Abstract
Inhibition of the mTOR (mechanistic target of rapamycin) signaling pathway by the FDA-approved drug rapamycin promotes life span in numerous model organisms and delays age-related disease in mice. However, the utilization of rapamycin as a therapy for age-related diseases will likely prove challenging due to the serious metabolic and immunological side effects of rapamycin in humans. We recently identified an intermittent rapamycin treatment regimen—2mg/kg administered every 5 days—with a reduced impact on glucose homeostasis and the immune system as compared with chronic treatment; however, the ability of this regimen to extend life span has not been determined. Here, we report for the first time that an intermittent rapamycin treatment regimen starting as late as 20 months of age can extend the life span of female C57BL/6J mice. Our work demonstrates that the anti-aging potential of rapamycin is separable from many of its negative side effects and suggests that carefully designed dosing regimens may permit the safer use of rapamycin and its analogs for the treatment of age-related diseases in humans.
Keywords: Anti-aging, Life span, Mice, mTOR, Rapamycin
Inhibition of the mechanistic target of rapamycin (mTOR) complex 1 (mTORC1) by rapamycin extends life span in mice even when treatment begins late in life (1,2). Excitingly, rapamycin also prevents or delays the onset of age-related diseases, rejuvenates the aging heart, ameliorates age-related cognitive decline, and promotes health in mouse models of Alzheimer’s disease and progeria (3–11). Unfortunately, rapamycin and its analogs have a number of potentially serious side effects in humans, including immunosuppression and metabolic dysfunction, which may preclude the long-term use of rapamycin as an anti-aging therapy (12–14). One possible strategy to decrease the side effects of chronic rapamycin treatment is to allow for frequent drug holidays, and indeed rapamycin administration for 2 weeks out of every 4 also extends life span in mice (15,16). However, the impact of rapamycin on glucose homeostasis persists for at least 2 weeks following cessation of treatment, and thus relatively short drug holidays likely do not substantially limit the duration of side effects (17,18).
Although the canonical target of rapamycin, mTORC1, is acutely sensitive to rapamycin, we recently discovered that long-term treatment with rapamycin also inhibits mTOR complex 2 (mTORC2), in vivo, resulting in glucose intolerance and hepatic insulin resistance (19–21). Rapamycin inhibits mTORC2 in many metabolically active tissues, including the liver, adipose tissue, skeletal muscle, and pancreas and also potently inhibits mTORC2 in immune cells (20,22–25). Selective genetic inhibition of mTORC1 or mTORC2 demonstrates that inhibition of mTORC1 is sufficient to promote longevity, whereas inhibition of mTORC2 is associated with negative metabolic and immunological consequences, and even decreases the life span of male mice (12,26).
Importantly, disruption of mTORC2 occurs only after prolonged exposure to rapamycin (27). We recently hypothesized that the differential kinetics of mTORC1 and mTORC2 inhibition by rapamycin might create a therapeutic window through which intermittent administration of rapamycin might more specifically target mTORC1 (28). We determined that intraperitoneal administration of 2mg/kg rapamycin once every 5 days had a substantially reduced impact on glucose homeostasis and the immune system in C57BL/6J mice. Although we observed sustained inhibition of mTORC1 in some tissues, the ultimate efficacy of this alternative treatment regimen as an anti-aging intervention was not determined.
In this study, we treated C57BL/6J female mice with 2mg/kg rapamycin once every 5 days starting at 20 months of age. We assessed glucose homeostasis, body composition, and energy expenditure and determined mean and maximal life span. We find that this alternative treatment regimen significantly extends both average and maximum life span in C57BL/6J female mice. Importantly, we did not observe negative impacts of this regimen on glucose homeostasis, body composition, or metabolism.
Materials and Methods
Animals and Treatments
Animal studies were approved by the Institutional Animal Care and Use Committee of the University of Wisconsin–Madison and the William S. Middleton Memorial Veterans Hospital, Madison, WI. C57BL/6J.Nia female mice were purchased from the National Institute of Health Aging Rodent Colony at approximately 19 months of age and were left unperturbed for 1 month. At approximately 20 months of age, mice were randomly assigned to receive either vehicle (3.3mL/kg of 0.9% NaCl, 5% PEG400, 5% Tween 20, and 3% ethanol) or 2mg/kg rapamycin (in vehicle) intraperitoneally once every 5 days. Glucose and insulin tolerance tests were performed by fasting the mice overnight for 16 hours and then injecting either glucose (1g/kg) or insulin (0.75U/kg) intraperitoneally. Glucose measurements were performed using a Bayer Contour blood glucose meter and test strips. Mouse body composition was determined using an EchoMRI 3-in-1 Body Composition Analyzer (Houston, TX) according to the manufacturer’s procedures. For assays of food consumption, metabolic parameters, and activity tracking, mice were acclimated to housing in a Columbus Instruments Oxymax/CLAMS metabolic chamber system (Columbus, OH) for approximately 24 hours, and data from a continuous 24-hour period were then recorded and analyzed.
