Abstract
Clostridium difficile infection (CDI) is the most common cause of antibiotic-associated diarrhea and a significant burden on the health care system. Aging has been identified in the literature as a risk factor for CDI as well as adverse outcome from CDI. Although this effect of advanced age on CDI could be partially explained by clinical factors associated with aging, biologic factors are important. Innate immune system, responsible for immediate response to acute infections, plays a major role in CDI pathogenesis. Impairment in function of innate immunity with aging, demonstrated in other infection models, may lead to worse outcome with CDI. C. difficile toxin-specific antibody response protects the host against initial and recurrent infections as shown in observational studies and clinical trial. Effect of aging on antibody response to CDI has not been demonstrated, but the results from vaccine studies in other infections suggest a negative effect on humoral immunity from aging. Although intestinal microbiota from healthy people confers resistance to CDI by preventing C. difficile colonization, changes in composition of microbiota with aging may affect that resistance and increase risk for CDI. There are also age-associated changes in physiology, especially of the gastrointestinal tract, that may play a role in CDI risk and outcomes. In this review, we will first discuss the epidemiology of CDI in the elderly people, then the alteration in innate immunity, humoral response, and microbiota that increases susceptibility to CDI and severe disease and lastly, the physiological and functional changes that may modify outcomes of infection.
Keywords: Immune Function, Infection, Inflammation, Epidemiology, Functional Performance.
Clostridium difficile infection (CDI) is the most commonly recognized cause of infectious diarrhea in health care settings and accounts for 20%–30% of cases of antibiotic-associated diarrhea (1). Pathogenesis of CDI involves disruption of normal colonic microbiota by antibiotics, colonization with toxigenic C. difficile, elaboration of C. difficile toxin A (TcdA) or toxin B (TcdB), and mucosal injury and inflammation (2). The clinical manifestations range from symptomless carriage, to mild or moderate diarrhea, to fulminant and sometimes fatal pseudomembranous colitis. Some of the clinical factors implicated in adverse outcomes are enumerated in Supplementary Table 1. Complications of severe CDI include dehydration, toxic megacolon, bowel perforation, renal failure, sepsis, and death. In the past decade there has been a dramatic increase in the incidence of CDI. From 1993 to 2009, the absolute number of U.S. hospital stays with CDI listed as a diagnosis increased fourfold, while the rate of stays with a CDI diagnosis increased threefold (3). Multistate prevalence survey revealed that by 2010 C. difficile was the most common pathogen to cause health care-associated infections, causing 12.1% of infections, and more common than Staphylococcus aureus, Klebsiella spp. or Escherichia coli (4). CDI poses a significant burden on the healthcare system. Estimates of total economic burden of CDI in acute care hospitals in the United States range from 1.0 to 4.9 billion dollars (5). This has prompted the Centers for Disease Control and Prevention (CDC) to identify C. difficile as one of only three bacteria categorized as “urgent” antibiotic resistance threats in its report in 2013 (Threat Report 2013 | Antimicrobial Resistance | CDC http://www.cdc.gov/drugresistance/threat-report-2013).
Clinical practice guidelines for CDI from Society for Healthcare Epidemiology of America and Infectious Diseases Society of America lists advanced age as a strong risk factor for symptomatic CDI and inferior clinical outcomes—both worse outcome from initial infection and relapse after apparent successful treatment (1). In this review, we will first discuss the epidemiologic trends associating advanced age and CDI. Next we will examine age-related alterations in host immune response and intestinal microbiota that increase susceptibility to CDI. Lastly, we will also consider the contribution of physiological and functional changes in the aged host that may contribute to worse outcomes of CDI.
Aging and CDI Epidemiology
A number of studies demonstrate advanced age as a risk factor for CDI and severe outcome of CDI. In a survey of hospital stays for CDI in U.S. hospitals in 2009, the rate of CDI was 350 per 10,000 hospital stays for patients 65 or older compared with 122 in younger age groups (3).
The effect of aging on severity of outcome has been especially well documented during the epidemic increase of NAP1/BI/027 strain in North America (6). It was found that attributable 30-day mortality rate increased significantly after age 60 and especially steeply above age 80. A similar phenomenon was noted on a subsequent surveillance in 2004 in Canada, where the percentage of CDI-related death and severe CDI were significantly higher in the older population (7). In a study of 336 patients with stool positive for C. difficile in Brigham and Women’s Hospital between 2004 and 2005, the odds ratio for severe disease was 3.35 for ages 70 and older (8). One might speculate that comorbid illness, not age, is driving adverse outcomes of CDI in seniors. However, even after controlling for comorbidity burden, in a review of 161 patients with CDI in a hospital in Quebec, Pépin and colleagues demonstrated that 30-day and 1-year mortality increased with older age, especially above the age 75 (9).
