Abstract
Background:
Slow gait is a robust biomarker of health and a predictor of functional decline and death in older adults, yet factors contributing to the decline in gait speed with aging are not well understood. Previous research suggests that the energetic cost of walking at preferred speed is inversely associated with gait speed, but whether individuals with a rising energetic cost of walking experience a steeper rate of gait speed decline has not been investigated.
Methods:
In participants of the Baltimore Longitudinal Study of Aging, the energetic cost of overground walking at preferred speed (mL/kg/m) was assessed between 2007 and 2014 using a portable indirect calorimeter. The longitudinal association between the energetic cost of walking and usual gait speed over 6 meters (m/s) was assessed with multivariate linear regression models, and the risk of slow gait (<1.0 m/s) was analyzed using Cox proportional hazards models.
Results:
The study population consisted of 457 participants aged 40 and older who contributed 1,121 person-visits to the analysis. In fully adjusted models, increases in the energetic cost of walking predicted the rate of gait speed decline in those older than 65 years (β = −0.008 m/s, p < .001). Moreover, those with a higher energetic cost of walking (>0.17mL/kg/m) had a 57% greater risk of developing slow gait compared with a normal energetic cost of walking (≤0.17mL/kg/m; adjusted hazard ratio = 1.57, 95% confidence interval: 1.01–2.46).
Conclusions:
These findings suggest that strategies to maintain walking efficiency hold significant implications for maintaining mobility in late life. Efforts to curb threats to walking efficiency should focus on therapies to treat gait and balance impairments, and reduce clinical disease burden.
Keywords: Functional performance, Gait, Metabolism, Physical function, Physiology
Slow gait speed is a well-established predictor of multiple adverse health outcomes, including physical function and cognitive decline, disability, and death in older adults (1–3). Assessing changes in gait speed over time helps track clinical progression of chronic diseases, establish prognoses, and make decisions about treatment regimens in older patients (4). Although gait speed is a valid biomarker of health and functional status that is widely used in research and clinical settings, the physiological mechanisms that determine the decline in gait speed with aging and disease are not well defined due to their complex, multifaceted nature (5).
Throughout adulthood, usual—or preferred—gait speed tends to average 1.1–1.3 m/s (6). A well-established body of literature has suggested that this speed is unconsciously selected to minimize energy expenditure and maximize walking efficiency (6–9). This is consistent with comparative studies across species showing that preferred speed of mobility has evolved to maximize energy efficiency and endurance and preserve life (10,11). Indeed, recent cross-sectional studies in relatively large cohorts of older men and women have suggested that the energetic cost of walking at preferred speed is higher with advancing age and is associated with gait speed decline (12–15).
A high energetic cost of walking may be particularly problematic in frail older persons with multiple chronic diseases who have both limited aerobic capacity and greater basal energy demands from chronic medical conditions (16–18). It has been hypothesized that individuals who face incremental increases in the energetic cost of walking (and of movement in general) may be compelled to reduce energy consumption, for example, by walking slower and/or curtailing unnecessary physical activity (8,12,19). This hypothesis cannot be fully tested in cross-sectional studies because of the strong possibility of reverse causality, namely that decline in gait speed defines low energetic efficiency because of reduced biomechanical optimization or similar factors. Thus, it is important to examine whether a higher energetic cost of walking is a risk factor for age-related gait speed decline or, in other terms, whether those individuals who develop a high energetic cost of walking are at a greater risk of developing slow gait—and eventually mobility limitations—compared with those who maintain a normal energetic cost of walking.
Because of the robust association between gait speed and age, and the independent, clinical association between gait speed and multiple health outcomes, defining physiologic predictors of gait speed decline is of utmost importance to elucidating opportunities for maintaining mobility and preventing disability. Consequently, the purpose of this study was to assess the longitudinal association between the energetic cost of walking at preferred speed and usual gait speed in community-dwelling adults aged 40–97 years participating in the Baltimore Longitudinal Study of Aging (BLSA).
Methods
The BLSA is a study of normative human aging, established in 1958 and supported by the National Institute on Aging Intramural Research Program (NIA–IRP). General descriptions of the sample and the enrollment procedures and criteria have been previously reported (20). Briefly, the BLSA constitutes a continuously enrolled cohort with some targeted recruitment (eg, women, racial minorities) over its 57-plus–year history. All participants are community volunteers who must pass a comprehensive health and functional screening evaluation and be free of all major chronic conditions and cognitive and functional impairment at enrollment. Once enrolled, participants undergo extensive testing every 1–4 years depending on their age and are followed for life. The BLSA began collecting data on the energetic cost of walking at preferred speed in 2007. The population for the current study consists of 457 men and women with two or more clinic visits assessed between July 2007 and March 2014. The Internal Review Board of the National Institute of Environmental Health Sciences approved the study protocol, and all participants provided written informed consent.