Life span Analysis
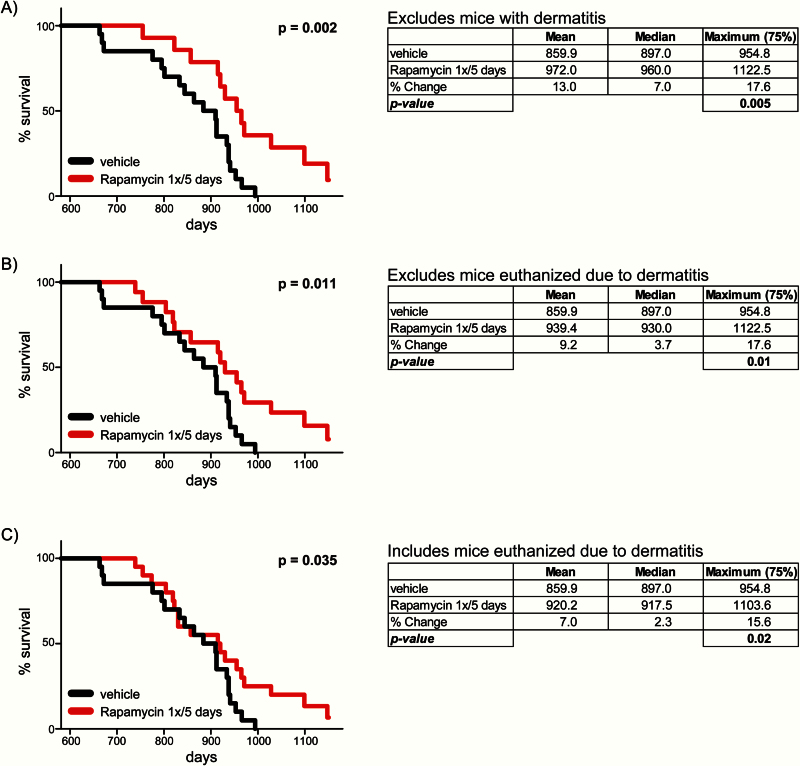
A total of 40 mice were used in the life span study with 20 mice in the control group and 20 mice in the rapamycin-treated group. Mice provided by the NIA Aging Rodent Colony have a specified birth month but not a specified birthdate, and our analyses assume that all mice were born on the 15th day of the birth month. Mice were maintained in specific pathogen-free housing with at least two mice per cage and were euthanized for humane reasons if moribund. Mice found dead were also noted at each daily inspection. Six rapamycin-treated mice—all of the mice from two cages received from the NIA Aging Rodent Colony—demonstrated severe dermatitis and were removed from the study; necropsy revealed fatal neoplastic disease in four of these animals. These animals were excluded from the analysis in Figure 1A but were included in the analysis in Figure 1B and C. Kaplan–Meier survival analysis was performed and a one-tailed log-rank test was performed using Prism 6 (GraphPad Software). We computed maximum life span at the 75th percentile using a one-tailed Boschloo’s unconditional exact test (29).
Figure 1.
Intermittent administration of rapamycin extends life span. (A) Kaplan–Meier plot showing life span of C57BL/6J.Nia female mice administered rapamycin (2mg/kg) or vehicle once every 5 days (n = 20 vehicle-treated mice, 14 rapamycin-treated mice; p = .002, one-tailed log-rank test). Mean, median, and maximum (75th percentile, p = .005, one-tailed Boschloo exact test) life span are shown; this analysis excludes six rapamycin-treated mice that developed dermatitis. (B) Life span analysis excluding only three rapamycin-treated mice euthanized due to dermatitis (n = 20 vehicle-treated mice, 17 rapamycin-treated mice; p = .011, one-tailed log-rank test; 75th percentile life span p = .01, one-tailed Boschloo exact test). (C) Life span analysis inclusive of every mouse that received vehicle or rapamycin, included those euthanized because of dermatitis (n = 20 vehicle-treated mice, 20 rapamycin-treated mice; p = .035, one-tailed log-rank test; 75th percentile life span p = .02, one-tailed Boschloo exact test).