In contrast to these findings, there are some studies showing that aging itself is not an independent risk factor for CDI or for worse disease. A logistic regression analysis of 69 patients with stool positive for C. difficile from University of Michigan showed that age was not found to be an independent risk factor after controlling for comorbidities (10). Further, in 121 patients with CDI diagnosis at a community hospital in New York City, age was not an independent risk factor when examined as a continuous or discrete variable (11). Finally, a post hoc analysis of the randomized clinical trial comparing fidaxomicin and vancomycin showed similar results for both agents regardless of age (12). Although each additional decade raised the risk of recurrence by 17% and decreased clinical cure by 13%, multivariate regression analysis showed that the odds ratio was not significant after controlling for other risk factors, such as concomitant antibiotic exposure, infection with NAP1 strain type, renal insufficiency, serum albumin less than 3g/dL, inpatient status, leukocytosis, and proton pump inhibitor–histamine receptor antagonist exposure.
An explanation of these conflicting findings may involve differences in the infecting strain of C. difficile. Many of the larger studies cited earlier were during the period of epidemic NAP1 strain outbreak in Quebec. Most earlier or smaller studies did not have strain information. Miller and colleagues outline the influence of C. difficile strain by stratifying the population by both age and strain, and there appears to be a notable interaction between the two variables. When CDI is due to the more virulent NAP1 strains, mortality by age begins to differ at age 60 and above, but in non-NAP1 strain infection the difference is seen only above age 90 (7). This interaction may be a factor in other studies cited above where the cutoff for defining advanced age ranges from 60 to 90 years between studies. There may also be an interaction between site of residence and CDI strain. The proportion of CDIs that are due to NAP1 strains is variable between 13% and 45% among residents of long-term care institutions, but is around 29% overall in the United States (13).
Regardless of whether the effect of aging on outcome is an independent factor or not, and strain-dependent or not, the elderly population remains the most vulnerable to CDI. Approximately 92% of deaths from C. difficile occurred in people aged 65 and older (14). CDI is also the 18th leading cause of death for the population aged 65 and older. The only other infections that cause more deaths are pneumonia and septicemia.
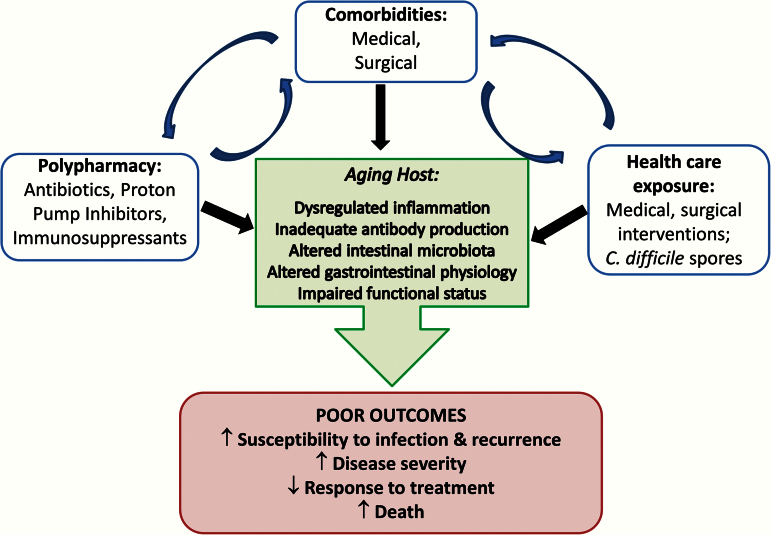
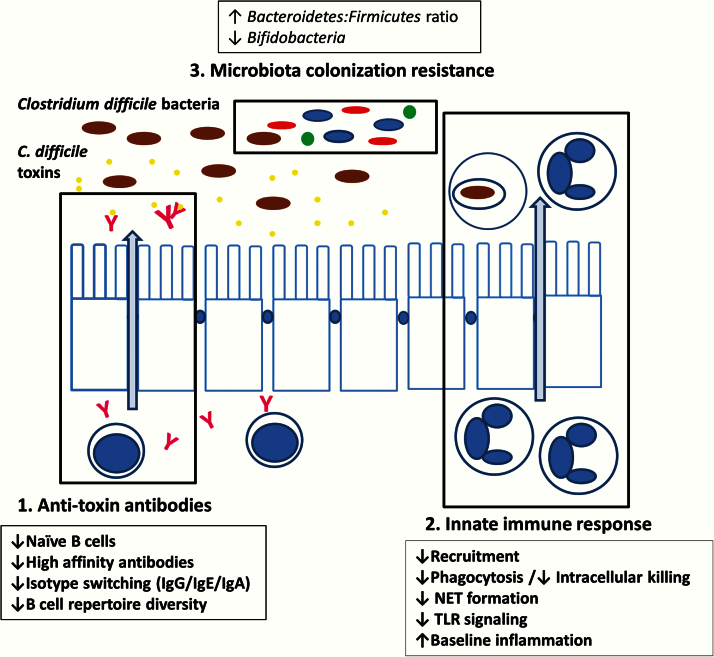
The underlying mechanisms of why CDI is more common and severe in the elderly population are unclear. Understanding the role of aging in CDI is challenging because of complexity of the elderly host. Multiple interacting factors such as comorbidities, polypharmacy, and health care exposure may be contributory to the observed outcomes of infection (Figure 1). However, there are three potential biological factors that have been marginally investigated but may be critical: humoral response, innate immunity, and intestinal microbiota (Figure 2).
