Abstract
Background:
Emerging evidence suggests that mildly down-regulated thyroid function in older persons may protect and/or reflect maintained health.
Methods:
Using observational data collected between January 2006 and March 2014 on a volunteer sample of 602 men and women aged 68–97 years with normal thyroid function participating in the Baltimore Longitudinal Study of Aging, this study examines the concurrent relationship between reported walking ability, usual and rapid gait speed, endurance walk performance, fatigability, and reported energy level with respect to free thyroxine (FT4) within the normal range (0.76–1.50ng/dL) as a continuous variable and categorized as low (lower quartile), medium (interquartile), or high (upper quartile).
Results:
Adjusting for sex, age, race, height, weight, exercise and smoking, reported walking ability, usual and rapid gait speed, 400-m time, fatigability, and reported energy level were less favorable with increasing FT4 (p = .013 to <.001). In sex-strata, similar associations were observed except for walking ability in men and energy level in women. Categorical analyses revealed that persons with low FT4 exhibited better functional mobility, fitness, and reported energy than persons with intermediate or high levels (p < .05 for all). Persons with high-normal versus medium FT4 had slower usual and rapid gait speed (p < .05) only.
Conclusion:
Older adults with low-normal FT4 exhibit better mobility, fitness, and fatigue profiles. Mildly down-regulated thyroid function appears to align with better function in old age and may serve as a biomarker of healthy longevity.
Keywords: Free thyroxine, Physical function, Aging, Fitness, Fatigue
Thyroid stimulating hormone (TSH) levels gradually increase beginning around age 70 at both the population (1) and individual level while free thyroxine (FT4) levels remain relatively stable (2). The clinical meaning and significance of this phenomenon remain under investigation (3–5), but emerging evidence suggests that mildly down-regulated thyroid function may protect and/or indicate maintained health in old age. Older persons with TSH levels between 4.5 and 10.0 mU/L have been found to demonstrate better physical function and lower mortality relative to those with TSH levels in the euthyroid range (6,7). Although this protective association has not been consistently observed (8–10), TSH may be a less valid indicator of thyroid function at older ages as the typical inverse log-linear relationship between TSH and FT4, a more direct indicator of thyroid hormone status at the tissue level, appears to be more complex with increasing age especially at the extremes of the normal distribution of FT4 (11–13).
In turn, studies of FT4 have been limited to persons in their mid-80s or men in their 70s and older and have largely focused on mortality (4,5,7–9). Three studies evaluated associations with dementia or cognitive decline in men and women (5,10,14), one single site and one meta-analysis examined respectively incident atrial fibrillation and heart failure (5) and coronary heart disease events and mortality (15) and only one considered physical performance and grip strength which was restricted to men (8). All these studies found high-normal FT4 to be a risk factor for poor outcomes, providing at best indirect support for low-normal FT4 as potentially protective or reflective of good health and functioning.
This study extends previous work in examining FT4 levels in both men and women encompassing a 30-year age range from 68 to 97 years and the association with functional mobility, fitness, and fatigue. These specific outcomes reflect overall health status (16–19) and figure prominently in the lives of older adults. The analyses consider the relationship over the full normal range of FT4 as well as categories that distinguish low and high-normal levels to better clarify protective versus risk associations. For comparison, associations with TSH levels are also examined.
Methods
Study Population
The study population consists of participants in the Baltimore Longitudinal Study of Aging (BLSA) who met the following criteria at one or more clinic visits: age from 68 to 97 years, TSH between 0.40 and 10.0 mU/L, and FT4 between 0.076 and 1.50ng/dL, not taking thyroid medications and valid assessment of usual gait speed. Because participants could meet eligibility criteria at multiple visits, the analytic sample was selected as follows. First, all participant–visit combinations in which the participant met above eligibility criteria with the exception of thyroid medication use were identified yielding 1,692 observations involving 703 participants (381 men and 322 women). Next, all participant–visit combinations involving thyroid medication use were excluded leaving 1,456 observations and 623 participants (347 men and 276 women). Lastly, to maximize the number of participants with data on newly introduced outcome measures, the most recent visit with available data for all covariates was selected to yield a sample of 602 (335 men and 267 women).
The BLSA was initiated in 1958 as a continuous enrollment cohort study of normative aging with eligibility at enrollment restricted to persons free of cognitive impairment, functional limitations, chronic diseases, and cancer within the past 10 years. Once enrolled, participants are followed until death through regularly scheduled comprehensive health, cognitive, and functional evaluations conducted during a 3-day visit to the National Institute on Aging Clinical Research Unit in Baltimore, Maryland. Visits occur biannually for persons aged 60–79 years and annually for persons aged 80 and older. Participants in the current study were seen between January 2006 and March 2014 and all provided informed consent. The current BLSA protocol was approved by the National Institute of Environmental Health Sciences Internal Review Board.