All participants completed a physical examination and health history assessment. Weight and height were measured according to standard protocols, and body mass index was calculated as weight in kilograms divided by height in meters squared. Total body dual-energy x-ray absorptiometry was performed using a Prodigy Scanner (GE, Madison, WI) and analyzed with version 10.51.006 software. The presence of chronic conditions was assessed by nurse practitioners and established according to information on medical history, drug treatment, and physical examination. Chronic conditions included in the analysis were cardiovascular disease (history of heart disease or cardiac surgery, including myocardial infarction, congestive heart failure, angina, coronary artery bypass graph, and angioplasty), cerebrovascular disease (history of stroke or transient ischemic attack), pulmonary disease (history of chronic bronchitis, emphysema, chronic obstructive pulmonary disease, or asthma), diabetes (self-reported past diagnosis and current medication for diabetes), arthritis (self-reported lower extremity arthritis pain), and balance difficulty (reported balance difficulty while walking on a level surface).
Assessment of Usual Gait Speed
Usual gait speed was assessed by asking participants to walk at their “usual, comfortable pace” over a 6-meter course in an uncarpeted corridor. Participants stood with their feet behind a taped starting line. After a command of “Go,” timing was initiated with the first foot-fall over the starting line and stopped after the first foot-fall over the finish line. Two timed trials were conducted to derive usual gait speed in m/s, with the faster used for analysis.
Assessment of Walking Energy Expenditure at Preferred Speed
Walking energy expenditure (mL/kg/minute) was assessed via a portable indirect calorimeter (Cosmed k4b2, Cosmed, Rome, Italy) during 2.5 minutes of overground walking at a preferred pace, the warm-up component of a modified long-distance corridor walk, a validated measure of cardiorespiratory fitness in older adults (21). Each participant performed the test on a 20-meter course marked by traffic cones at each end in an uncarpeted corridor while wearing the Cosmed k4b2. Participants were instructed to walk at their “usual comfortable pace” around the course in a continuous loop until directed to stop. Participants stood with their feet behind a taped starting line. After a command of “Go,” timing was initiated with the first foot-fall over the line and stopped after 2.5 minutes of walking. Distance covered in meters was recorded.
The Cosmed k4b2 unit continuously collects and analyzes oxygen consumption and carbon dioxide production using breath-by-breath measurement and averages these measures over 30-second intervals to reduce variability. Preferred walking energy expenditure was calculated as the average volume of oxygen consumed per kilogram of body weight per minute (VO2 mL/kg/minute) during the 2.5-minute walking test. To calculate average VO2 (mL/kg/minute), readings from the first 1.5 minutes of testing were discarded to allow the participant to adjust to the workload and achieve a metabolic steady state, and data from the remaining minute were averaged to arrive at a single measure of the average VO2 (mL/kg/minute) at preferred walking speed (14).
Calculation of the Energetic Cost of Walking at Preferred Speed
The energetic cost of walking at preferred speed (mL/kg/m) was derived by taking the average VO2 (mL/kg/minute) at preferred walking speed and standardizing it by the number of meters walked during the 2.5-minute walking test:
Statistical Analysis
Descriptive analyses were performed to assess general characteristics of the study population. Fitted plots were created to assess trends among age, the energetic cost of walking, and usual gait speed at baseline (Figures 1 and 2). Based on the appearance of these figures, correlation analyses between age (stratified at 65 years) and the energetic cost of walking and gait speed (stratified at 1.0 m/s) and the energetic cost of walking were performed to assess the magnitude and trends of the associations between these variables.
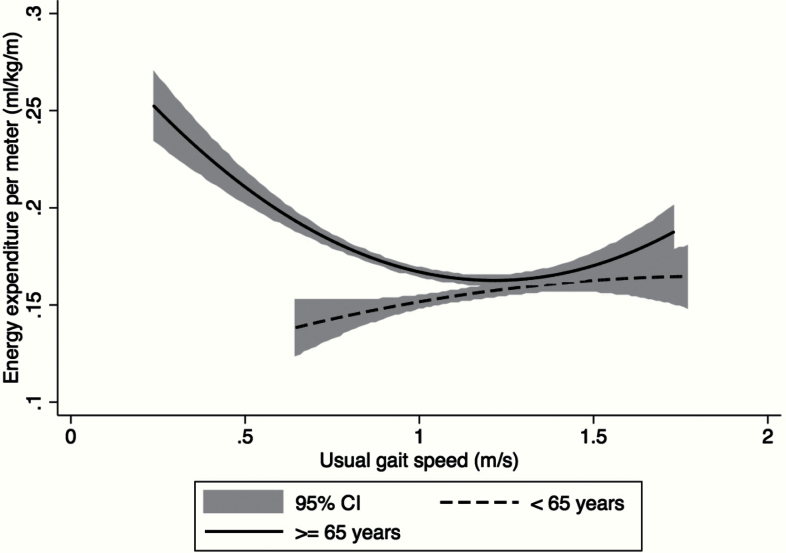
Figure 1.
