Abstract
Rapamycin extends mouse life span, but the extent to which rapamycin prevents aging-associated changes in specific tissues remains unclear. Stiffness increases and collagen turnover decreases in mouse tendon with aging; thus, our aim was to determine the effect of long-term rapamycin treatment on the mechanical and structural properties of tendons from old mice. Tendons were harvested from female UM-HET3 mice maintained on a standard chow diet for 4 (adult) or 22 (old) months or fed chow containing polymer-encapsulated rapamycin (eRAPA) from 9 to 22 months of age (old RAPA). Stiffness was twofold higher for tendons of old compared with adult mice, but in old RAPA mice, tendon stiffness was maintained at a value not different from that of adults. Additionally, expression of collagen decreased, expression of matrix metalloproteinase-8 increased, and total hydroxyproline content trended toward decreased levels in tendons of old eRAPA-fed mice compared with controls. Finally, age-associated calcification of Achilles tendons and accompanying elevations in expression of chondrocyte and osteoblast markers were all lower in old eRAPA-fed mice. These results suggest that long-term administration of rapamycin alters the molecular pathways responsible for aging of tendon extracellular matrix, resulting in tissue that is structurally and mechanically similar to tendons in adult mice.
Keywords: mTOR, Mice, Biology of aging
Tendon provides a critical functional link between muscle and bone, transferring forces produced by the contractile elements of muscle to the skeleton, ideally with high fidelity and infrequent injury. The mechanical properties of tendon therefore have a meaningful influence on mobility, especially in older adults. In old age, declines in muscle performance (1,2) and bone mass (3) have been well established as major contributors to impairments in mobility and elevated risk of musculoskeletal injury (4), but the influence of changing tendon properties on age-associated disability is not fully understood. In humans, aging is associated with increased incidence of tendon pathology, including abnormal thickening, degeneration, and calcification (5). At least one third of persons older than the age of 70 years suffer from some degree of tendon degeneration (6,7), which increases the risk of full tendon rupture (8). In addition, although tendon ruptures can be painful and debilitating injuries (9), tendon degeneration even without frank rupture can lead to decreased muscle power (10), reduced joint torque (11), and loss in fidelity of force transfer to the skeleton (12). Finally, surgical treatment of tendon disorders in older individuals is associated with a high rate of complications, and most patients do not return to their preinjury activity level (13).
The high prevalence of tendinopathy in the older adults coupled with limited options for successful treatments highlights the need for greater knowledge of the mechanisms underlying the progression of the changes. Age-associated increases in tendon dysfunction may be linked causally to alterations in the mechanical properties of tendons with aging (6,14). We have previously shown that the load-bearing tibialis anterior (TA) and plantaris tendons from mouse hind limbs stiffen in a region-dependent manner with aging, with the most pronounced changes occurring in the region of the tendon nearest the muscle (15,16). Emerging evidence suggests that age-related tendon stiffening also occurs in the load-bearing human Achilles tendon (14). The mechanical properties of tendons are determined by the underlying composition, which is primarily fibrillar type I collagen (COL1). In tendons of young, healthy individuals, the balance between the deposition of new collagen and collagen degradation via matrix metalloproteinase (MMPs) collagenases helps to maintain the mechanical integrity of the collagen fibrils. With aging, however, gene expression of both COL1 and MMPs is severely impaired (16,17), likely resulting in a reduction in collagen turnover and allowing for the accumulation of post-translation modifications to collagen that result in inter- and intramolecular crosslinks that can increase collagen fibril stiffness and compromise the structural and mechanical integrity of tendon (18). Spontaneous calcification of tendon also occurs with age (16,19).
Mechanistic target of rapamycin (mTOR) is an important protein kinase that is a component of two complexes, mTORC1 and mTORC2, which have critical, and distinct, roles in regulating numerous cellular functions. One major function of mTORC1 is the regulation of collagen and other extracellular matrix proteins at the translational level (20). The drug rapamycin is a potent suppressor of mTORC1 activity that, when administered throughout life, results in robust life-span extension in both male and female genetically homogeneous (21) and heterogeneous (22,23) mice. We previously showed that the original dose of rapamycin shown to extend life span also delayed the age-associated increase in tendon stiffness in the proximal region of TA tendons (24), but whether there is a dose-dependent effect of rapamycin on tendon mechanical properties has not been explored, nor have the mechanisms underlying the protective effects been established. The present study thus aimed to compare the mechanical and structural characteristics of tendons of old mice fed a diet containing a polymer-encapsulated rapamycin (eRAPA) with those of age-matched mice fed standard chow. Our hypothesis was that long-term administration of eRAPA would slow the progression of age-associated changes in tendon mechanical properties by (i) enhancing collagen turnover and (ii) slowing the progression of spontaneous tendon calcification into old age at levels observed in adult animals.