Figure 1.
Factors leading to poor outcome of Clostridium difficile infection in aging host. Multiple host and environmental factors contribute to poor clinical outcome. Health care-related factors have significant impact, but host factors related to the biologic changes associated with aging may play a part as well.
Figure 2.
Changes in immune response and microbiome with aging. Aging leads to impairment in function of both innate immune response and adaptive humoral response to infections. Microbiota component also changes with aging. These changes may lead to worse clinical outcome in elderly patients with Clostridium difficile infections.
Aging and Humoral Response to TcdA and TcdB
A possible cause of inferior CDI outcomes in the aged is impaired antibody response. Inadequate humoral response may be secondary to declining quantity or function, or both, of antibody-producing cells. There are a number of changes in B-cell function and antibody response with aging as described in detail by McGlauchlen and Vogel and Frasca and Blomberg (15,16). Both the percentage and number of naϊve human B cells in blood decrease with age. Numbers of memory B cells are less consistently affected by advancing age depending on whether CD19 or CD20 are used to define the B-cell population (17,18). There is, however, an age-related contraction of the B-cell repertoire with age that correlates with poor health status (18–20).
When the host encounters a pathogen, the antigen-presenting cells stimulate the T cells and B cells to start the adaptive immune response. Although the number of antigen-presenting cells does not appear to change substantially with aging, there is reduction in their function (see Innate Immunity later) and in the effect of stimulating T cells on B-cell differentiation/maturation. The most significant change with aging in humoral immunity is impairment in the ability to produce high affinity immunoglobulins and undergo immunoglobulin class switch, which allows the production of high affinity IgG, IgA, and IgE (21). This may be associated with the decrease in specific antibody responses in humans vaccinated against Salmonella, S. pneumoniae, and influenza (20). In regard to mucosal immunity, serum IgA levels are elevated in the elderly people as compared with younger controls, but these IgA are predominantly monomeric IgA which are not transported to the mucosal surface as dimeric secretory IgA (22). No age-related differences in total immunoglobulin titers in the intestinal lumen or in the cultures of duodenal biopsies have been reported.
The critical role of the antibody response in CDI has been demonstrated by a number of cohort studies. A randomized, double-blind, placebo-control trial has shown that monoclonal antibodies specific to TcdA and TcdB, administered with standard antibiotic treatment, reduced recurrence of CDI indicating the role of humoral immunity in modulating outcomes (23). Many healthy adults (∼60%) have detectable serum IgG and IgA to TcdA and TcdB despite only a small population (2%–3%) being colonized (24). It is not known if the prevailing responses in adults are a reflection of childhood exposure or subclinical infections as adults. The ability to mount an effective immune response following exposure to C. difficile appears to affect the course of disease expression. Indeed, only a small proportion of high-risk hospitalized patients develop symptomatic infection while up to 31% are colonized with C. difficile (25). In a prospective study of hospitalized patients, it was found that in patients who were colonized with C. difficile, those who became asymptomatic carriers had significantly greater increases in serum levels of IgG antibody against TcdA than did patients in whom CDI developed (25). The odds ratio for diarrhea was 48.0 among patients with colonization who had a serum level of IgG antibody against TcdA of 3.00 ELISA units or less, as compared with patients with colonization who had a level of more than 3.00 ELISA units (25).
Following symptomatic infection, many individuals develop antitoxin antibodies in serum, including toxin neutralizing IgA, as well as in stool and this response appears to be associated with protection from subsequent infection (24). The inability of the host to mount an adequate IgM and IgG response to an initial infection was associated with higher likelihood of a recurrent CDI in a prospective study (26). After adjustment for disease severity, the odds ratio for recurrent diarrhea associated with a low IgM anti-TcdA value on Day 3 was 9.0, whereas the unadjusted odds for recurrent diarrhea associated with a serum concentration of IgG against TcdA of less than 1.29 ELISA units (<75th percentile) on Day 12 was 48.0. Age did not seem to affect antibody production, however. While age greater than 65 had an odds ratio of 10.4 for recurrent disease, IgG concentrations on Day 12 against TcdA were not affected by age. In another prospective study, lower anti-TcdB IgA levels at Day 0 and lower anti-TcdA and anti-TcdB IgA levels at Days 18–21 were associated with higher recurrence (27). Although age greater than 73 was an independent risk factor for recurrence in a multivariate logistic regression analysis with an odds ratio of 4.8, possible associations between age and antibody levels were not explored in this study.