The baseline association between the energetic cost of walking (mL/kg/m) and usual gait speed (m/s) stratified at age 65. Two fitted lines with 95% confidence intervals (CIs) display the associations between the energetic cost of walking (mL/kg/m) and usual gait speed (m/s) in those: (i) younger than 65 years (dashed line) and (ii) 65 years and older (solid line).
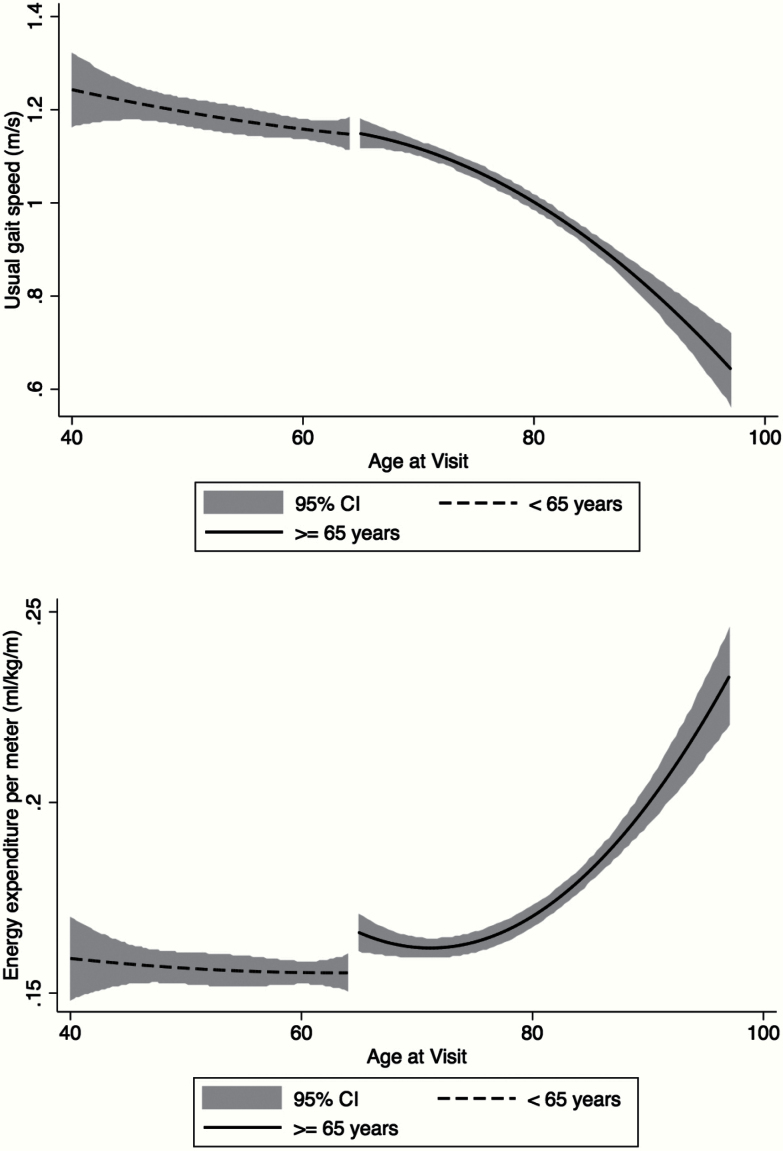
Figure 2.
(A) The baseline association between age and gait speed decline. A fitted curve with 95% confidence intervals (CIs) displays the association between usual gait speed (m/s) and advancing age in those: (i) younger than 65 years (dashed line) and (ii) 65 years and older (solid line). (B) The baseline association between age and the energetic cost of walking at preferred speed. A fitted curve with 95% CIs displays the association between the energetic cost of walking at preferred speed (mL/kg/m) and advancing age in those: (i) younger than 65 years (dashed line) and (ii) 65 years and older (solid line).
Based on these results, the longitudinal association between the energetic cost of walking and usual gait speed was modeled continuously using age-stratified generalized estimating equations and a working autoregressive correlation (Order 1) to take into account within-subject autocorrelation of repeated measures. Covariates included age (centered at 40 years [Model A] and 65 years [Model B]), sex, body mass index, body composition (ratio of fat-to-lean mass from dual-energy x-ray absorptiometry), race, history of smoking, coronary heart disease, stroke, diabetes, pulmonary disease, arthritis, and balance difficulty. The selection of variables introduced in the multivariable model was guided by multiple criteria: (i) confounders with face validity; (ii) confounders identified in previous studies; and (iii) confounders significantly associated with usual gait speed in univariate analyses. Variables included in the final models were restricted to those with statistical significance (p < .05) (Table 2).