Methods
Animals
The mice used in this investigation were genetically heterogeneous UM-HET3 females, produced by a cross between (BALB/cByJ × C57BL/6J)F1 mothers and (C3H/HeJ × DBA/2J)F1 fathers. All procedures were approved by the University of Michigan Committee for the Use and Care of Animals. Animals were housed in a specific pathogen-free barrier facility in the Unit for Laboratory Animal Medicine at the University of Michigan, with food and water provided ad libitum. Weanlings were fed a diet of standard chow until 270 days of age, when a subset of the mice was switched to chow containing eRAPA at concentrations of 4.7, 14, or 44 ppm (21). Ingestion of eRAPA was confirmed by measuring the concentration of rapamycin in serum, which increased in proportion to the eRAPA dose (24). eRAPA-fed mice were euthanized at 22 months of age (“RAPA”). Twenty-two-month-old (“Old”) and 4-month-old (“Adult”) UM-HET3 mice fed standard chow served as controls. Body masses (mean ± SD) for adult and old mice were 23.5±0.2g and 37.8±6.2g, respectively. Body mass for the RAPA mice was 42.6±9.0g and did not differ from the old control group but was significantly greater than in the adult group (p < .05).
Tissue Extraction
Mice were typically sacrificed in the morning, and TA muscle-tendon units (tibialis anterior muscle, tibialis anterior tendon, and 1st metatarsal) were dissected from one hind limb, and overlying connective tissue was carefully removed. Samples were stored in sterile phosphate-buffered saline at 4°C until testing, which occurred within 3 hours of tissue removal. The contralateral TA tendon and both Achilles tendons were also extracted and immediately coated with Tissue Tek and frozen in liquid nitrogen-cooled isopentane or prepared for mRNA isolation.
Determination of Tendon Mechanical Properties
TA tendon mechanical properties were determined in six tendons from adult mice, nine tendons from old mice, and sixteen tendons from old mice fed the various eRAPA diets. The stress–strain response of each TA tendon was determined as described previously (15). Briefly, the TA muscle-tendon unit was placed in a room temperature phosphate-buffered saline bath, and the TA muscle and 1st metatarsal were secured to custom grips so that the tendon was free standing. Twenty-five-micrometer polystyrene beads (IMT Laboratories, Irvine, CA) were brushed along the tendon to serve as optical strain markers. At three points along the length of the tendon, diameters were measured using a calibrated microscope eyepiece at both 0° and 90° rotation. Cross-sectional area (CSA) was calculated for each point assuming an elliptical cross section. Tendons were stretched to a preload of ~0.03N that served as the zero point and were then subjected to a single load–unload cycle of 10% grip-to-grip strain at a constant strain rate of 0.01/second. Force recordings were obtained using two force transducers, and images of the entire test were captured with a camera positioned directly above the tendon. Synchronized force and image recordings were recorded using LabVIEW (Austin, TX). Bead positions were tracked with MetaMorph image analyzing software (Molecular Devices, Sunnyvale, FL), and nominal strains in the proximal (near the muscle) and distal (near the bone) tendon regions were calculated as the change in separation between two beads in each region divided by their initial separation. Regional stress was determined by dividing raw load data by the local CSA. Overall tendon strain was calculated using beads that were positioned one tendon diameter length proximal to the bone insertion and one tendon diameter length distal to the muscle insertion. Overall tendon stress was calculated using average tendon CSA. Stress–strain data were fit to a third-degree polynomial, and maximum tangent modulus of each tendon region was calculated as the maximum slope of the curve. Tangent modulus served as our measure of tendon stiffness. Hysteresis area was calculated as (Area under the loading curve – Area under the unloading curve) / (Area under the loading curve) * 100.
Stiffness in all tendon regions as well as end-to-end values were independent of eRAPA dose (Supplementary Figure 1). Thus, mechanics data from mice fed each of the three concentrations of eRAPA were pooled for the final analyses of mechanical properties. Histological and gene expression data were examined in tendons only from mice fed the diet of mid dose of 14 ppm eRAPA.
Hematoxylin and Eosin Staining
Based on the role of mTOR as a regulator of cell division and cell survival (25), and the relationship between cell density and tendinopathy (26), cell densities were determined from two TA tendons of each group. Ten-micrometer cryosections were taken ~1.5mm proximal to the bone insertion (distal region) and ~1.5mm distal to the muscle insertion (proximal region). Sections were stained with hematoxylin and eosin using standard procedures and viewed under an Olympus BX-51 light microscope. Cell density was measured using ImageJ (NIH) by counting the number of nuclei present in three random regions in each section and normalizing by the area of the region (each region 6,000 µm2). For each section, nuclei counts from each region were averaged to obtain the cell density for the sample. All nuclei counts were determined by a single investigator blinded to the identity of the sample.
Tendon Collagen Content
Hydroxyproline is an amino acid found almost exclusively in collagen. Thus, hydroxyproline content was determined in four TA tendons of each group as a measure of collagen content. The method of Woessner (27) was used for these measures. Briefly, tendons were dried at 110°C for 1 hour, weighed immediately, and then digested overnight at 100°C in 6N hydrochloric acid. The next day the samples were neutralized with sodium hydroxide. Chloramine T was added, and the tubes were incubated at room temperature for 20 minutes, after which the chloramine T was inactivated with perchloric acid. Ehrlich’s solution was added, the tubes were incubated at 60°C for 20 minutes and then cooled, and the absorbance was measured at 560nm. Hydroxyproline concentration was determined by normalizing total hydroxyproline content to tendon dry mass. As an additional marker of tendon collagen dynamics, mRNA levels for collagen I (COL1A1) and for a proteinase important in collagen degradation, matrix metalloproteinase-8 (MMP-8), were also determined in TA tendons using methods described in the following sections.