Measures
Thyroid function
On the morning of the first clinic visit day after an overnight fast, participants undergo venipuncture. The sample analyzed for serum TSH and FT4 levels is processed the same day at the MedStar Harbor Hospital clinical laboratory by immunoassay (Vista Chemiluminescence, Siemens Tarrytown, NY). Because the reference ranges varied slightly over the 8-year data collection period, we defined normal as the middle 97.5% of the distribution for BLSA participants with no history of thyroid disease or taking any type of thyroid medication. This approach yielded a normal range of FT4 of 0.76–1.50ng/dL which is similar to the current reference range of 0.76–1.46ng/dL. For analysis, FT4 was examined as a continuous and categorical variable with three levels corresponding to the sex-specific lower quartile, interquartile, and upper quartile ranges. Persons with a TSH outside of the current reference range encompassing normal to mild subclinical hypothyroidism (0.40–10.0 mU/L) were excluded.
Functional mobility
Perceived walking ability was determined from responses to a series of questions beginning with, “Because of a health or physical problem, do you have any difficulty walking a quarter of a mile that is about 2 or 3 blocks, without stopping?” Those reporting difficulty were asked whether they had a little, some or a lot of difficulty or were unable to walk. Persons denying difficulty were asked how easy it is for them to walk a quarter of a mile—very, somewhat, or not so easy—followed by whether they have any difficulty in walking one mile and the ease of walking one mile if no difficulty was reported. Responses were combined to create a walking ability index ranging from 0 to 9, where 0 represents unable to walk ¼ mile and 9 indicates walking one mile is very easy (6,18).
Usual and rapid gait speeds were assessed over 6 m with participants asked to walk at their “usual walking pace” for two trials and then “as fast as [they] can” for two trials. Total time recorded to the hundredth of a second was divided into 6 to obtain respectively usual and rapid gait speed in meters per second. The fastest of each trial was used in the analyses.
Fitness
Time to walk 400 m as quickly as possible, a measure of cardiorespiratory fitness in older adults (19), was determined from the long distance corridor walk, a two-stage, endurance walk test performed over a 20-m course (19). The first stage consists of a 2.5-minute usual pace walk followed immediately by a 400-m walk “done as quickly as possible.” The long distance corridor walk was implemented in the BLSA in April 2007. Of the 314 men and 254 women in the analytic sample seen since April 2007, 6 men and 5 women were not tested for administrative reasons, 5 men and 8 women met test exclusion criteria (eg, severe electrocardiogram abnormalities, systolic or diastolic blood pressure exceeding 199 mmHg or 109 mmHg, respectively or reporting a myocardial infarction, angioplasty, or heart surgery in the prior 3 months or experiencing new or worsening symptoms of chest pain, shortness of breath, or angina), and 22 men and 26 women could not complete the full 400 m due to calf or joint pain, shortness of breath or excessive fatigue leaving 281 men and 215 women with valid 400-m walk times.
Fatigability and fatigue
Fatigability connotes the degree of fatigue experienced following performance of a standardized activity (17). In the BLSA, the standardized activity comprises a slow paced 5-minute walk (1.5 mph; 0.67 m/s) performed on a treadmill at zero percent grade with fatigability assessed using the Borg Rating of Perceived Exertion which ranges from 6 to 20 (20). This fatigability performance test was implemented in July 2007 and was recently validated (21). Of the 305 men and 244 women in the analytic sample seen since July 2007, 8 men and 12 women were ineligible (eg, needed a walking aid), 5 men and 5 women were not tested for technical reasons (eg, treadmill malfunction), and 2 men and 3 women refused, yielding an analytic sample of 290 men and 224 women.
Fatigue or perceived energy level was evaluated using two different self-report questions. The first asks participants to describe their usual energy level in the past month on an 11-point scale where zero represents no energy at all and 10 the most energy ever had. The second comes from the Short Form-12 (22), and asks participants to report how much of the time they had “a lot of energy” in the past 4 weeks: all (1), most (2), a good bit (3), some (4) a little (5), or none (6). A low score represents higher energy or lower fatigue.
Covariates
Covariates encompass socio-demographic factors including age, self-designated black or non-black race, and behavioral factors known to affect functional mobility and fatigue in late life including physical activity and smoking status (current and persons who quit within 10 years vs never and former smokers who quit more than 10 years before their clinic visit). Physical activity level was categorized as sedentary, low, moderate, or high based on reported frequency and duration of vigorous and moderate physical activity including brisk walking. For walking-based performance measures, whether a walking aid, typically a cane, was used were accounted for as well. Even though walking aids were not permitted for the fatigability test, walking aid use was included to account for possible gripping of handrails. Measured height and weight were also accounted for in the analyses.