Kaplan–Meier survival estimates and adjusted Cox proportional hazard models were used to estimate differences in time from baseline (time of first walking energy expenditure assessment) to slowed gait stratified by a “high” or “low” energetic cost of walking (Figure 3). Slow gait speed was defined as gait speed below 1.0 m/s because this threshold has been associated with increased risk of mobility disability, hospitalization, and death in previous work (2). The energetic cost of walking was stratified at 0.17mL/kg/m, to be consistent with previous laboratory studies of walking efficiency in older adults (7,8). All analyses were conducted using Stata SE version 13 (Statacorp, College Station, TX).
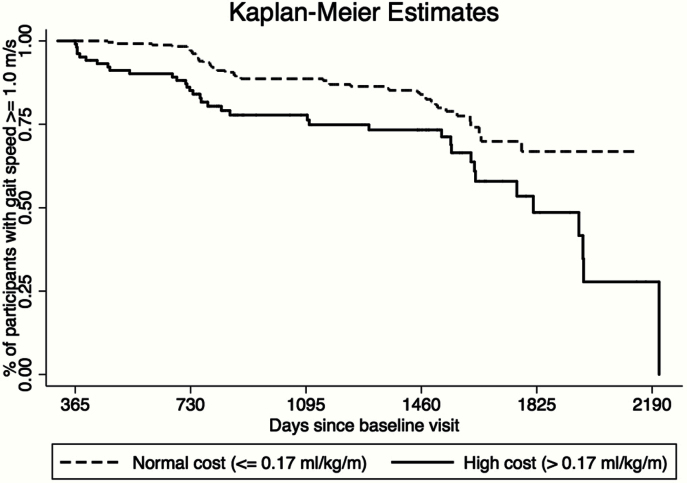
Figure 3.
Kaplan–Meier estimates of the time to slow gait (<1.0 m/s) stratified by the energetic cost of walking. A Kaplan–Meier estimate shows the difference in time to slow gait speed stratified by the energetic cost of walking. Those who have a normal cost (<0.17mL/kg/m) are shown using a dashed line and those who have a high cost (≥0.17mL/kg/m) are shown using a solid line. The log-rank test to compare differences in survival distributions revealed a significantly faster time to slow gait among those with a higher energetic cost of walking (p < .001).
Results
The study population consisted of 457 participants aged 40 and older who had two or more clinic visits between July 2007 and March 2014. These participants contributed 1,121 person-visits to the analysis. The mean number of visits per participant was 2.5 (range: 2–6). Baseline characteristics are outlined in Table 1. The most common chronic condition was lower extremity arthritis, with 38.3% of the sample reporting arthritis pain in the hips, knees, or feet. All other chronic conditions were relatively rare, with no more than 13% reporting a history of cardiovascular disease, stroke, pulmonary disease, diabetes, or balance difficulty. All participants were able to complete 2.5 minutes of walking at preferred speed without stopping and without a walking aid. Those with a higher energetic cost of walking at baseline were older, and were more likely to be male, of white race, and have slightly less fat mass. There was also a nonsignificant trend toward a greater prevalence of cardiovascular disease and lower extremity arthritis in those with a higher energetic cost of walking (p < .15).
Table 1.
Baseline Demographics of Study Population (N = 457)
| Characteristic | Overall (SD / %) | Normal Cost (SD / %) | High Cost (SD / %) | p Value |
|---|---|---|---|---|
| N = 457 | n = 182 | n = 275 | ||
| Age (y) | 70.3 (10.3) | 67.0 (9.8) | 72.2 (9.9) | <.001 |
| Male sex (no.) | 233 (51.0%) | 79 (43.6%) | 154 (56.1%) | .008 |
| Height (cm) | 169.4 (9.2) | 169.1 (9.0) | 169.6 (9.4) | .48 |
| Weight (kg) | 77.1 (15.3) | 78.1 (16.3) | 76.5 (14.7) | .30 |
| Fat mass (kg) | 26.7 (10.0) | 28.0 (10.4) | 25.9 (9.6) | .03 |
| Lean mass (kg) | 47.6 (9.8) | 47.1 (9.7) | 47.9 (9.8) | .33 |
| Non-White race | 146 (32.5%) | 74 (40.3%) | 72 (25.3) | .001 |
| Usual gait speed (m/s) | 1.07 (0.2) | 1.08 (0.2) | 1.06 (0.2) | .33 |
| Walking O2 consumption (mL/minute) | 973.6 (252.0) | 855.6 (244.1) | 1050.2 (239.5) | <.001 |
| Walking CO2 production (mL/minute) | 786.7 (210.7) | 694.9 (180.5) | 846.4 (207.7) | <.001 |
| Walking O2 consumption per kg (mL/kg/minute) | 12.5 (2.5) | 10.8 (1.8) | 13.7 (2.3) | <.001 |
| Energetic cost of walking (mL O2/kg/m) | 0.17 (0.03) | 0.14 (0.01) | 0.19 (0.02) | <.001 |
| Smoking (no.)a | 9 (1.9%) | 3 (1.6%) | 6 (2.1%) | .71 |
| Cardiovascular disease (no.)b | 56 (12.3%) | 17 (9.4%) | 39 (14.0%) | .12 |
| Cerebrovascular disease (no.)c | 21 (4.6%) | 8 (4.8%) | 13 (4.7%) | .73 |
| Pulmonary disease (no.)d | 19 (4.2%) | 7 (3.7%) | 12 (4.4%) | .37 |
| Diabetes (no.)e | 37 (8.1%) | 15 (8.5%) | 22 (8.0%) | .81 |
| Arthritis (no.)f | 175 (38.3%) | 62 (34.0%) | 113 (41.1%) | .11 |
| Poor balanceg | 49 (10.7%) | 16 (8.8%) | 33 (11.8%) | .32 |
Notes. aSelf-reported history of smoking on a regular basis.