Tendon Calcification
Because significant age-associated calcification occurs in the Achilles tendon in BL6 mice (16), the extent of calcification was determined in Achilles tendons from the UM-HET3 mice used in the study. Four frozen Achilles tendons from each group were sectioned at 12 µm at the central tendon region and stained with 0.5% Alizarin Red to visualize calcium deposits. Percent area calcified was determined optically using ImageJ by a single, blinded investigator.
mRNA Levels
Total RNA was isolated by homogenizing tendons in Qiazol tissue lysis reagent (Qiagen, Valencia, CA). mRNA was isolated using a miRNeasy Mini Kit (Qiagen) and treated with DNAse I (Qiagen). Reverse transcription was performed using a QuantiTect Reverse Transcription kit (Qiagen), and cDNA was amplified in a CFX96 real-time thermal cycler (Bio-Rad, Hercules, CA) using a QuantiTect SYBR Green I PCR Kit (Qiagen). Reactions were performed in duplicate. The specificity of amplification was verified with melting curve analysis. Relative copy number of each sample was determined using the linear regression of efficiency method, which captures amplification efficiency for each individual reaction (28).
Fibroblasts from calcified avian tendons show chondrogenic and osteogenic phenotypes (29). To explore whether the deposition of ectopic calcium in the tendons of old mice is also associated with the emergence of a chondrogenic or osteogenic phenotype, we examined levels of transcripts for markers for osteoblast and chondrocyte differentiation, Sp7 and SOX9, respectively, and bone formation (Bglap). Additionally, because hypoxia has been suggested to be a driving factor in tendon calcification (30), we also examined mRNA levels for hypoxia-inducible factor 1-alpha (Hif1a). Achilles tendons from n = 5 adult mice, 4 old mice, and 4 old eRAPA-fed mice were analyzed for mRNA levels for SOX9, Sp7, Bglap, and Hif1a due to the extensive calcification observed Achilles tendons. mRNA levels for COL1A1 and MMP-8 were determined in TA tendons from n = 5 adult mice, n = 4 old mice, and 4 old eRAPA-fed mice. All primers were obtained from Qiagen.
Statistics
Values are reported as means ± 1 SEM. Statistical analysis was performed using SigmaPlot software (San Jose, CA). Differences in mean values for regional mechanical and histological data were determined using two-way analysis of variance, with treatment group and tendon region as independent factors. Differences in gene expression, hydroxyproline content, and tendon calcification were determined using a one-way analysis of variance with treatment group as the independent factor. In cases where the analysis of variance indicated significance, individual differences were determined using a Holm Sidak post hoc test. Significance was set at p < .05.
Results
Tendon Morphology
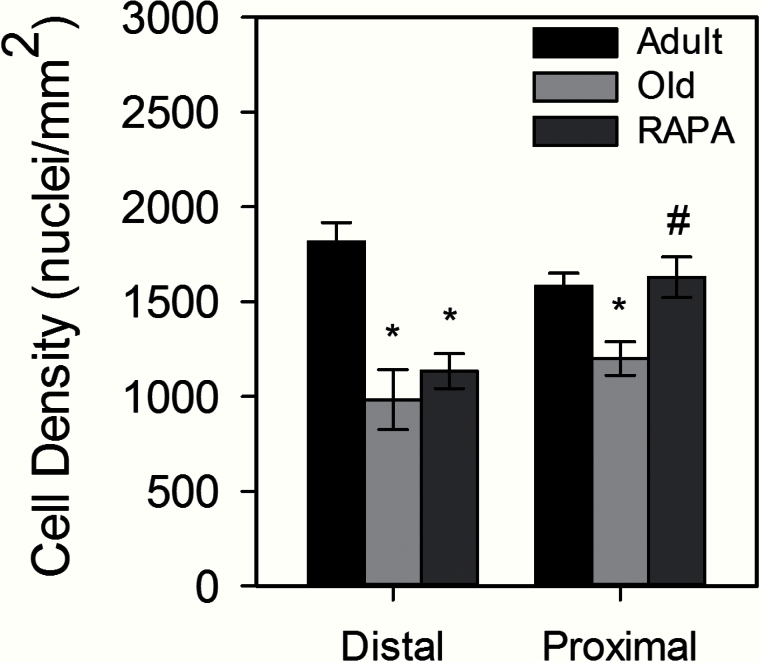
Within each age group, TA tendon CSA was smallest in the distal tendon region, but no age-related or eRAPA-induced changes were seen for CSA in any region of the tendon (Table 1). Likewise, tendon length remained constant among all experimental groups. Fibroblast density decreased with age in both the distal and proximal regions of the tendon. Although cellularity in the distal tendon was unaffected by eRAPA treatment, in the proximal region, cellularity was significantly greater in tendons from eRAPA-fed mice compared with old mice fed a standard diet. The increase in cellularity in the proximal tendons of old eRAPA treated mice resulted in values in that region that were not different from those for tendons of adult mice (Figure 1).
Table 1.
TA Tendon Morphological Properties From Adult, Old, and Old RAPA-Fed UM-HET3 Female Mice
| Adult (n = 6) | Old (n = 9) | RAPA (n = 16) | |
|---|---|---|---|
| Length, mm | 6.5±0.2 | 6.6±0.2 | 6.7±0.2 |
| CSA, mm2 | |||
| Distal | 0.087±0.008 | 0.082±0.010 | 0.082±0.012 |
| Central | 0.136±0.014* | 0.132±0.013* | 0.133±0.012* |
| Proximal | 0.118±0.011* | 0.112±0.009* | 0.116±0.016* |
| Overall | 0.113±0.007 | 0.108±0.007 | 0.110±0.013 |
Notes: Values are means ± SD.