In summary, there is an association demonstrated in multiple studies between aging and recurrent CDI. There is also an association between lower antibody levels, although different components—IgM, IgG, or IgA—were measured in different studies, and recurrent disease. Despite evidence from vaccine studies of other infections showing an association between aging and lower antibody response, this has not been shown in CDI so far. Therefore, overall, it is suspected that decreased antibody response may lead to increased susceptibility to infection and recurrence in CDI with aging, but the evidence is insufficient.
Aging and Innate Immune Response to CDI
Acute colitis with pseudomembrane formation and heavy neutrophil infiltration of the colonic epithelium and submucosa is characteristic of severe CDI. Marked increase in neutrophils in the peripheral blood is also a feature of severe CDI and linked to poor prognosis (28). Such observations point to an important role for innate immune response in CDI pathogenesis.
Aging is known to cause dysregulation of the innate immune system (for a more detailed review refer to Shaw and colleagues (29)). The numbers of innate immune cells including neutrophils remain stable or only slightly decrease with age. The functions, however, have been noted to be significantly altered in aged hosts (Supplementary Table 2). Chemotaxis, directional movement of cells in response to a gradient of a stimulus, is impaired with age in neutrophils as demonstrated in vitro with human and mouse neutrophils (30–32). This leads not only to decreased recruitment of neutrophils to infected tissues, but also defective neutrophil egress from inflamed tissues. Micro-environmental and tissue-related factors, such as production of chemokines and cytokines, may also be subject to the dysregulation related to aging. With pulmonary Pseudomonas aeruginosa infection, aged mice had higher mortality and lower neutrophil count in infected tissue despite higher circulating numbers of neutrophils (30). The level of the chemokine keratinocyte-derived chemokine (KC), which corresponds to IL-8 in humans, both strong neutrophil chemoattractants, was elevated in the infected tissue of the aged mice compared with young mice. When cutaneous Staphylococcus aureus infection was studied, clearance of bacteria in the wound was impaired in aged mice starting approximately 3 days after infection (31). Neutrophil count at the wound site was decreased on Day 3 postinfection, whereas levels of chemokines KC and MIP-2 were increased on Day 1, all compared with young mice. These studies show lower numbers of neutrophil recruitment to tissue but higher levels of chemokines in these tissues demonstrating dysregulation of innate immune response.
In addition to recruitment, immune cell effector functions are decreased. Neutrophils from aged hosts show impaired phagocytosis of bacteria, intracellular killing, and formation of neutrophil extracellular traps—scaffolds of extruded chromatin—that facilitate capture and killing of pathogens (29). Pattern recognition receptor signaling pathways, including Toll-like receptors and pro-inflammatory cytokine production, have a crucial role in linking innate and adaptive immune responses. These functions in monocytes and macrophages seem to be diminished in aged mice and humans, but these impairments occur in the presence of dysregulated or inappropriately persistent inflammatory responses such as production of tumor necrosis factor, IL-8, and IL-6 (33,34). The baseline increase in levels of pro-inflammatory cytokines and chemokines with aging is referred to as inflamm-aging (35). This phenomenon has been linked with increased inflammatory disorders and frailty.
The importance of the innate immune response to C. difficile or its toxins has been demonstrated in experiments blocking key signaling pathways. As discussed in a recent review by Cowardin and Petri, studies show conflicting results (36). The experiments using toxin instillation into ileal loop models seem to show improved outcome with less edema and permeability in bowel walls when innate immune pathways are blocked (37). However, whole bacteria animal infection models show worse outcome by abrogating key innate immune signaling pathways such as Nod1 and MyD88 or depleting neutrophils (38,39).
The effect of aging on the innate immune response in the context of CDI has not been studied in in vivo animal experiments, but changes with aging have been noted in vitro. Neutrophil chemotaxis is increased in response to C. difficile TcdA at very high concentrations of 50–100 μg/mL (40). However, TcdA concentration is unlikely to be so high in the colon, and a lower concentration of 15 μg/mL was not found to act as a chemoattractant for neutrophils (41). In the same study, it was found that the presence of TcdA impaired the movement of neutrophils to chemotactic stimuli. In vitro phagocytosis of C. difficile bacteria was decreased in the neutrophils from healthy elderly participants compared with young participants (42). Curiously, this effect was almost reversed when serum from young participants were used with neutrophils from elderly participants, which suggests that alteration in opsonization function may be involved as well. There were no additional efforts to characterize these opsonins in the same study, but in a separate study looking at phagocytosis of C. difficile bacteria, opsonization efficacy of human serum seemed to diminish when heat-inactivated at 56 °C, which indicates that complements may play a role in opsonization and clostridial clearance (43). Intracellular killing of C. difficile does not seem to be affected by aging (42).