bSelf-reported history of heart disease or cardiac surgery, including myocardial infarction, congestive heart failure, angina pectoris, coronary artery bypass graft, and angioplasty.
cSelf-reported history of stroke or transient ischemic attack.
dSelf-reported history of pulmonary disease.
eSelf-reported past diagnosis of and current medication for diabetes.
fSelf-reported lower extremity arthritis pain.
gReported difficulty keeping balance when walking on a level surface.
Figure 1 displays the age-stratified, fitted associations (and 95% confidence intervals) between average usual gait speed and the energetic cost of walking at preferred speed at baseline. The curvilinear association in those aged 65 and older (solid line) has been suggested by previous studies in older populations (6,22) and confirms that a higher energetic cost of walking is associated with slower gait speed. Figure 2 displays the age-stratified, fitted associations (and 95% confidence intervals) between: (a) average usual gait speed and age and (b) the average energetic cost of walking at preferred speed and age at baseline. Gait speed was substantially constant through age 65 (Figure 2A, dashed line) and then markedly slower at older ages (Figure 2A, solid line). The energetic cost of walking at preferred speed displayed a similar trend to the gait speed curve, with a sharp rise in the energetic cost of walking after the age of 65 (Figure 2B, solid line).
Unadjusted correlation analyses between age (stratified at 65 years) and the energetic cost of walking at preferred speed showed no association in those younger than 65 years (r = −.03, p = .47) and a positive association in those aged 65 and older (r = .28, p < .001). Moreover, correlation analyses between usual gait speed (stratified at 1.0 m/s) and the energetic cost of walking at preferred speed revealed a mild positive association in those who walked faster (r = .07, p = .04) but a strong negative association in those who walked slower (r = −.30, p < .001). Based on these results, generalized estimating equations stratified at age 65 were used to assess the longitudinal association between the energetic cost of walking at preferred speed and usual gait speed in middle-aged and older adults.
Among those aged 40–65 years at baseline (Table 2, Model A), the energetic cost of walking did not significantly affect change in gait speed (p = .55). The only variables that significantly contributed to the model were body composition, race, and history of lower extremity arthritis pain. However, among those aged 65 and older at baseline (Table 2, Model B), gait speed declined to an average of 0.008 m/s for each one-unit (0.01mL/kg/m) increase in the energetic cost of walking (p < .001), after adjusting for age (centered at 65 years), body composition, race, history of lower extremity arthritis pain, and reported balance difficulty. Variables for sex, body mass index, smoking, and history of coronary heart disease, stroke, diabetes, and pulmonary disease did not contribute significantly to the model fit in either age group and were removed from the final models.
Table 2.
Association Between the Energetic Cost of Walking and Gait Speed Decline Modeled Using Generalized Estimating Equations With a Working Autoregressive Correlation Structure and Stratified at a Baseline Age of 65 Years
| Dependent Variable: Usual Gait Speed | Model A | Model B | ||
|---|---|---|---|---|
| 40–65 years; n = 140 | 65 years and older; n = 317 | |||
| Independent Variables | β | p Value | β | p Value |
| Intercept | 1.234 | <.001 | 1.456 | <.001 |
| EEM (per 0.01mL/kg/m) | 0.002 | .55 | −0.008 | <.001 |
| Centered age | 0.001 | .69 | −0.012 | <.001 |
| Body composition | −0.140 | .01 | −0.191 | <.001 |
| Non-White race | −0.050 | .03 | −0.060 | .01 |
| Lower extremity arthritis pain | −0.058 | .01 | −0.020 | .15 |
| Poor balance | −0.039 | .34 | −0.055 | <.001 |
Notes. The model shows the β coefficient and p value from a population average model assessing the decline in usual gait speed (m/s) for each 1-year increase in age, adjusting for body composition (ratio of fat-to-lean mass), race, lower extremity arthritis pain, and self-reported balance difficulty. Age was centered at 40 years in Model A and at 65 years in Model B. Variables for sex, BMI, history of smoking, cardiovascular disease, cerebrovascular disease, pulmonary disease, and diabetes were not significant and were not included in the final model.