Statistical Analyses
The relationship between FT4 and functional mobility and fatigability and fatigue was examined using multiple linear regression models treating FT4 as a continuous variable and general linear models when FT4 level was categorized as low, medium, and high based on sex-specific quartiles with the interquartile range defining the medium group. The cutpoints were (0.76–0.89, 0.90–1.09, and 1.10–1.50) for men and (0.76–0.90, 0.91–1.07, and 1.08–1.50) for women. Analyses were performed in the overall sample and in sex-strata to facilitate comparison with other studies. All models were adjusted for age, race, reported activity level, smoking status, weight and height, and walking aid use for walking-based measures only and sex in non-sex-stratified analyses. TSH was examined in a similar fashion except the highest category in the categorical analysis was defined using the reference range for mild subclinical hypothyroidism (4.50–9.99 mU/L) and the lowest was defined as 0.40–2.49 mU/L.
Results
The 602 participants consisted of 335 men with a mean age of 78.6 years and 267 women with a mean age of 77.4 who were generally healthy. As shown in Table 1, two thirds of participants reported very good to excellent self-rated health, less than 24% had a body mass index consistent with obesity, 40% and 27% of men and women respectively engaged in at least 150 minutes per week of moderate to vigorous physical activity and less than 5% of either men or women reported fair to poor health, used a walking aid, or had smoked within the past 10 years. Mean TSH and FT4 levels were 2.76 and 2.69 mU/L and 1.02 and 1.01ng/dL respectively for men and women, with 11.3% and 10.1% meeting criteria for subclinical hypothyroidism. Distributions of mobility and fatigue assessments reflect a generally robust study population.
Table 1.
Baseline Characteristics by Sex in the BLSA Analytic Sample
| Characteristic | Men | Women |
|---|---|---|
| N = 602, No. | 335 | 267 |
| Age, mean (SD), y | 78.6 (6.6) | 77.4 (6.7) |
| Black, % | 17.0 | 31.1 |
| Health status and behaviors | ||
| Current or recent smoker, % | 2.1 | 4.1 |
| Uses a walking aid, % | 3.0 | 3.4 |
| Fair to poor self-rated health, % | 4.2 | 3.4 |
| Very good to excellent self-rated health, % | 66.9 | 65.9 |
| Obese (BMI ≥30kg/m2), % | 23.9 | 23.6 |
| Sedentary (active < 30min/wk), % | 37.6 | 47.9 |
| Physically active at least 150min/wk, % | 40.3 | 27.3 |
| Thyroid hormone status | ||
| Thyroid stimulating hormone, mean (SD), mU/L | 2.76 (1.52) | 2.69 (1.44) |
| Subclinical hypothyroidism (TSH = 4.50–9.99 mU/L), % | 11.3 | 10.1 |
| Free thyroxine, mean (SD), ng/Dl | 1.02 (0.14) | 1.01 (0.13) |
| Mobility and fatigue | ||
| Walking ability index score, mean (SD), points | 7.66 (2.13) | 7.40 (2.40) |
| Usual gait speed, mean (SD), m/s | 1.09 (0.25) | 1.06 (0.24) |
| Rapid gait speed, mean (SD), m/s | 1.66 (0.41) | 1.52 (0.36) |
| 400-m time*, mean (SD), s | 293 (70.9) | 312 (81.7) |
| Fatigability score†, mean (SD), points | 9.14 (2.33) | 9.17 (2.14) |
| Usual energy level past month, mean (SD), points | 7.44 (1.61) | 7.42 (1.61) |
| Frequency a lot of energy past 4 weeks, mean (SD) | 2.60 (1.05) | 2.61 (1.05) |
Notes: BLSA = Baltimore Longitudinal Study of Aging; BMI = body mass index.
*Persons able to complete the full 400-m walk only (281 men and 215 women).
†Subsample administered the fatigability test which was implemented later in the study (290 men and 224 women).
In analyses adjusted for sex, age, race, height, weight, exercise, and smoking displayed in Table 2, increasing FT4 examined as a continuous variable in increments of 0.1ng/dL was associated with worse reported walking ability, slower usual and rapid gait speed, and poorer fitness as reflected by more time needed to walk 400 m (p = .013 to <.001). Fatigability and reported energy level using either measure were also less favorable with increasing FT4 (p ≤ .004 for all). In sex-stratified analyses, the same associations were observed and statistically significant in men with the exception of reported walking ability and in women with the exception of reported energy level and 400-m time which was borderline (p = .061).
Table 2.