CSA = cross-sectional area; RAPA = Rapamycin; TA = tibialis anterior.
*Significantly different from distal region within an experimental group. No effects of age or RAPA treatment were seen for regional CSA.
Figure 1.
Cell density in adult, old, and rapamycin (RAPA) fed mice. There was an age-associated decrease cell density in both the distal and proximal tendon regions. In the distal region, cell density remained lower following RAPA treatment but was similar to adult values in the proximal region. Data are means ± SEM. *Significantly different from adult. #Significantly different from old.
Changes in Tendon Viscoelastic Properties
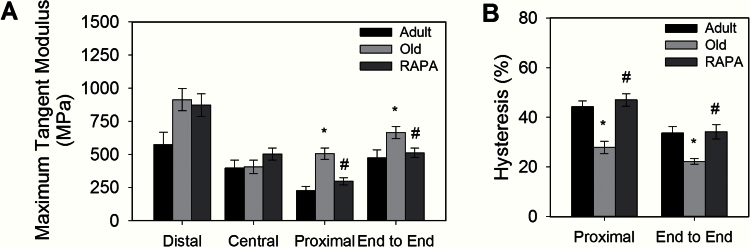
Maximum tangent modulus and hysteresis area of TA tendons for all groups are shown in Figure 2A and B, respectively. Maximum tangent modulus of the proximal tendon region increased twofold with age in the control group (Adult: 226±77MPa, Old: 507±128MPa) but did not change from the adult level in the old eRAPA treated mice (RAPA: 297±110MPa). The lack of an increase in modulus with age in the proximal tendons of eRAPA treated mice resulted in significantly lower moduli for old eRAPA-fed compared with old control mice. The overall end-to-end tendon modulus showed the same pattern, with no difference between tendons from adult and old eRAPA-fed mice for end-to-end stiffness and lower values for tendons of old eRAPA-fed mice compared with old control mice (Adult: 475±148MPa, Old: 666±137MPa, RAPA: 512±143MPa). Similarly, hysteresis area in the proximal tendon region decreased with age for control mice, but not for eRAPA treated mice (Adult: 44.3±5.6%, Old: 27.9±7.4%, RAPA: 47.0±9.9%). Thus, in the proximal tendon region, hysteresis area was significantly greater in tendons from old eRAPA-fed mice compared with those from old mice fed a standard diet and not different for the proximal tendon regions of adult and old eRAPA-fed mice. End-to-end hysteresis response showed the same pattern, with the hysteresis of tendons from old eRAPA-fed mice being significantly greater than that of the old mice (RAPA: 47.0±9.9%, Old: 22.2±3.5%), and not different from that of the adult control mice (Adult: 33.7±6.4%).
Figure 2.
Effect of aging and rapamycin (RAPA) on the (A) tangent modulus and (B) hysteresis area of mouse tibialis anterior tendons. Modulus increased with aging in the proximal tendon region and overall tendon response but was reduced to adult control levels in age-matched RAPA mice (p < .05). Hysteresis area decreased with age in both the proximal region and overall end-to-end response (p < .05). Hysteresis areas were increased for RAPA mice compared with age-matched controls (p < .05). Data are means ± SEM. *Significantly different from adult. #Significantly different from old.
Markers of Collagen Synthesis and Degradation
mRNA levels for key proteins associated with turnover of tendon extracellular matrix, and hydroxyproline content, are shown in Figure 3. Analysis of gene expression showed unchanged levels of COL1A1 mRNA with aging, but a 40% lower COL1A1 expression in tendons from old eRAPA-fed mice compared with age-matched controls. MMP-8, which was expressed in extremely low levels in tendons from adult and old mice, showed a 110-fold increase following eRAPA treatment. Total hydroxyproline content in TA tendons was unchanged with aging, but TA tendons of old eRAPA-fed mice showed a trend toward decreased hydroxyproline content compared with old controls (p = .07).
Figure 3.
Expression of (A) COL1A1 and (B) MMP-8 and (C) hydroxyproline content in tibialis anterior tendons from rapamycin (RAPA) fed mice and age-matched old and adult controls. COL1A1 expression was unchanged with aging, but was decreased by 48% in tendons from RAPA-fed mice compared with age-matched controls. MMP-8 was expressed in extremely low levels in adult and old mice and was highly elevated in tendons from RAPA-fed mice. No age-associated changes in hydroxyproline concentration were seen, although tendons from old RAPA-fed mice trended toward decreased hydroxyproline content as compared with age-matched controls (p = .07). Data are presented as means ± SEM. *Significantly different from adult. #Significantly different from old.
