, reduced incidence of tumors (6,9,11,50) and increased life span of tumor-prone mice (13,14,16,17,19,20).
The toxicity of rapamycin in the db/db mice was not totally unexpected because chronic rapamycin treatment has been shown to result in insulin resistance in mice (10,26) and rats (51). In a recent study, Liu and colleagues (52) compared the effect of rapamycin on insulin sensitivity in UM-HET3 and C57BL/6 mice fed either a low- or high-fat diet. Chronic rapamycin treatment resulted in insulin resistance in mice fed both diets; no significant interaction between rapamycin and diet was observed suggesting that rapamycin promoted metabolic dysfunction equally in mice on both low- and high-fat diets. While a high-fat diet significantly increased fasting blood levels of glucose and insulin, rapamycin did not significantly alter blood glucose or insulin levels on either the low- or high-fat diet. Thus, in “normal” wild type mice, the increase in insulin resistance by rapamycin does not lead to a diabetic phenotype, that is, increased blood levels of glucose and insulin. However, it is possible that rapamycin exacerbates the diabetic phenotype in the db/db mice. We recently showed that rapamycin increased fasting blood glucose in female but not in male db/db mice (53). Interestingly, resveratrol, which has also been touted as an anti-aging therapy, increases the life span and improves insulin sensitivity of obese C57BL/6 fed a high-fat diet (54); however, resveratrol has no effect on the life span of UM-HET3 or C57BL/6 mice (7,55).
In summary, this is the first study to show that life-long rapamycin treatment is harmful to a strain of mice. Currently, 15 studies have shown that rapamycin increases the life span of a wide variety of mouse models (6–20), suggesting that rapamycin has an anti-aging phenotype and would delay/prevent a variety of age-related diseases. Indeed, since showing that rapamycin increased the life span of mice, rapamycin has been shown to have a broad anticancer effect in a wide range of mouse models (13,14,16,17,19,20), improve cognition and prevent neurodegeneration in various mouse models [for a review see reference (56)], and improve cardiac function in aging mice (57). Because of these data in mice and because rapamycin is FDA approved, rapamycin has become one of the prime anti-aging candidates to take to humans. Although the current literature in mice show many potential benefits of rapamycin, our study points to potential side effects which might occur in obese/diabetic individuals. Therefore, it is important that the effect of rapamycin be studied in other animal models of diabetes, which more closely mimic diabetes observed in humans, for example, nonhuman primates. Also, because of the high incidence of obesity and diabetes in the United States, it is important that current and future clinical trials closely follow the effect of rapamycin or its rapalogues on metabolic syndrome in obese and diabetic individuals.
Funding
This work was supported in whole or in part by grants from the National Institutes of Health [RC2AG036613 (to B.S.K., A.R., Y.I.), DK077295 (B.S.K.), DK050190 (G.G.C)]; Merit Grants from the Veterans Administration (B.S.K, G.G.C., J.L.B, A.R.); the Juvenile Diabetes Research Foundation (D.F., G.G.C.), and the Department of Medicine Chairman’s Fund (B.S.K.).
Acknowledgments
We thank Dr. H. E. Abboud for his helpful comments on the manuscript; he unexpectedly passed away in January, 2015. G.G.C. and A.R. are recipients of Senior Research Career Scientist Award from the Veterans Administration. An abstract containing some of the data was presented at the annual meeting of the American Society of Nephrology, 2013.