Cross-sectional Associations* Between FT4 and TSH and Functional Mobility, Fitness, and Fatigue
| Measures | Overall | Men | Women | |||
|---|---|---|---|---|---|---|
| No. | 602 | 335 | 267 | |||
| β (SE) p-Value | β (SE) p-Value | β (SE) p-Value | ||||
| FT4 (units = 0.1ng/dL) | ||||||
| Walking ability index score | −.214 (.066) | .001 | −.141 (.082) | .087 | −.331 (.110) | .003 |
| Usual gait speed | −.025 (.006) | <.001 | −.029 (.008) | <.001 | −.022 (.009) | .019 |
| Rapid gait speed | −.040 (.010) | <.001 | −.033 (.013) | .015 | −.046 (.014) | .001 |
| 400-m time† | 4.93 (1.97) | .013 | 5.89 (2.53) | .021 | 5.66 (3.05) | .061 |
| Fatigability rating‡ | .243 (.073) | <.001 | .226 (.098) | .022 | .278 (.111) | .013 |
| Energy level past month | −.147 (.051) | .004 | −.160 (.066) | .016 | −.134 (.081) | .099 |
| Frequency a lot of energy past 4 weeks | .100 (.033) | .002 | .134 (.042) | .002 | .047 (.053) | .375 |
| TSH (units = 1.0 mU/L) | ||||||
| Walking ability index score | .044 (.057) | .441 | −.001 (.071) | .999 | .143 (.010) | .135 |
| Usual gait speed | −.002 (.005) | .752 | −.007 (.007) | .315 | .007 (.008) | .372 |
| Rapid gait speed | .004 (.008) | .636 | −.006 (.012) | .616 | .017 (.012) | .171 |
| 400-m time† | 0.67 (1.67) | .689 | 2.25 (2.27) | .154 | −4.17 (2.38) | .082 |
| Fatigability rating‡ | −.009 (.061) | .884 | −.044 (.085) | .609 | .007 (.090) | .937 |
| Energy level past month | .078 (.044) | .077 | .088 (.058) | .128 | .080 (.070) | .254 |
| Frequency a lot of energy past 4 weeks | −.035 (.029) | .228 | −.044 (.037) | .244 | −.021 (.045) | .649 |
Notes: FT4 = free thyroxine; TSH = thyroid stimulating hormone.
*Adjusted for age, race, exercise level, smoking status, weight in kilograms, and height in centimeters for all and walking aid use for usual and rapid gait speed, 400-m time and fatigability only.
†Persons able to complete the full 400-m walk only (281 men and 215 women).
‡Subsample administered the fatigability test which was implemented later in the study (290 men and 224 women).
TSH and FT4 showed a significant inverse correlation overall (r = −0.18; p < .001) and in men and women separately (r = −0.17; p = .001; r = −0.19; p = .002). In models examining TSH as a continuous variable, no associations were observed with any outcome overall or in men and women separately.
Categorical analyses in Table 3 reveal in the overall sample, persons with low FT4 had better reported walking ability, faster usual and rapid gait speed, higher fitness level, and greater frequency of reporting a lot of energy in the past 4 weeks than persons with intermediate FT4 levels. Persons in the low FT4 group had more favorable values on all parameters than persons in the high FT4 group. Those with high-normal FT4 showed worse functioning than persons with intermediate FT4 for usual and rapid gait speed only. Sex-specific findings were less consistent. Both men and women with low FT4 had faster rapid gait speed than those with medium FT4. In men only, those with low FT4 had better reported walking ability and more favorable reported energy levels than the medium group; whereas, in women only fitness (time needed to walk 400 m) was superior in the low versus medium FT4 group. Comparing low versus high FT4, both men and women had better reported walking ability and faster usual and rapid gait speed. In men only, reported energy level (both questions) and in women, only fitness and fatigability were superior in the low versus high groups. Lastly, the high versus medium FT4 group exhibited slower usual gait speed in men and poorer reported walking ability and energy level in the past month solely in women.
Table 3.