Tendon Calcification
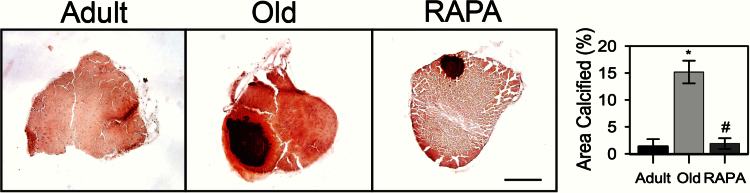
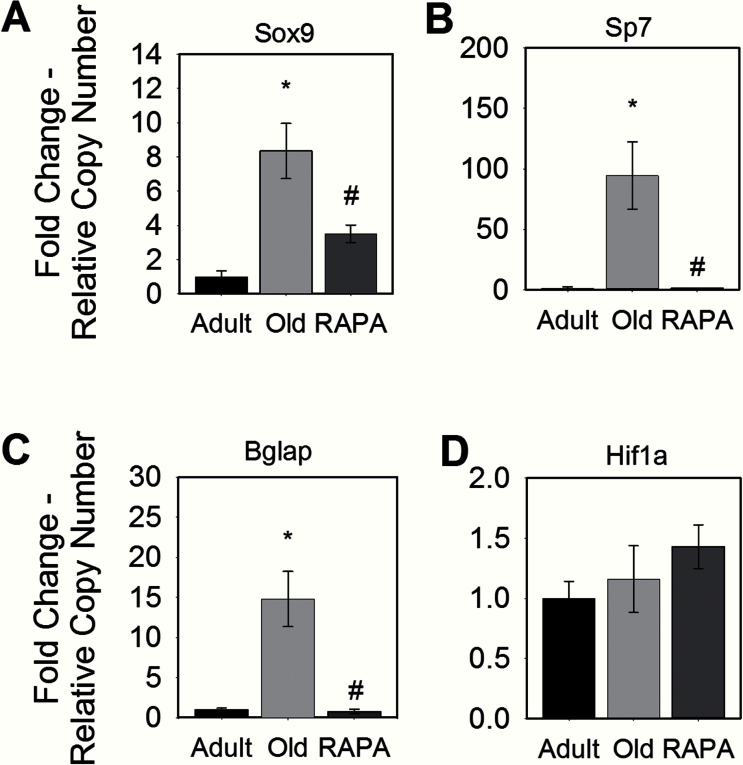
Alizarin Red staining revealed minimal calcification of adult Achilles tendons, with 1.4±1.6% of the tendon area calcified. With aging, the calcified area of Achilles tendons of old mice significantly increased to 15.2±4.1% (Figure 4). Tendons from old eRAPA mice, however, showed significantly decreased calcification as compared with age-matched controls, such that calcification of tendons of old eRAPA mice was not different from that observed for tendons of adult controls. Consistent with the observation that fibroblasts from calcified avian tendon show chondrogenic and osteogenic phenotypes (29), mRNA levels for proteins indicative of bone (Sp7 and Bglap) and cartilage (SOX9) formation were elevated in tendons of old mice compared with adult mice, in which none of these three markers was detected (Figure 5). The reduced calcium deposition in tendons from old eRAPA-fed mice was associated with twofold lower levels of Sox9 expression compared with age-matched controls, and expression of Sp7 and Bglap was nearly eliminated in tendons from old eRAPA-fed mice. Although hypoxia has been proposed to be a driving factor in tendon calcification (30), no differences in Hif1 mRNA levels were observed in the present study between any of the groups.
Figure 4.
Area calcified in mouse Achilles tendons, as revealed with Alizarin Red staining. Tendons from old mice were extensively calcified, whereas tendons from young mice showed minimal calcification. Rapamycin-fed mice exhibited minimal tendon calcification equal to that from tendons of adult mice. Scale bar = 250 μm. Data are presented as means ± SEM. *Significantly different from adult. #Significantly different from old.
Figure 5.
The effect of rapamycin (RAPA) on mouse Achilles tendon gene expression of proteins important in bone and cartilage formation. Compared with age-matched controls, tendons from RAPA-fed mice showed decreased expression of the chondrocyte and osteoblast markers (A) Sox9 and (B) Sp7, respectively. RAPA also attenuated the expression of the bone marker (C) Bglap. (D) Expression of the hypoxia marker Hif1a was unchanged with RAPA. Data are presented relative to adult expression levels and are presented as means ± SEM. *Statistically different from adult. #Statistically different from old.
Discussion
A major finding of the present study was that age-associated changes in tendon mechanics previously observed at 28–30 months of age in the mouse TA tendon (15) occur much earlier in the life span. In the present study, the changes in mechanical properties were observed a full 6 months sooner than previous studies, with the mechanical changes developing first in the proximal tendon region. In addition, the previous findings were in C57BL/6 mice, whereas the present study was performed on UM-HET3 mice, indicating that the previous observations generalize beyond a single mouse strain. We also demonstrated that long-term administration of rapamycin delayed age-associated changes in both the elastic and viscous properties of tendons while maintaining cell density the tissue. In addition, our observations of a dramatically elevated expression of MMP-8 and a moderately decreased expression of collagen I for tendons of old eRAPA treated mice compared with old control mice are consistent with the conclusion that rapamycin has an effect to modulate the expression of proteins important in collagen turnover throughout the life span. Finally, treatment with eRAPA reduced the extent of age-associated spontaneous calcification of the Achilles tendons. Overall, rapamycin appears to affect numerous cellular and molecular processes that regulate the structural and functional properties of tendon extracellular matrix resulting in the prevention or delay of detrimental changes typically observed with aging.