References
- 1. Cafferkey R, McLaughlin MM, Young PR, Johnson RK, Livi GP. Yeast TOR (DRR) proteins: amino-acid sequence alignment and identification of structural motifs. Gene. 1994;141:133–136. [DOI] [PubMed] [Google Scholar]
- 2. Brown EJ, Albers MW, Shin TB, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. [DOI] [PubMed] [Google Scholar]
- 3. Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. [DOI] [PubMed] [Google Scholar]
- 4. Caron E, Ghosh S, Matsuoka Y, et al. A comprehensive map of the mTOR signaling network. Mol Syst Biol. 2010;6:453. doi:10.1038/msb.2010.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. [DOI] [PubMed] [Google Scholar]
- 6. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi:10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi:10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anisimov VN, Zabezhinski MA, Popovich IG, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi:10.4161/cc.10.24.18486 [DOI] [PubMed] [Google Scholar]
- 9. Neff F, Flores-Dominguez D, Ryan DP, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi:10.1172/JCI67674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller RA, Harrison DE, Astle CM, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi:10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Bokov A, Gelfond J, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69:119–130. doi:10.1093/gerona/glt056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fok WC, Chen Y, Bokov A, et al. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS ONE. 2014;9:e83988. doi:10.1371/journal.pone.0083988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci U S A. 2008;105:13544–13549. doi:10.1073/pnas.0800041105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anisimov VN, Zabezhinski MA, Popovich IG, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176:2092–2097. doi:10.2353/ajpath.2010.091050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramos FJ, Chen SC, Garelick MG, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. doi:10.1126/scitranslmed.3003802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Komarova EA, Antoch MP, Novototskaya LR, et al. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/- mice. Aging. 2012;4:709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Livi CB, Hardman RL, Christy BA, et al. Rapamycin extends life span of Rb1+/- mice by inhibiting neuroendocrine tumors. Aging. 2013;5:100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi:10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hasty P, Livi CB, Dodds SG, et al. eRapa restores a normal life span in a FAP mouse model. Cancer Prev Res (Phila). 2014;7:169–178. doi:10.1158/1940-6207.CAPR-13-0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Comas M, Toshkov I, Kuropatwinski KK, et al. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53-/- mice by delaying carcinogenesis. Aging. 2012;4:715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bhattacharya A, Bokov A, Muller FL, et al. Dietary restriction but not rapamycin extends disease onset and survival of the H46R/H48Q mouse model of ALS. Neurobiol Aging. 2012;33:1829–1832. doi:10.1016/j. neurobiolaging.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 22. Zhang X, Li L, Chen S, et al. Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Autophagy. 2011;7:412–425. [DOI] [PubMed] [Google Scholar]
- 23. Caspersen CJ, Thomas GD, Boseman LA, Beckles GL, Albright AL. Aging, diabetes, and the public health system in the United States. Am J Public Health. 2012;102:1482–1497. doi:10.2105/AJPH.2011.300616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sataranatarajan K, Mariappan MM, Lee MJ, et al. Regulation of elongation phase of mRNA translation in diabetic nephropathy: amelioration by rapamycin. Am J Pathol. 2007;171:1733–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deblon N, Bourgoin L, Veyrat-Durebex C, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012;165:2325–2340. doi:10.1111/j.1476-5381.2011.01716.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi:10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gyurus E, Kaposztas Z, Kahan BD. Sirolimus therapy predisposes to new-onset diabetes mellitus after renal transplantation: a long-term analysis of various treatment regimens. Transplant Proc. 2011;43:1583–1592. doi:10.1016/j.transproceed.2011.05.001 [DOI] [PubMed] [Google Scholar]
- 28. Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol. 2003;284:F1138–F1144. [DOI] [PubMed] [Google Scholar]
- 29. Ikeno Y, Bronson RT, Hubbard GB, Lee S, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci. 2003;58:291–296. [DOI] [PubMed] [Google Scholar]