Mean Functional Mobility, Fitness, and Fatigue Parameter Values* by FT4 and TSH Category† Overall and Within Sex-Strata
| Measures | FT4 Level† | ||
|---|---|---|---|
| Low (Q1) | Medium (Q2–Q3) | High (Q4) | |
| Overall | |||
| N = 602, No. | 141 | 295 | 166 |
| Walking ability index score‡, points | 8.06§,‖ | 7.51 | 7.17 |
| Usual gait speed‡, m/s | 1.12§,¶ | 1.08 | 1.03‖ |
| Rapid gait speed‡, m/s | 1.70§,** | 1.60 | 1.52‖ |
| 400-m time††,‡‡, s | 290¶,§§ | 304 | 309 |
| Fatigability rating‡‡,‖‖, points | 8.90¶¶ | 9.12 | 9.50 |
| Energy level past month‡‡, points | 7.70§§ | 7.43 | 7.21 |
| Frequency a lot of energy past 4 weeks*** | 2.39§,¶ | 2.61 | 2.78 |
| Men | |||
| N = 335, No. | 76 | 165 | 94 |
| Walking ability index score, points | 8.12¶,¶¶ | 7.56 | 7.47 |
| Usual gait speed***, m/s | 1.14§ | 1.09 | 1.03¶ |
| Rapid gait speed***, m/s | 1.75§,¶ | 1.66 | 1.58 |
| 400-m time††,s | 283 | 295 | 300 |
| Fatigability rating‖‖, points | 9.00 | 9.06 | 9.46 |
| Energy level past month***, points | 7.93‖,§§ | 7.31 | 7.28 |
| Frequency a lot of energy past 4 weeks*** | 2.27§,‖ | 2.64 | 2.79 |
| Women | |||
| N = 267, No. | 65 | 130 | 72 |
| Walking ability index score***, points | 7.98§§ | 7.46 | 6.79¶ |
| Usual gait speed‡‡, m/s | 1.11§§ | 1.07 | 1.02 |
| Rapid gait speed‡, m/s | 1.63§,‖ | 1.52 | 1.45 |
| 400-m time††,***, s | 297¶,§§ | 314 | 327 |
| Fatigability rating‖‖, points | 8.79¶¶ | 9.20 | 9.56 |
| Energy level past month, points | 7.47 | 7.58 | 7.10¶ |
| Frequency a lot of energy past 4 weeks | 2.52 | 2.57 | 2.77 |
| Measures | TSH Level† | ||
| Low Normal | High Normal | Subclinical Hypothyroid | |
| Overall | |||
| N = 602, No. | 320 | 217 | |
| Walking ability index score, points | 7.45 | 7.67 | 7.57 |
| Usual gait speed, m/s | 1.08 | 1.07 | 1.07 |
| Rapid gait speed, m/s | 1.59 | 1.62 | 1.59 |
| 400-m time††, s | 302 | 299 | 309 |
| Fatigability rating‖‖, points | 9.21 | 9.04 | 9.27 |
| Energy level past month, points | 7.31¶ | 7.63 | 7.40 |
| Frequency a lot of energy past 4 weeks | 2.64 | 2.58 | 2.53 |
| Men | |||
| N = 335, No. | 171 | 126 | 38 |
| Walking ability index score, points | 7.61 | 7.81 | 7.39 |
| Usual gait speed, m/s | 1.10 | 1.09 | 1.06 |
| Rapid gait speed, m/s | 1.65 | 1.69 | 1.60 |
| 400-m time††,s | 291 | 292 | 308 |
| Fatigability rating‖‖, points | 9.32 | 8.84 | 9.32 |
| Energy level past month‡, points | 7.19** | 7.86 | 7.21¶ |
| Frequency a lot of energy past 4 weeks | 2.70¶ | 2.46 | 2.61 |
| Women | |||
| N = 267, No. | 149 | 91 | 27 |
| Walking ability index score, points | 7.24 | 7.49 | 7.99 |
| Usual gait speed, m/s | 1.06 | 1.05 | 1.08 |
| Rapid gait speed, m/s | 1.52 | 1.52 | 1.58 |
| Measures | TSH Level† | ||
| Low Normal | High Normal | Subclinical Hypothyroid | |
| 400-m time††, s | 317 | 308 | 303 |
| Fatigability rating‖‖, points | 9.12 | 9.30 | 9.05 |
| Energy level past month, points | 7.44 | 7.32 | 7.71 |
| Frequency a lot of energy past 4 weeks | 2.56 | 2.74 | 2.43 |
Notes: *Adjusted for age, race, exercise level, smoking status, weight in kilograms, and height in centimeters for all and walking aid use for usual and rapid gait speed, 400-m time and fatigability only.
†FT4 categories defined by sex-specific quartiles, with low comprising the lowest quartile, medium the two middle quartiles, and high the highest quartile. Cutpoints are (0.76–0.89, 0.90–1.09, 1.10–1.50) for men and (0.76–0.90, 0.91–1.07, 1.08–1.50) for women. TSH categories for low normal, high normal, and subclinical hypothyroid are defined respectively as 0.40–2.49, 2.50–4.49, and 4.50–9.99.
‡ p < .001 for trend.
§ p < .001 from high group.
‖ p < .01 from medium group.
¶ p < .05 from medium group.
** p < .001 from medium group.
††Persons able to complete the full 400-m walk only (281 men and 215 women).
‡‡ p < .05 for trend.
§§ p < .01 from high group.
‖‖Subsample administered the fatigability test which was implemented later in the study (290 men and 224 women).
¶¶ p < .05 from high group.