The ability of rapamycin to extend life span has been investigated extensively, with increased longevity seen in response to treatment with rapamycin in flies (31) and mice (22,23,32). Given that neoplastic disease is responsible for the death of ~80% of UM-HET3 mice (23), the effect of rapamycin to extend life span in these mice likely represents an effect limiting the development of cancers. Although rapamycin may indeed have important effects on processes affecting neoplasia, the observations in the present study of RAPA’s ability to regulate extracellular matrix composition, coupled with previous reports on modulation of aging effects on myocardial, uterine, and thyroid pathology (24) by rapamycin, strongly suggest that the drug has a general effect to modulate a wide range of age-related processes. New evidence also suggests that rapamycin may have a positive influence on age-associated behavior and physiological outcomes as well, even when fed late in life (33). Additional compounds, such as acarbose, nordihydroguaiaretic acid, and 17-alpha-estradiol, have been shown to enhance life span in mice (34), and examination of the ability of these compounds to modulate a wide array of age-related processes in a manner similar to that of rapamycin is an important line for future investigation.
Our observation that dietary administration of eRAPA slowed or prevented both the age-associated increase in tendon stiffness and the loss of capacity for energy storage as indicated by the decrease in hysteresis indicates that the underlying structure and/or composition of the tendon extracellular matrix is altered by rapamycin. Tendon is composed primarily of fibrillar type I collagen. Collagen has an extremely slow turnover rate (35), allowing for the accumulation of post-translation modifications that can compromise the structural and mechanical integrity of collagen-rich tissues, like tendons. These modifications include glucose-mediated permanent crosslinks such as pentosidine and carboxymethyllysine, both of which increase in concentration in a linear fashion with aging (36). The presence of these permanent crosslinks also reduces collagen turnover (37), which is consistent with reports of reduced mRNA expression with aging for type I collagen (16,17) and MMP-8 (17) in old rodents. Although the current study did not show an age-associated reduction in the expression of type I collagen or MMP-8 in tendons from 22-month-old UM-HET3 mice, previous reports used older, inbred strains of rodents (16,17). It is therefore likely that age-related attenuation of collagen and MMP-8 expression occurs at a later age in UM-HET3 strain of mice than was examined in the present study.
The hypothesis that rapamycin may act to alter the expression profile of proteins important for collagen turnover in aging through the suppression of mTOR is supported by previous reports that mTOR is an important regulator of extracellular matrix proteins, including MMPs (20). Indeed, COL1 expression and protein levels have been shown to decrease, and MMP-1 expression increased in cultured fibroblasts following treatment with rapamycin (20,38). The present investigation demonstrated similar changes in tendon tissue, with a 40% decrease in COL1A1 and a 110-fold increase in MMP-8 expression in tendons of old eRAPA-fed mice compared with age-matched controls. Coupled with decreased expression of COL1A1, increased MMP-8 levels may accelerate the degradation of existing collagen, reducing the potential for the development of post-translational modifications in the collagen and enhancing collagen turnover. eRAPA also prevented the age-associated decline in cell density in the region of the tendon in which the most dramatic protective effects of rapamycin on mechanical properties were observed. Overall, these data suggest that rapamycin contributes to the preservation of pathways responsible for the maintenance of extracellular matrix mechanical integrity through 22 months of age in UM-HET3 mice. This maintenance is likely due to the preservation of cell density within the tissue, allowing for the increased levels of collagen degradation and thus reducing the accumulation of detrimental post-translational modifications. It should be noted that the expression profiles of COL1A1 and MMP-8 resulting in the maintenance of mechanical properties in the old eRAPA-fed mice were not identical to those seen in adult control mice. This observation indicates that multiple molecular pathways can similarly influence the mechanical properties of collagen-rich tissue.
In addition to the observations in the present study of an impact of long-term eRAPA administration on processes regulating the structural and functional properties of tendon extracellular matrix, there is emerging evidence that rapamycin could serve as a promising therapeutic agent for other soft tissue pathologies. For example, short-term treatment with rapamycin reverses pulmonary artery cell proliferation in a rodent model of pulmonary hypertension (39). In addition, 2 weeks of rapamycin administration has been shown to improve motor performance as measured using a rotarod coordination test in mice deficient in the nuclear envelope protein lamin A (Lmna−/− mice) that exhibit severe skeletal muscular dystrophy (40). Moreover, in an injury model of murine osteoarthritis, 10 weeks of RAPA treatment reduced the severity of the disease (41). Of great interest with respect to aging processes is the observation that the effects of rapamycin on life span are very similar whether the drug is initiated at 20 months or at 9 months of age (42). Thus, studies to determine whether the age-dependent changes in tendon properties might be reversed in mice given rapamycin at advanced ages are warranted.
The observation in the present study of age-associated calcification of Achilles tendons of genetically heterogeneous UM-HET3 mice expands on our previous observation of extensively calcified tendons in 28- to 30-month-old C57BL/6 mice (16) and demonstrates the presence of tendon calcification at an even earlier age and in mice of a genetically heterogeneous stock. Although the mechanisms underlying age-associated calcification of tendon are largely unclear, previous reports have suggested that fibroblasts from calcified avian tendons show chondrogenic and osteogenic phenotypes (29). Consistent with this possibility is the present observation of highly elevated expression of markers for chondrocytes (Sox9), osteoblasts (Sp7), and bone formation (Bglap) in calcified tendons from old mice compared with adult mice, which showed minimal expression of all three markers. Notably, in tendons from old mice fed eRAPA, expression of all three genes was maintained at levels not different from adult tendons. Hypoxia has also been suggested as a driving factor in tendon calcification (30); however, we saw no difference in Hif1a expression in tendons from any group. Overall, our findings indicate that age-associated tendon calcification likely involves chondrogenic and/or osteogenic processes but not hypoxia.