- 30. Ikeno Y, Hubbard GB, Lee S, et al. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517. [DOI] [PubMed] [Google Scholar]
- 31. Ikeno Y, Hubbard GB, Lee S, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529. doi:10.1093/gerona/glp017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cox DR. Regression models and life-tables. J R Stat Soc Ser B. 1972;34:187–220. [Google Scholar]
- 33. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 34. Bates D, Chambers JM, Dalgaard P, et al. A Language and Environment for Statistical Computing, v2.15.3. Vienna, Austria: R Core Team; 2013. http://www.R-project.org/. [Google Scholar]
- 35. Therneau TM. A Package for Survival Analysis in S v2.37-2. Fochester, MN: Mayo Clinic; 2012. http://CRAN.R-project.org/package=survival. [Google Scholar]
- 36. Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. Berlin: Springer-Verlag; 2000. [Google Scholar]
- 37. Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi:10.1002/bimj.200810425 [DOI] [PubMed] [Google Scholar]
- 38. Pinheiro JD, Bates DM. Mixed-effects Models in S and S-LUS. Berlin: Springer-Verlag; 2000. [Google Scholar]
- 39. Ladiges W, Ikeno Y, Liggitt D, Treuting PM. Pathology is a critical aspect of preclinical aging studies. Pathobiol Aging Age Relat Dis. 2013;3. doi:10.3402/pba.v3i0.22451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kobayashi K, Forte TM, Taniguchi S, Ishida BY, Oka K, Chan L. The db/db mouse, a model for diabetic dyslipidemia: molecular characterization and effects of Western diet feeding. Metabolism. 2000;49:22–31. [DOI] [PubMed] [Google Scholar]
- 41. Garris DR, Garris BL. Cytochemical analysis of pancreatic islet hypercytolipidemia following diabetes (db/db) and obese (ob/ob) mutation expression: influence of genomic background. Pathobiology. 2004;71:231–240. [DOI] [PubMed] [Google Scholar]
- 42. Bokov AF, Garg N, Ikeno Y, et al. Does reduced IGF-1R signaling in Igf1r+/- mice alter aging? PLoS One. 2011;6:e26891. doi:10.1371/ journal.pone.0026891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mandel MA, Mahmoud AA. Impairment of cell-mediated immunity in mutation diabetic mice (db/db). J Immunol. 1978;120:1375–1377. [PubMed] [Google Scholar]
- 44. Zhang Y, Ikeno Y, Bokov A, et al. Dietary restriction attenuates the accelerated aging phenotype of Sod1(-/-) mice. Free Radic Biol Med. 2013;60:300–306. doi:10.1016/j.freeradbiomed.2013.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi:10.1111/j.1474-9726.2009.00533.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davis RC, Schadt EE, Cervino AC, Péterfy M, Lusis AJ. Ultrafine mapping of SNPs from mouse strains C57BL/6J, DBA/2J, and C57BLKS/J for loci contributing to diabetes and atherosclerosis susceptibility. Diabetes. 2005;54:1191–1199. [DOI] [PubMed] [Google Scholar]
- 47. Umemura A, Park EJ, Taniguchi K, et al. Liver damage, inflammation, and enhanced tumorigenesis after persistent mTORC1 inhibition. Cell Metab. 2014;20:133–144. doi:10.1016/j.cmet.2014.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hinojosa CA, Mgbemena V, Van Roekel S, et al. Enteric-delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Exp Gerontol. 2012;47:958–965. doi:10.1016/j.exger.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mannick JB, Del Giudice G, Lattanzi M, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179. doi:10.1126/scitranslmed.3009892 [DOI] [PubMed] [Google Scholar]
- 50. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi:10.1111/j.1474-9726.2012.00832.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Houde VP, Brule S, Festuccia WT, et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi:10.2337/db09-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Y, Diaz V, Fernandez E, et al. Rapamyin-induced metabolic defects are transient in both lean and obese mice. Aging. 2014;6:742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tabatabai-Mir H, Sataranatarajan K, Lee HJ, et al. Rapamycin selectively alters serum chemistry in diabetic mice. Pathobiol Aging Age Relat Dis. 2012;2. doi:10.3402/pba.v2i0.15896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi:10.1016/j.cmet.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Richardson A, Galvan V, Lin AL, Oddo S. How longevity research can lead to therapies for Alzheimer’s disease: The rapamycin story. Exp Gerontol. 2015; 68:164–176. doi:10.1016/j.cmet.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Flynn JM, O’Leary MN, Zambataro CA, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell. 2013;12:851–862. doi:10.1111/acel.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]