*** p < .01 for trend.
TSH category was not associated with any outcome overall except for reported energy level in the past month which was higher in the high-normal versus the low-normal group (p = .024). In men only, reported energy level was higher in those with high-normal TSH than those with low-normal TSH or TSH in the subclinical hypothyroid range. Reported frequency of a lot of energy was also marginally more favorable in men with high-normal TSH versus the low-normal group (p = .049).
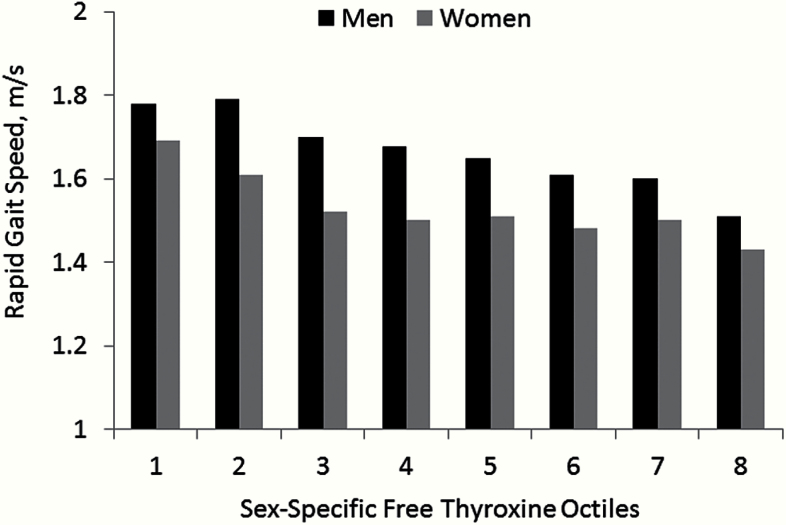
Figure 1 illustrates an approximate “dose–response” relationship between FT4 level categorized in sex-specific octiles and functional mobility using rapid gait speed. In age-adjusted models, men and women in the lowest FT4 octile respectively had significantly (p < 0.05) faster rapid gait speed than men in the sixth through eighth octiles and women in octiles three through eight. Men and women in the highest FT4 octile were significantly slower than men in octiles one through four and women in octiles one and two.
Figure 1.
Mean rapid gait speed by free thyroxine sex-specific octile.
Discussion
Generally in healthy persons aged 68–97 years with normal thyroid function, lower FT4 was uniformly associated with better functional mobility and fitness and a more favorable fatigue profile. Little previous work has investigated the relationship between FT4 and functional mobility, fitness, and fatigue specifically. Nonetheless, findings are consistent with observations that high or increasing FT4 in the normal range in older adults is associated with less desirable health outcomes including mortality, heart failure, coronary heart disease, dementia, and cognitive decline (4,5,7–10,14,15). Although most of this work treated high FT4 as a risk factor, the finding of increased dementia among men in the upper three quartiles versus the lower quartile of FT4 (10), effectively indicates a protective association for low FT4. The work reported herein extends observed associations between higher FT4 and poorer physical function from just men (8) to women and to other functional and health-related entities including fatigue. The inverse association between FT4 and reported energy levels and fatigability is especially noteworthy, given that tiredness is a hallmark symptom of hypothyroidism and suggests that low-normal FT4 is a distinctly different condition.
The less consistent sex-specific findings examining FT4 as a continuous variable may reflect differences in how men and women respond to self-report questions on walking ability and energy level as results were remarkably similar across the four performance-based measures. Inconsistent sex-specific findings using categories of FT4 likely derive from reduced statistical power due to stratification compounded by categorization as mean values of all measures for both men and women generally followed the same pattern—best in the low and worst in the high with the medium group in-between.
The effectively absent association between TSH level and physical function and fitness stands in contrast to two previous studies, one using data on 2,200 men and women covering a 10-year age span (6) and the other examining 599 men and women within a 2-year birth cohort. As noted above, TSH may be a less accurate or precise indicator of thyroid function at older ages (11–13), and thus a larger more homogeneous sample would be required to detect a protective association between mildly down-regulated thyroid function defined by TSH level alone. The current study of 602 men and women spanning 30 years of age simply may be underpowered to uncover an association.
Together this and other work suggest that FT4 at the lower end of normal may indicate more robust health, but whether this reflects a predisposition for healthy longevity or lifestyle-related adaptation or some combination is unclear. Offspring of long-lived populations provide some evidence of a genetic underpinning, however. For example, middle-aged (55–64 years) offspring of nonagenarians have lower FT4 levels on average than their partners (23) and offspring (aged 59–79 years) of Ashkenazi Jewish centenarians have higher TSH levels than age-matched controls without familial longevity (24). Other work, restricted to euthyroid men between 20 and 45 years, indicates a heritability of FT4 between 80% and 90% (25). In contrast, with the exception of smoking (25,26), few lifestyle factors have been associated with down-regulated thyroid function. In the current study, for instance, neither body mass index nor exercise level varied by FT4 category.