Role of various innate immune cells, pattern recognition receptors, and inflammatory signaling pathways are yet to be investigated. Moreover, contribution of circulating immune cells and molecular signals to local tissue injury from C. difficile intoxication or infection is unclear. Although published evidence remain limited, innate immune system changes may be critical to understanding the effect of aging on C. difficile infection outcomes.
Aging and the Intestinal Microbiota in CDI
The term microbiota refers to the community of microorganisms that inhabit a particular region of the body (44). In the human gut, intestinal microbiota consists of 300–500 species of microorganisms with roughly 1012 bacterial cells per gram of stool. These organisms aid in several functions, including digestion of complex carbohydrates, energy storage, immune functions, and protection against invasion by pathogens. Intestinal microbiota may change as the host ages. Analysis of the bacterial population in the gut shows alterations with aging (45). At the phylum level the microbiota of elderly participants is dominated by Bacteroidetes, whereas in younger subjects the Firmicutes phylum is dominant. In the same study, increased relative abundance of Bacteroidetes and increase in the Bacteroidetes:Firmicutes ratio were recorded in antibiotic users, with decreases in the relative abundance of both Firmicutes and Proteobacteria. These findings may suggest that aging has similar effects on intestinal microbiota as antibiotic usage, though a limitation in these data was high interindividual variability. The proportion of the Bacteroidetes phylum ranged from 3% to 92%, while the Firmicutes ranged from 7% to 94% in the elderly population.
The widely accepted model for C. difficile pathogenesis is that use of broad spectrum antimicrobials alters the balance of the intestinal microbiota, allowing pathogenic strains of C. difficile to infect the intestine. The ability of normal intestinal human microbiota to suppress C. difficile colonization was demonstrated in a study, where C. difficile bacteria were inoculated in fecal emulsions from healthy adult volunteers, infants, children, and geriatric patients (46). All human stools had inhibitory effect on C. difficile growth and toxin production. This effect of the intestinal microbiota is called colonization resistance, and is the premise for the wide use for fecal transplantation as a treatment strategy for relapsing CDI in seniors, reviewed in Burke and colleagues (47).
When the microbiota was compared between healthy young and healthy elderly participants, and with patients with CDI, there were some notable differences (48). The number of Bifidobacteria decreased with age but much more so in CDI patients. CDI patients had lower numbers of Bacteroidetes and higher number of Clostridia. These studies show that the intestinal microbiotas in the aged host and in CDI patients are different, although a consistent trend has yet to be defined from various experiments. In the colonization resistance study by Borriello and Barclay, stool from elderly participants was found to have smaller inhibitory effect on C. difficile than stool from young participants, although the difference was not statistically significant (46). These results suggest that changes in the microbiota related to age may lead to differences in CDI incidence or outcome between age groups.
Microbiota presents a promising lead to prevention of initial and recurrent infections as demonstrated in recent studies showing benefits of fecal microbiota transplantation (47). However, specific changes associated with aging that may lead to increased risk of CDI is not characterized uniformly due to difference in measurement or targeted taxonomic groups or species, as well as the large interindividual variations in published studies.
Additional Factors That Change With Age and May Contribute to CDI Incidence and Severity
There are a number of important factors unrelated to microbiology or immunology which vary with age that may also contribute to CDI in seniors. The aging gastrointestinal tract demonstrates altered physiology all along its length (reviewed in Bhutto and colleagues (49)). In the stomach, acid secretion has been generally thought to decline with age and atrophic gastritis is a common diagnosis in seniors. However, this diagnosis may have been confounded by Helicobacter-induced gastritis and may be changing (50). Decreased mucus and bicarbonate secretion, reduced prostaglandin production, and impaired mucosal blood flow have also been reported with age. Reduced colonic motility is well described in those with advanced age and could contribute to more severe CDI. Up to 50% of community-dwelling elderly report constipation as do >70% of those residing in nursing homes (51). Underlying mechanisms of general aging include slow and poorly coordinated colonic transit, and multiple contributing factors frequently co-exist including: poor mobility, comorbidities (eg, stroke, diabetes, Parkinson’s disease), medications (especially anticholinergics), and impaired anorectal sensation (51,52).
Impaired functional status—poor mobility, dependence on others for activities of daily living (ADL; eg, impaired dressing, bathing, feeding), and cognitive impairment—is a common feature of advanced age. There is increasing recognition that functional status should be included as an important risk factor in studies investigating elderly patients and infections based primarily on studies of respiratory infections (53). Poor functional status has been identified as a risk factor for nosocomial infections in general and more recently, as a risk factor for CDI and worse outcome from CDI (54–56). In a retrospective case–control study of diabetic patients in a hospital in Israel, it was found that bedridden state was a risk factor for CDI with a odds ratio of 9.31 (p < .001) (57). In a study by Rao and colleagues., of 99 hospitalized patients with CDI (mean age 67 years), 25 (28%) required assistance with ADLs (55). On univariate analysis, ADL class of full assistance was associated with severe CDI (odds ratio = 7, 95% confidence interval 1.83–26.79). In a multivariable model including age, ADL class, congestive heart failure, diabetes mellitus, depression, weighted Charlson-Deyo comorbidity score, immunosuppression, prior CDI, and proton pump inhibitor use, an ADL class of full assistance retained its association with severe CDI (odds ratio = 8.1, 95% confidence interval 1.24–52.95). Another study by Kyne and colleagues showed a significant association between lower Bathel score (ability to perform ADL) at the onset of symptoms and prolonged symptoms and more severe disease from CDI as well (56).