BMI = body mass index; EEM = energy expenditure per meter.
To provide a clinical perspective, we dichotomized the energetic cost of walking as higher than 0.17mL/kg/m (high energetic cost of walking) versus 0.17mL/kg/m or less (normal energetic cost of walking) and tested the hypothesis that a high cost of walking would predict time to development of slow gait speed (<1.0 m/s) compared with normal energetic cost of walking. As shown in Figure 3, time to slow gait was significantly different between the groups, and those in the “high cost” were significantly more likely to develop slow gait speed than those in the “low cost” group (log-rank p < .001). Among the 359 participants whose usual gait speed was ≥1.0 m/s at baseline, Cox proportional hazard models were used to examine differences in the risk of slow gait using days since baseline as the time metric and adjusting for the variables significant in Model B (age, body composition, race, and reported balance difficulty). Participants with a high energetic cost of walking showed a 57% greater risk of slow gait compared with those with a low cost of walking (adjusted hazard ratio = 1.57, 95% confidence interval: 1.01–2.46).
Discussion
The findings from this study indicate that the energetic cost of walking at preferred speed rises progressively with advancing age and that this increase contributes to the age-related decline in gait speed. Over the last few years, efforts to understand and quantify age-associated mobility loss have focused on gait speed due to its strong predictive power on multiple adverse health outcomes (4,23,24). Yet, until the mechanisms of mobility loss are understood and methods to assess those mechanisms are identified, it will be difficult to develop preventive and therapeutic strategies that effectively delay mobility loss with aging. One of the interesting findings of this study was that the energetic cost of walking at preferred speed almost mirrored the decline in gait speed (Figure 2A and B), which is consistent with the hypothesis that walking efficiency is causally linked to future gait speed decline.
Although changes in gait speed are part of the aging process (6,8), an accumulation of comorbidities and conditions with advancing age provides avenues for pathological changes in gait speed that may accelerate functional decline (8). Recent findings from the Cardiovascular Health Study indicate that a faster rate of gait speed decline is accounted for by physiologic impairments over multiple organ systems (25). Similarly, a higher comorbidity burden has been linked to a higher resting metabolic rate, suggesting that in the presence of multiple chronic conditions, a greater percentage of daily energy expenditure is needed to maintain life (16,17), with less energy available for physical functioning (12). To this end, slowed gait may be a compensatory mechanism to counteract the accumulation of age-related changes in mitochondrial function, biomechanical factors, and neuromuscular function induced by aging and disease, while maintaining vital function and the ability to perform the most basic activities of daily living (8,22,26,27).
Different directions of the correlation between age and the energetic cost of walking in younger versus older adults suggest altered ability to recruit and utilize energy with age (28,29). The fully adjusted regression models demonstrate the detrimental association of incremental increases in the energetic cost of walking, particularly in those older than 65 years. It has been suggested that a change in gait speed of 0.05 m/s or more is clinically meaningful (30). Given the current results, an increase in the energetic cost of walking at preferred speed of 0.06mL/kg/m may contribute to meaningful gait speed decline. In daily life, this may contribute to an inability to perform activities related to independent living such as heavy household tasks, crossing the street, or climbing several flights of stairs, leading to an accelerated pathway to mobility disability (31).
Previous research on the association between the energetic cost of walking at preferred speed and gait speed decline has been limited to cross-sectional data, smaller samples, or confined to laboratory settings and the use of a treadmill, which can affect gait mechanics and thus may not accurately reflect energy expenditure while walking at preferred speed in the free-living environment (12,13,15,32–34). Our study examined the longitudinal relationship between the energetic cost of overground walking at preferred speed and usual gait speed decline over a wide age spectrum to provide a more accurate assessment of the energetic cost of mobility, and how it changes with age. Although the implications of this research suggest that slowing appears to be a compensatory mechanism in the presence of increasing energetic cost, whether this slowing actually moderates or reduces the energetic cost of walking was not directly tested as participants were asked to walk at their self-selected preferred walking speed. Future research should investigate the effectiveness of slowing down as a compensation for higher energetic cost.