Our study does have some limitations. The mice used in this study were part of a larger investigation that required animal sacrifice at 22 months of age. The use of this age group precluded direct comparison between these data and previously published reports on 28-month-old TA tendons in inbred mice (15,16). However, given the observed age-associated mechanical and structural changes in the tendons, we are confident that the reported data adequately describe the effect of eRAPA on aged tendon mechanical and structural properties. The small size and low mRNA yields from mouse tendons precluded regional gene expression data from being obtained. Additionally, although our gene expression data are consistent with an increase in collagen turnover in eRAPA treated mice compared with age-matched control mice, we have not measured collagen synthesis or degradation directly. Finally, the present study only examined the influence of long-term eRAPA administration on tendon properties.
In summary, we demonstrated that long-term administration of rapamycin through dietary eRAPA delays the age-associated changes in TA tendon mechanical properties, as evidenced by the maintenance of adult levels of stiffness through at least 22 months of age for the rapamycin-treated mice, in contrast to tendons of age-matched untreated mice that showed increased stiffness levels. The protective effects appear to be mediated through the maintenance of fibroblast density and collagen degradation, which is dramatically suppressed with normal aging. Additionally, we provide evidence that age-associated tendon calcification is likely driven by chondrogenic and osteogenic processes that are both inhibited by rapamycin. This study has provided evidence that the mechanisms underlying the aging of tendon tissue are dependent at least in part by the actions of mTOR and that the inhibition of this pathway results in delayed aging of the tissue through the maintenance of structural and mechanical integrity of the tissue from adulthood to old age.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by the National Institute of Health (NIH) Grant AG020591 to S.V.B., AG022303 to R.A.M., and AG000114, which provided fellowship support to L.W.Z.
Supplementary Material
Acknowledgements
We thank Sabrina van Roekel and Lisa Burmeister for help with obtaining the tissue.
References
- 1. Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi:10.1113/jphysiol.1988.sp017279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat. 2006;208:433–443. doi:10.1111/j.1469-7580.2006.00548.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gomez-Cabello A, Ara I, Gonzalez-Aguero A, Casajus JA, Vicente-Rodriguez G. Effects of training on bone mass in older adults. Sports Med. 2012;42:301–325. doi:10.2165/11597670-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 4. Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi:10.1152/physrev.00031.2003 [DOI] [PubMed] [Google Scholar]
- 5. Rees JD, Wilson AM, Wolman RL. Current concepts in the management of tendon disorders. J Rheumatol. 2006;45:508–521. doi:10.1093/ rheumatology/kel046 [DOI] [PubMed] [Google Scholar]
- 6. Tempelhof S, Rupp S, Seil R. Age-related prevalence of rotator cuff tears in asymptomatic shoulders. J Shoulder Elbow Surg. 1999;8:296–299. doi:10.1016/S1058-2746(99)90148-9 [DOI] [PubMed] [Google Scholar]
- 7. Yamamoto A, Takagishi K, Osawa T, et al. Prevalence and risk factors of a rotator cuff tear in the general population. J Shoulder Elbow Surg. 2010;19:116–120. doi:10.1016/j.jse.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 8. Tallon C, Maffulli N, Ewen SW. Ruptured Achilles tendons are significantly more degenerated than tendinopathic tendons. Med Sci Sports Exerc. 2001;33:1983–1990. doi:10.1097/00005768-200112000-00002 [DOI] [PubMed] [Google Scholar]
- 9. Kearney RS, Achten J, Lamb SE, Parsons N, Costa ML. The Achilles tendon total rupture score: a study of responsiveness, internal consistency and convergent validity on patients with acute Achilles tendon ruptures. Health Qual Life Outcomes. 2012;10:24. doi:10.1186/1477-7525-10-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schibany N, Zehetgruber H, Kainberger F, et al. Rotator cuff tears in asymptomatic individuals: a clinical and ultrasonographic screening study. Eur J Radiol. 2004;51:263–268. doi:10.1016/S0720-048X(03)00159-1 [DOI] [PubMed] [Google Scholar]
- 11. Mackey DC, Robinovitch SN. Mechanisms underlying age-related differences in ability to recover balance with the ankle strategy. Gait Posture. 2006;23:59–68. doi:10.1016/j.gaitpost.2004.11.009 [DOI] [PubMed] [Google Scholar]
- 12. Bojsen-Møller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol (1985). 2005;99:986–994. doi:10.1152/japplphysiol.01305.2004 [DOI] [PubMed] [Google Scholar]
- 13. Nestorson J, Movin T, Möller M, Karlsson J. Function after Achilles tendon rupture in the elderly: 25 patients older than 65 years followed for 3 years. Acta Orthop Scand. 2000;71:64–68. doi:10.1080/00016470052943928 [DOI] [PubMed] [Google Scholar]
- 14. Turan A, Teber MA, Yakut ZI, Unlu HA, Hekimoglu B. Sonoelastographıc assessment of the age-related changes of the Achilles tendon. Med Ultrason. 2015;17:58–61. doi:10.11152/mu.2013.2066.171.ayt [DOI] [PubMed] [Google Scholar]