These physiological changes with aging may contribute to the effect on C. difficile acquisition and outcome and should be taken into consideration for further aging research.
Conclusion
Advanced age is an important factor affecting CDI risk and outcome. Various age-related changes in neutrophil function, antibody response, intestinal microbiota, gastrointestinal physiology, and functional status may contribute to development of severe disease in the elderly people. Moreover, comorbidities, medications, medical and surgical interventions, and health care exposure potentially further facilitate acquisition of infection and poor clinical outcomes. The complexity of the aging host and the adverse consequences of infections such as CDI necessitate more studies to dissect underlying mechanisms in order to develop management approaches appropriate for this vulnerable population. Further studies on how to enhance antibody responses to C. difficile toxins, what the roles of various innate immune cells, pattern recognition receptors and inflammatory signaling pathways are during development of infection, what the impact of the intestinal microbiota on both humoral and innate immunity is, and what alterations in the intestinal microbiota may be potential targets for intervention, are just few of the research questions that need to be addressed. Research into the interaction of CDI with aging may provide insight into the aged host in context of other infections, particularly illnesses controlled by mucosal immunity.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by the National Institutes of Health Training Grant (5T32AI007046-39).
Conflict of Interest
The authors have no conflicts of interest.
Supplementary Material
References
- 1. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol Off J Soc Hosp Epidemiol Am. 2010;31(5):431–455. doi:10.1086/651706 [DOI] [PubMed] [Google Scholar]
- 2. Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases . 8th ed. Philadelphia, PA: Elsevier/Saunders; 2015. [Google Scholar]
- 3. Lucado J, Gould C, Elixhauser A. Clostridium Difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #124. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville, MD: Agency for Health Care Policy and Research (US); 2006. [cited July 28, 2014]. http://www.ncbi.nlm.nih.gov/books/NBK92613/ [PubMed] [Google Scholar]
- 4. Magill SS, Edwards JR, Bamberg W, et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N Engl J Med. 2014;370(13):1198–1208. doi:10.1056/NEJMoa1306801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis. 2012;55(suppl 2):S88–S92. doi:10.1093/cid/cis335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Loo VG, Poirier L, Miller MA, et al. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005;353(23):2442–2449. doi:10.1056/NEJMoa051639 [DOI] [PubMed] [Google Scholar]
- 7. Miller M, Gravel D, Mulvey M, et al. Health care-associated Clostridium difficile infection in Canada: patient age and infecting strain type are highly predictive of severe outcome and mortality. Clin Infect Dis Off Publ Infect Dis Soc Am. 2010;50(2):194–201. doi:10.1086/649213 [DOI] [PubMed] [Google Scholar]
- 8. Henrich TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile-associated disease. Emerg Infect Dis. 2009;15:415–422. doi:10.3201/eid1503.080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pépin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ Can Med Assoc J J Assoc Medicale Can. 2005;173(9):1037–1042. doi:10.1503/cmaj.050978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rao K, Walk ST, Micic D, et al. Procalcitonin levels associate with severity of Clostridium difficile infection. PLoS One. 2013;8(3):e58265. doi:10.1371/journal.pone.0058265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dharmarajan TS, Sipalay M, Shyamsundar R, Norkus EP, Pitchumoni CS. Co-morbidity, not age predicts adverse outcome in clostridium difficile colitis. World J Gastroenterol. 2000;6(2):198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Louie TJ, Miller MA, Crook DW, et al. Effect of age on treatment outcomes in Clostridium difficile infection. J Am Geriatr Soc. 2013;61(2):222–230. doi:10.1111/jgs.12090 [DOI] [PubMed] [Google Scholar]
- 13. Waslawski S, Lo ES, Ewing SA, et al. Clostridium difficile ribotype diversity at six health care institutions in the United States. J Clin Microbiol. 2013;51(6):1938–1941. doi:10.1128/JCM.00056-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep Cent Dis Control Prev Natl Cent Health Stat Natl Vital Stat Syst. 2011;59(10):1–126. [PubMed] [Google Scholar]