Findings are also consistent with the notion that a lower metabolic demand or having a “cooler engine” measured as a lower resting metabolic rate or core body temperature is consistent with better health (27,28) and longevity (29,30). Thyroid hormone is known to regulate metabolic rate (31) and lower FT4 may facilitate functional independence by sparing energy necessary for other purposes and to cope with stress and illness. Analogously, recent work found a lower metabolic cost of walking, which includes basal metabolism, associated with faster gait speed (32) and a lower rate of decline (33) in older adults.
Although our findings suggest that high-normal FT4 may be undesirable, they have no direct implication for exogenous treatment with thyroxine as the study population consisted of individuals free of overt thyroid disease and not taking any thyroid medication. Nevertheless, our findings along with recent evaluations of the changing relationship between TSH and FT4 with age (11–13) suggest that assessment of TSH alone may be insufficient to determine thyroid function and evaluation of FT4 and other thyroid hormones may be necessary to derive an accurate clinical profile. The clinical relevance of lower FT4 is unclear and whether its association with better function is merely correlational or there exists a protective relationship remains to be demonstrated using longitudinal data. Whether higher FT4 reflects increasing FT4 as a possible indicator of declining health warrants further investigation as well.
The study population consists of volunteer participants in a long running observational study that exhibit on average better health than similarly aged adults, thus findings best apply to community residents in robust health. All associations are from cross-sectional data and measurements taken at a single time point. Whether associations hold over time or reflect or confer different aging trajectories remain to be demonstrated. Given the reduced statistical power in the sex-stratified and categorical analyses, lack of a statistically significant difference should not be interpreted as definitive evidence for absence of an association.
In summary, in community resident generally healthy persons aged 68–97 years with normal thyroid function, both men and women with FT4 levels at the lower end of normal exhibit better mobility functioning, fitness, and fatigue profiles. Findings add to evidence that low-normal FT4 aligns with better health status in old age and thus may serve as a biomarker of healthy longevity.
Funding
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health.
Acknowledgments
Author contributions: E.M.S. had full access to all of the data and takes full responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: E.M.S., C.W.C., J.S.M., and L.F. Acquisition of data: E.M.S., C.W.C, J.M.E., and L.F. Analysis and interpretation of data: E.M.S., J.S.M., and L.F. Drafting of the manuscript: E.M.S. Critical revision of the manuscript for important intellectual content: E.M.S., C.W.C., J.M.E., and L.F. Statistical analysis: E.M.S. and L.F. Obtained Funding: L.F. Administrative, technical, and material support: E.M.S., C.W.C., J.M.E., and L.F. Financial disclosure: None reported.
References
- 1. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab. 2007;92:4575–4582. [DOI] [PubMed] [Google Scholar]
- 2. Bremner AP, Feddema P, Leedman PJ, et al. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab. 2012;97:1554–1562. doi:10.1210/jc.2011-3020 [DOI] [PubMed] [Google Scholar]
- 3. Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379:1142–1154. doi:10.1016/S0140-6736(11)60276-6 [DOI] [PubMed] [Google Scholar]
- 4. Waring AC, Arnold AM, Newman AB, Bùzková P, Hirsch C, Cappola AR. Longitudinal changes in thyroid function in the oldest old and survival: the cardiovascular health study all-stars study. J Clin Endocrinol Metab. 2012;97:3944–3950. doi:10.1210/jc.2012-2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. J Clin Endocrinol Metab. 2015;100:1088–1096. doi:10.1210/jc.2014-3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simonsick EM, Newman AB, Ferrucci L, et al. Subclinical hypothyroidism and functional mobility in older adults. Arch Intern Med. 2009;169:2011–2017. doi:10.1001/archinternmed.2009.392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA. 2004;292:2591–2599. [DOI] [PubMed] [Google Scholar]
- 8. van den Beld AW, Visser TJ, Feelders RA, Grobbee DE, Lamberts SW. Thyroid hormone concentrations, disease, physical function, and mortality in elderly men. J Clin Endocrinol Metab. 2005;90:6403–6409. [DOI] [PubMed] [Google Scholar]
- 9. Yeap BB, Alfonso H, Hankey GJ, et al. Higher free thyroxine levels are associated with all-cause mortality in euthyroid older men: the Health In Men Study. Eur J Endocrinol. 2013;169:401–408. doi:10.1530/EJE-13-0306 [DOI] [PubMed] [Google Scholar]