This study is not without limitations. First, the participants in the BLSA are healthier than the general population, particularly the oldest participants. Although this may underestimate the true association between the energetic cost of walking and gait speed decline, particularly in those with joint and gait impairments, it reduces confounding of the association by comorbidities and conditions. Second, because testing is performed overground, there is no guarantee that participants walked at a uniform pace throughout the duration of the test. However, because individuals tend to walk at a speed that minimizes energy consumption (8,34), participants were able to achieve a metabolic steady state during testing, which logically translates to a nearly constant rate on a flat surface (14). Finally, it should be noted that the timeframe of this test (2.5 minutes) is shorter than what is typically used to assess the energetic cost of walking in laboratory studies and thus may reflect elements of aerobic and anaerobic metabolism. However, respiratory quotient values during testing consistently averaged 0.82 at 1.5 minutes, 0.81 at 2.0 minutes, and 0.82 at 2.5 minutes, and VO2/kg values were within 10% of each other (average: 11.5–12.2mL/kg/minute), providing good evidence of a consistent metabolic pattern.
Loss of mobility with age poses a substantive threat to quality of life and seriously impacts the capacity to live independently. Slow or slowing gait speed frequently proceeds and portends mobility limitations and disability (4,35,36). Identifying and reducing sources of walking inefficiency could lead to targeted interventions aimed at preventing the burden of mobility decline that affects many older individuals. Appropriate evaluation, training, and rehabilitation of the energetic cost of walking may provide a gateway by which to prevent mobility loss and preserve independence in an aging population.
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging. J.A.S was supported by K01AG048765 and HHSN311210300177P.
Conflict of Interest
There are no conflicts of interest for any authors to report.
Acknowledgments
Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging. J.A.S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Fried LP, Bandeen-Roche K, Chaves PH, Johnson BA. Preclinical mobility disability predicts incident mobility disability in older women. J Gerontol A Biol Sci Med Sci. 2000;55:M43–M52. [DOI] [PubMed] [Google Scholar]
- 2. Cesari M, Kritchevsky SB, Penninx BW, et al. Prognostic value of usual gait speed in well-functioning older people–results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi:10.1111/j.1532-5415.2005.53501.x [DOI] [PubMed] [Google Scholar]
- 3. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi:10.1001/jama.2010.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI Study. JAGS. 2000;48:1618–1625. doi:10.1111/j.1532-5415.2000.tb03873.x [DOI] [PubMed] [Google Scholar]
- 6. Larish DD, Martin PE, Mungiole M. Characteristic patterns of gait in the healthy old. Ann N Y Acad Sci. 1988;515:18–32. [DOI] [PubMed] [Google Scholar]
- 7. Waters RL, Lunsford BR, Perry J, Byrd R. Energy-speed relationship of walking: standard tables. J Orthop Res. 1988;6:215–222. [DOI] [PubMed] [Google Scholar]
- 8. Waters RL, Mulroy S. The energy expenditure of normal and pathologic gait. Gait Posture. 1999;9:207–231. doi:10.1016/S0966-6362(99)00009-0 [DOI] [PubMed] [Google Scholar]
- 9. Saunders JB, Inman VT, Eberhart HD. The major determinants in normal and pathological gait. J Bone Joint Surg Am. 1953;35:543–558. [PubMed] [Google Scholar]
- 10. Priede IG. Natural selection for energetic efficiency and the relationship between activity level and mortality. Nature. 1977;267:610–611. doi:10.1038/267610a0 [DOI] [PubMed] [Google Scholar]
- 11. Wax TM, Goodrick CL. Nearness to death and wheelrunning behavior in mice. Exp Gerontol. 1978;13:233–236. doi:10.1016/0531-5565(78)90017-7 [DOI] [PubMed] [Google Scholar]
- 12. Schrack JA, Simonsick EM, Ferrucci L. The energetic pathway to mobility loss: an emerging new framework for longitudinal studies on aging. J Am Geriatr Soc. 2010;58(suppl 2):S329–S336. doi:10.1111/j.1532-5415.2010.02913.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Richardson CA, Glynn NW, Ferrucci LG, Mackey DC. Walking energetics, fatigability, and fatigue in older adults: the study of energy and aging pilot. J Gerontol A Biol Sci Med Sci. 2015;70:487–494. doi:10.1093/gerona/glu146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schrack JA, Simonsick EM, Chaves PH, Ferrucci L. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc. 2012;60:1811–1816. doi:10.1111/j.1532-5415.2012.04153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fiser WM, Hays NP, Rogers SC, et al. Energetics of walking in elderly people: factors related to gait speed. J Gerontol A Biol Sci Med Sci. 2010;65A:1332–1337. doi:10.1093/gerona/glq137 [DOI] [PubMed] [Google Scholar]