- 15. McGlauchlen KS, Vogel LA. Ineffective humoral immunity in the elderly. Microbes Infect Inst Pasteur. 2003;5(13):1279–1284. [DOI] [PubMed] [Google Scholar]
- 16. Frasca D, Blomberg BB. Aging affects human B cell responses. J Clin Immunol. 2011;31(3):430–435. doi:10.1007/s10875-010-9501-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ademokun A, Wu YC, Dunn-Walters D. The ageing B cell population: composition and function. Biogerontology. 2010;11:125–137. doi:10.1007/s10522-009-9256-9 [DOI] [PubMed] [Google Scholar]
- 18. Dunn-Walters DK, Ademokun AA. B cell repertoire and ageing. Curr Opin Immunol. 2010;22(4):514–520. doi:10.1016/j.coi.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 19. Gibson KL, Wu Y-C, Barnett Y, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8(1):18–25. doi:10.1111/j.1474-9726.2008.00443.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi:10.1038/ni.2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frasca D, Landin AM, Lechner SC, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol Baltim Md 1950. 2008;180(8):5283–5290. [DOI] [PubMed] [Google Scholar]
- 22. Penn ND, Purkins L, Kelleher J, Heatley RV, Mascie-Taylor BH. Ageing and duodenal mucosal immunity. Age Ageing. 1991;20:33–36. [DOI] [PubMed] [Google Scholar]
- 23. Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med. 2010;362(3):197–205. doi:10.1056/NEJMoa0907635 [DOI] [PubMed] [Google Scholar]
- 24. Giannasca PJ, Warny M. Active and passive immunization against Clostridium difficile diarrhea and colitis. Vaccine. 2004;22:848–856. doi:10.1016/j.vaccine.2003.11.030 [DOI] [PubMed] [Google Scholar]
- 25. Kyne L, Warny M, Qamar A, Kelly CP. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med. 2000;342:390–397. doi:10.1056/NEJM200002103420604 [DOI] [PubMed] [Google Scholar]
- 26. Kyne L, Warny M, Qamar A, Kelly CP. Association between antibody response to toxin A and protection against recurrent Clostridium difficile diarrhoea. Lancet. 2001;357(9251):189–193. doi:10.1016/S0140-6736(00)03592-3 [DOI] [PubMed] [Google Scholar]
- 27. Bauer MP, Nibbering PH, Poxton IR, Kuijper EJ, van Dissel JT. Humoral immune response as predictor of recurrence in Clostridium difficile infection. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2014. doi:10.1111/1469-0691.12769 [DOI] [PubMed] [Google Scholar]
- 28. Kelly CP, Kyne L. The host immune response to Clostridium difficile. J Med Microbiol. 2011;60(8):1070–1079. doi:10.1099/jmm.0.030015-0 [DOI] [PubMed] [Google Scholar]
- 29. Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. doi:10.1038/nri3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen MM, Palmer JL, Plackett TP, Deburghgraeve CR, Kovacs EJ. Age-related differences in the neutrophil response to pulmonary pseudomonas infection. Exp Gerontol. 2014;54:42–46. doi:10.1016/j.exger.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J Immunol Baltim Md 1950. 2013;190(4):1746–1757. doi:10.4049/jimmunol.1201213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sapey E, Greenwood H, Walton G, et al. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: toward targeted treatments for immunosenescence. Blood. 2014;123(2):239–248. doi:10.1182/blood-2013-08-519520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hearps AC, Martin GE, Angelovich TA, et al. Aging is associated with chronic innate immune activation and dysregulation of monocyte phenotype and function. Aging Cell. 2012;11(5):867–875. doi:10.1111/j.1474-9726.2012.00851.x [DOI] [PubMed] [Google Scholar]
- 34. Van Duin D, Allore HG, Mohanty S, et al. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis. 2007;195(11):1590–1597. doi:10.1086/516788 [DOI] [PubMed] [Google Scholar]
- 35. Fulop T, Franceschi C, Hirokawa K, Pawelec G, eds. Handbook on Immunosenescence [Internet]. Dordrecht: Springer Netherlands; 2009. [cited July 23, 2014]. http://www.springerlink.com/index/10.1007/978-1-4020-9063-9. Accessed July 23, 2014. [Google Scholar]
- 36. Cowardin CA, Petri WA. Host recognition of Clostridium difficile and the innate immune response. Anaerobe [Internet] 2014. Sep [cited November 10, 2014]. http://linkinghub.elsevier.com/retrieve/pii/S1075996414001206. doi:10.1016/j.anaerobe.2014.08.014
- 37. Kelly CP, Becker S, Linevsky JK, et al. Neutrophil recruitment in Clostridium difficile toxin A enteritis in the rabbit. J Clin Invest. 1994;93(3):1257–1265. doi:10.1172/JCI117080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hasegawa M, Yamazaki T, Kamada N, et al. Nucleotide-binding oligomerization domain 1 mediates recognition of Clostridium difficile and induces neutrophil recruitment and protection against the pathogen. J Immunol Baltim Md 1950. 2011;186(8):4872–4880. doi:10.4049/jimmunol.1003761 [DOI] [PubMed] [Google Scholar]