- 10. Yeap BB, Alfonso H, Chubb SA, et al. Higher free thyroxine levels predict increased incidence of dementia in older men: the Health in Men Study. J Clin Endocrinol Metab. 2012;97:E2230–E2237. doi:10.1210/jc.2012-2108 [DOI] [PubMed] [Google Scholar]
- 11. Hoermann R, Eckl W, Hoermann C, Larisch R. Complex relationship between free thyroxine and TSH in the regulation of thyroid function. Eur J Endocrinol. 2010;162:1123–1129. doi:10.1530/EJE-10-0106 [DOI] [PubMed] [Google Scholar]
- 12. Clark PM, Holder RL, Haque SM, Hobbs FD, Roberts LM, Franklyn JA. The relationship between serum TSH and free T4 in older people. J Clin Pathol. 2012;65:463–465. doi:10.1136/jclinpath-2011-200433 [DOI] [PubMed] [Google Scholar]
- 13. Hadlow NC, Rothacker KM, Wardrop R, Brown SJ, Lim EM, Walsh JP. The relationship between TSH and free T₄ in a large population is complex and nonlinear and differs by age and sex. J Clin Endocrinol Metab. 2013;98:2936–2943. doi:10.1210/jc.2012-4223 [DOI] [PubMed] [Google Scholar]
- 14. Hogervorst E, Huppert F, Matthews FE, Brayne C. Thyroid function and cognitive decline in the MRC Cognitive Function and Ageing Study. Psychoneuroendocrinology. 2008;33:1013–1022. doi:10.1016/j.psyneuen.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 15. Åsvold BO, Vatten LJ, Bjøro T, et al. Thyroid function within the normal range and risk of coronary heart disease: an individual participant data analysis of 14 cohorts. JAMA Intern Med. 2015;175:1037–1047. doi:10.1001/jamainternmed.2015.0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eldadah BA. Fatigue and fatigability in older adults. PM R. 2010;2:406–413. doi:10.1016/j.pmrj.20doi:10.03.022 [DOI] [PubMed] [Google Scholar]
- 18. Simonsick EM, Newman AB, Nevitt MC, et al. Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2001;56A:M644–M649. [DOI] [PubMed] [Google Scholar]
- 19. Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54:127–132. [DOI] [PubMed] [Google Scholar]
- 20. Borg G. Psychophysical scaling with applications in physical work and the perception of exertion. Scand J Work Environ Health. 1990;16(suppl 1):55–58. [DOI] [PubMed] [Google Scholar]
- 21. Simonsick EM, Schrack JA, Glynn NW, Ferrucci L. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–351. doi:10.1111/jgs.12638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 23. Rozing MP, Westendorp RG, de Craen AJ, et al. Low serum free triiodothyronine levels mark familial longevity: the Leiden Longevity Study. J Gerontol A Biol Sci Med Sci. 2010;65A:365–368. doi:10.1093/gerona/glp200 [DOI] [PubMed] [Google Scholar]
- 24. Atzmon G, Barzilai N, Surks MI, Gabriely I. Genetic predisposition to elevated serum thyrotropin is associated with exceptional longevity. J Clin Endocrinol Metab. 2009;94:4768–4775. doi:10.1210/jc.2009-0808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roef G, Taes Y, Toye K, et al. Heredity and lifestyle in the determination of between-subject variation in thyroid hormone levels in euthyroid men. Eur J Endocrinol. 2013;169:835–844. doi:10.1530/EJE-13-0265 [DOI] [PubMed] [Google Scholar]
- 26. Asvold BO, Bjøro T, Nilsen TI, Vatten LJ. Tobacco smoking and thyroid function: a population-based study. Arch Intern Med. 2007;167:1428–1432. [DOI] [PubMed] [Google Scholar]
- 27. Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. “IDEAL” aging is associated with lower resting metabolic rate: the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62:667–672. doi:10.1111/jgs.12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fabbri E, An Y, Schrack JA, et al. Energy metabolism and the burden of multimorbidity in older adults: results from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2015;70:1297–1303. doi:10.1093/geron/glu209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roth GS, Lane MA, Ingram DK, et al. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002;297:811. [DOI] [PubMed] [Google Scholar]
- 30. Ruggiero C, Metter EJ, Melenovsky V, et al. High basal metabolic rate is a risk factor for mortality: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2008;63:698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Silva JE. The thermogenic effect of thyroid hormone and its clinical implications. Ann Intern Med. 2003;139:205–213. [PubMed] [Google Scholar]
- 32. Schrack JA, Simonsick EM, Chaves PH, Ferrucci L. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc. 2012;60:1811–1816. doi:10.1111/j.1532-5415.2012.04153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schrack JA, Zipunnikov V, Simonsick EM, Studenski SA, Ferrucci L. Rising energetic cost of walking predicts gait speed decline with age. J Gerontol A Biol Sci Med Sci (Under review). [DOI] [PMC free article] [PubMed] [Google Scholar]