- 16. Fabbri E, An Y, Schrack JA, et al. Energy metabolism and the burden of multimorbidity in older adults: results from the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2014;70:1297–1303. doi:10.1093/gerona/glu209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. “IDEAL” aging is associated with lower resting metabolic rate: the Baltimore Longitudinal Study of Aging. J Am Geriatr Soc. 2014;62:667–672. doi:10.1111/jgs.12740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim S, Welsh DA, Ravussin E, et al. An elevation of resting metabolic rate with declining health in nonagenarians may be associated with decreased muscle mass and function in women and men, respectively. J Gerontol A Biol Sci Med Sci. 2014;69:650–656. doi:10.1093/gerona/glt150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malatesta D, Simar D, Dauvilliers Y, et al. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J Appl Physiol. 2003;95:2248–2256. [DOI] [PubMed] [Google Scholar]
- 20. Stone JL, Norris AH. Activities and attitudes of participants in the Baltimore longitudinal study. J Gerontol. 1966;21:575–580. [DOI] [PubMed] [Google Scholar]
- 21. Simonsick EM, Fan E, Fleg JL. Estimating cardiorespiratory fitness in well-functioning older adults: treadmill validation of the long distance corridor walk. J Am Geriatr Soc. 2006;54:127–132. doi:10.1111/j.1532-5415.2005.00530.x [DOI] [PubMed] [Google Scholar]
- 22. VanSwearingen JM, Studenski SA. Aging, motor skill, and the energy cost of walking: implications for the prevention and treatment of mobility decline in older persons. J Gerontol A Biol Sci Med Sci. 2014;69:1429–1436. doi:10.1093/gerona/glu153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cummings SR, Studenski S, Ferrucci L. A diagnosis of dismobility–giving mobility clinical visibility: a Mobility Working Group recommendation. JAMA. 2014;311:2061–2062. doi:10.1001/jama.2014.3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Studenski S. Bradypedia: is gait speed ready for clinical use? J Nutr Health Aging. 2009;13:878–880. doi:10.1007/s12603-009-0245-0 [DOI] [PubMed] [Google Scholar]
- 25. Rosso AL, Sanders JL, Arnold AM, et al. Multisystem physiologic impairments and changes in gait speed of older adults. J Gerontol A Biol Sci Med Sci. 2015;70:319–324. doi:10.1093/gerona/glu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–957. doi:10.1172/JCI64125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hortobágyi T, Finch A, Solnik S, Rider P, DeVita P. Association between muscle activation and metabolic cost of walking in young and old adults. J Gerontol A Biol Sci Med Sci. 2011;66:541–547. doi:10.1093/gerona/glr008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Voorrips LE, van Acker TM, Deurenberg P, van Staveren WA. Energy expenditure at rest and during standardized activities: a comparison between elderly and middle-aged women. Am J Clin Nutr. 1993;58:15–20. [DOI] [PubMed] [Google Scholar]
- 29. Jones LM, Waters DL, Legge M. Walking speed at self-selected exercise pace is lower but energy cost higher in older versus younger women. J Phys Act Health. 2009;6:327–332. [DOI] [PubMed] [Google Scholar]
- 30. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi:10.1111/j.1532-5415.2006.00701.x [DOI] [PubMed] [Google Scholar]
- 31. Knaggs JD, Larkin KA, Manini TM. Metabolic cost of daily activities and effect of mobility impairment in older adults. J Am Geriatr Soc. 2011;59:2118–2123. doi:10.1111/j.1532-5415.2011.03655.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pearce ME, Cunningham DA, Donner AP, Rechnitzer PA, Fullerton GM, Howard JH. Energy cost of treadmill and floor walking at self-selected paces. Eur J Appl Physiol. 1983;52:115–119. doi:10.1007/BF00429037 [DOI] [PubMed] [Google Scholar]
- 33. Parvataneni K, Ploeg L, Olney SJ, Brouwer B. Kinematic, kinetic and metabolic parameters of treadmill versus overground walking in healthy older adults. Clin Biomech. 2009;24:95–100. doi:10.1016/j.clinbiomech.2008.07.002 [DOI] [PubMed] [Google Scholar]
- 34. Martin PE, Rothstein DE, Larish DD. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J Appl Physiol (1985). 1992;73:200–206. [DOI] [PubMed] [Google Scholar]
- 35. Cunningham DA, Rechnitzer PA, Pearce ME, Donner AP. Determinants of self-selected walking pace across ages 19 to 66. J Gerontol. 1982;37:560–564. [DOI] [PubMed] [Google Scholar]
- 36. Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower extremity function in persons over the age of 70 years as a predictor of subsequent disability. New Engl J Med. 1995;332:556–561. doi:10.1056/NEJM199503023320902 [DOI] [PMC free article] [PubMed] [Google Scholar]