- 39. Jarchum I, Liu M, Shi C, Equinda M, Pamer EG. Critical role for MyD88-mediated neutrophil recruitment during Clostridium difficile colitis. Infect Immun. 2012;80:2989–2996. doi:10.1128/IAI.00448-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Triadafilopoulos G, Shah MH, Pothoulakis C. The chemotactic response of human granulocytes to Clostridium difficile toxin A is age dependent. Am J Gastroenterol. 1991;86:1461–1465. [PubMed] [Google Scholar]
- 41. Brito GA, Sullivan GW, Ciesla WP, Jr, Carper HT, Mandell GL, Guerrant RL. Clostridium difficile toxin A alters in vitro-adherent neutrophil morphology and function. J Infect Dis. 2002;185:1297–1306. doi:10.1086/340236 [DOI] [PubMed] [Google Scholar]
- 42. Bassaris HP, Lianou PE, Legakis NJ, Papavassiliou JT. Interaction between Clostridium difficile and polymorphonuclear leucocytes from the elderly and post-operative cancer patients: phagocytosis and bactericidal function. Med Microbiol Immunol. 1984;173:49–55. [DOI] [PubMed] [Google Scholar]
- 43. Dailey DC, Kaiser A, Schloemer RH. Factors influencing the phagocytosis of Clostridium difficile by human polymorphonuclear leukocytes. Infect Immun. 1987;55:1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis Off Publ Infect Dis Soc Am. 2011;53(10):994–1002. doi:10.1093/cid/cir632 [DOI] [PubMed] [Google Scholar]
- 45. Claesson MJ, Cusack S, O’Sullivan O, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci. 2011;108(suppl 1):4586–4591. doi:10.1073/pnas.1000097107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Borriello SP, Barclay FE. An in-vitro model of colonisation resistance to Clostridium difficile infection. J Med Microbiol. 1986;21:299–309. [DOI] [PubMed] [Google Scholar]
- 47. Burke KE, Lamont JT. Fecal transplantation for recurrent Clostridium difficile infection in older adults: a review. J Am Geriatr Soc. 2013;61:1394–1398. doi:10.1111/jgs.12378 [DOI] [PubMed] [Google Scholar]
- 48. Hopkins MJ, Macfarlane GT. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J Med Microbiol. 2002;51:448–454. [DOI] [PubMed] [Google Scholar]
- 49. Bhutto A, Morley JE. The clinical significance of gastrointestinal changes with aging. Curr Opin Clin Nutr Metab Care. 2008;11:651–660. doi:10.1097/MCO.0b013e32830b5d37 [DOI] [PubMed] [Google Scholar]
- 50. Poh CH, Navarro-Rodriguez T, Fass R. Review: treatment of gastroesophageal reflux disease in the elderly. Am J Med. 2010;123:496–501. doi:10.1016/j.amjmed.2009.07.036 [DOI] [PubMed] [Google Scholar]
- 51. Rao SS, Go JT. Update on the management of constipation in the elderly: new treatment options. Clin Interv Aging. 2010;5:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wiskur B, Greenwood-Van M, eerveld B. The aging colon: the role of enteric neurodegeneration in constipation. Curr Gastroenterol Rep. 2010;12:507–512. doi:10.1007/s11894-010-0139-7 [DOI] [PubMed] [Google Scholar]
- 53. High KP, Bradley S, Loeb M, Palmer R, Quagliarello V, Yoshikawa T. A new paradigm for clinical investigation of infectious syndromes in older adults: assessment of functional status as a risk factor and outcome measure. Clin Infect Dis Off Publ Infect Dis Soc Am. 2005;40(1):114–122. doi:10.1086/426082 [DOI] [PubMed] [Google Scholar]
- 54. Mylotte JM, Graham R, Kahler L, Young L, Goodnough S. Epidemiology of nosocomial infection and resistant organisms in patients admitted for the first time to an acute rehabilitation unit. Clin Infect Dis Off Publ Infect Dis Soc Am. 2000;30(3):425–432. doi:10.1086/313708 [DOI] [PubMed] [Google Scholar]
- 55. Rao K, Micic D, Chenoweth E, et al. Poor functional status as a risk factor for severe Clostridium difficile infection in hospitalized older adults. J Am Geriatr Soc. 2013;61(10):1738–1742. doi:10.1111/jgs.12442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kyne L, Merry C, O'Connell B, Kelly A, Keane C, O'Neill D. Factors associated with prolonged symptoms and severe disease due to Clostridium difficile. Age Ageing. 1999;28:107–113. [DOI] [PubMed] [Google Scholar]
- 57. Eliakim-Raz N, Fishman G, Yahav D, et al. Predicting Clostridium difficile infection in diabetic patients and the effect of metformin therapy: a retrospective, case-control study. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2015;34(6):1201–1205. doi:10.1007/s10096-015-2348-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.