- 15. Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol (1985). 2011;111:999–1006. doi:10.1152/japplphysiol.00460.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wood LK, Brooks SV. Ten weeks of treadmill running decreases stiffness and increases collagen turnover in tendons of old mice. J Ortho Res. In press. doi:10.1002/jor.22824 [DOI] [PubMed] [Google Scholar]
- 17. Kostrominova TY, Brooks SV. Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age (Dordr). 2013;35:2203–2214. doi:10.1007/s11357-013-9514-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit achilles tendon. Exp Diabesity Res. 2004;5:143–153. doi:10.1080/15438600490277860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iagnocco A, Filippucci E, Sakellariou G, et al. Ultrasound imaging for the rheumatologist XLIV. Ultrasound of the shoulder in healthy individuals. Clin Exp Rheumatol. 2013;31:165–171. [PubMed] [Google Scholar]
- 20. Goc A, Choudhary M, Byzova TV, Somanath PR. TGFβ- and bleomycin-induced extracellular matrix synthesis is mediated through Akt and mammalian target of rapamycin (mTOR). J Cell Physiol. 2011;226:3004–3013. doi:10.1002/jcp.22648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Bokov A, Gelfond J, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69:119–130. doi:10.1093/gerona/glt056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi:10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller RA, Harrison DE, Astle CM, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi:10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi:10.1111/j.1474-9726.2012.00832.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schmelzle T, Hall MN. TOR, a central controller of cell growth. Cell. 2000;103:253–262. doi:10.1016/S0092-8674(00)00117-3 [DOI] [PubMed] [Google Scholar]
- 26. Riley GP, Curry V, DeGroot J, et al. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002;21:185–195. doi:10.1016/S0945-053X(01)00196-2 [DOI] [PubMed] [Google Scholar]
- 27. Woessner JF. Determination of hydroxyproline in tissue and protein samples containing small properties of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi:10.1016/0003-9861(61)90291-0 [DOI] [PubMed] [Google Scholar]
- 28. Rutledge RG, Stewart D. A kinetic-based sigmoidal model for the polymerase chain reaction and its application to high-capacity absolute quantitative real-time PCR. BMC Biol. 2008;8:47. doi:10.1186/1472-6750-8-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Agabalyan NA, Evans DJ, Stanley RL. Investigating tendon mineralisation in the avian hindlimb: a model for tendon ageing, injury and disease. J Anat. 2013;223:262–277. doi:10.1111/joa.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jozsa L, Balint BJ, Reffy A. Calcifying tendinopathy. Arch Orthop Traum Surg. 1980;97:305–307. doi:10.1007/BF00380713 [DOI] [PubMed] [Google Scholar]
- 31. Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi:10.1242/dev.01255 [DOI] [PubMed] [Google Scholar]
- 32. Neff F, Flores-Dominguez D, Ryan DP, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi:10.1172/JCI67674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carter CS, Khamiss D, Matheny M, et al. Rapamycin versus intermittent feeding: dissociable effects on physiological and behavioral outcomes when initiated early and late in life. J Gerontol A Biol Sci Med Sci. Epub ahead of print. doi:10.1093/gerona/glu238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harrison DE, Strong R, Allison DB, et al. Acarbose, 17-α-estradiol, and nordihydroguaiaretic acid extend mouse lifespan preferentially in males. Aging Cell. 2014;13:273–282. doi:10.1111/acel.12170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Babraj JA, Cuthbertson DJ, Smith K, et al. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab. 2005;289:E864–E869. doi:10.1152/ajpendo.00243.2005 [DOI] [PubMed] [Google Scholar]
- 36. Hansen P, Haraldsson BT, Aagaard P, et al. Lower strength of the human posterior patellar tendon seems unrelated to mature collagen cross-linking and fibril morphology. J Appl Physiol (1985). 2010;108:47–52. doi:10.1152/japplphysiol.00944.2009 [DOI] [PubMed] [Google Scholar]
- 37. DeGroot J, Verzijl N, Wenting-Van Wijk MJ, et al. Age-related decrease in susceptibility of human articular cartilage to matrix metalloproteinase-mediated degradation: the role of advanced glycation end products. Arthritis Rheum. 2001;44:2562–2571. doi:10.1002/ 1529-0131(200111)44:11<2562::AID-ART437>3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- 38. Poulalhon N, Farge D, Roos N, et al. Modulation of collagen and MMP-1 gene expression in fibroblasts by the immunosuppressive drug rapamycin. A direct role as an antifibrotic agent? J Biol Chem. 2006;281:33045–33052. doi:10.1074/jbc.M606366200 [DOI] [PubMed] [Google Scholar]
- 39. Houssaini A, Abid S, Mouraret N, et al. Rapamycin reverses pulmonary artery smooth muscle cell proliferation in pulmonary hypertension. Am J Respir Cell Mol Biol. 2013;48:568–577. doi:10.1165/rcmb.2012-0429OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ramos FJ, Chen SC, Garelick MG, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. doi:10.1126/scitranslmed.3003802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caramés B, Hasegawa A, Taniguchi N, Miyaki S, Blanco FJ, Lotz M. Autophagy activation by rapamycin reduces severity of experimental osteoarthritis. Ann Rheum Dis. 2012;71:575–581. doi:10.1136/ annrheumdis-2011-200557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi:10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.