Materials
Chemicals were purchased from Sigma unless noted. Rapamycin was purchased from LC Labs.
Results
We obtained aged female C57BL/6J mice from the National Institute on Aging Aged Rodent Colony and administered either rapamycin (2mg/kg) or vehicle once every 5 days beginning at 20 months of age. The analysis of Kaplan–Meier life span curves found a significant extension of life span, and we also observed a significant extension of maximum life span (Figure 1A). Of the mice intermittently treated with rapamycin, 29% survived the death of the longest-lived mouse receiving vehicle alone. In this analysis, we excluded two cages of mice in which all six of the mice developed severe dermatitis; the effect of intermittent rapamycin administration on both overall life span and maximum life span remains significant if we include those mice which contracted dermatitis but were not euthanized as a result (Figure 1B), or if we include all six of these mice, including those euthanized for dermatitis (Figure 1C). Life span data for each mouse are listed in Supplementary Table 1.
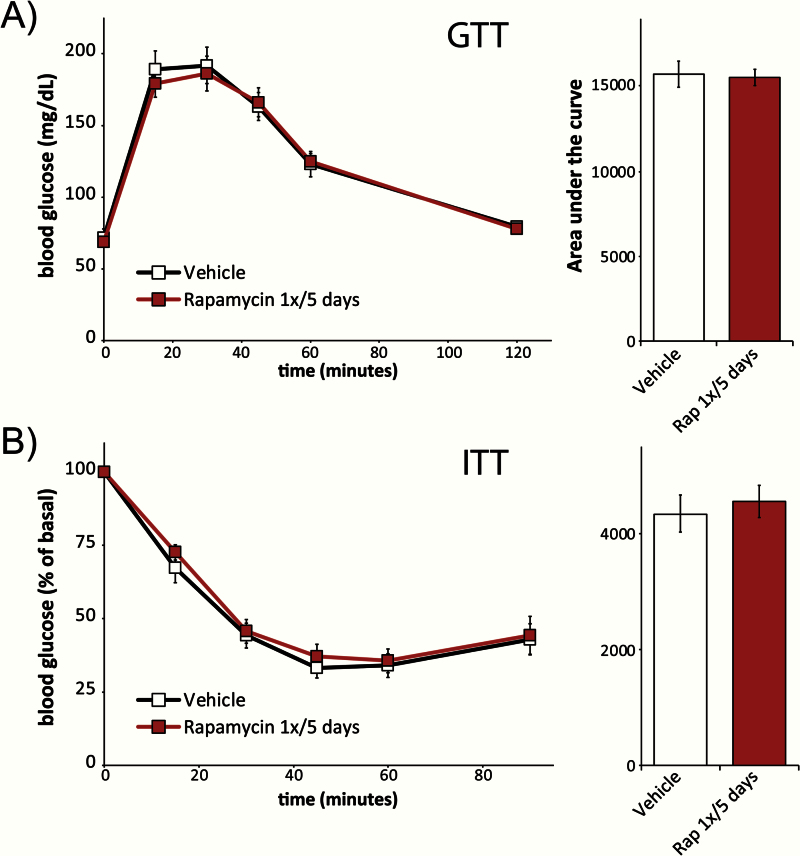
One of the most pronounced side effects of continuous rapamycin administration in rodents of all ages and genetic backgrounds is impaired glucose homeostasis (17–20,30–32). We assessed the effect of intermittent rapamycin administration on glucose homeostasis by performing fasting glucose (Figure 2A) and insulin (Figure 2B) tolerance tests after 8 weeks of treatment; each test was performed 5 days after the previous administration of rapamycin. Consistent with our recent demonstration that intermittent rapamycin administration had minimal impact on glucose homeostasis in young mice (28), we observed no difference in either glucose or insulin tolerance between vehicle-treated animals and animals treated intermittently with rapamycin. These results sharply contrast with the impaired glucose tolerance observed during continuous administration of rapamycin to mice (17–20,33).
Figure 2.
Intermittent administration of rapamycin does not impair glucose or insulin tolerance. (A) Glucose and (B) insulin tolerance test on female C57BL/6J.Nia mice treated with either vehicle or rapamycin (2mg/kg) once every 5 days for 8 weeks (n = 7–10 vehicle, n = 10 rapamycin, two-tailed t test). Tests were performed 5 days after the last administration of either vehicle or rapamycin, at the conclusion of an overnight fast. Error bars represent standard error.
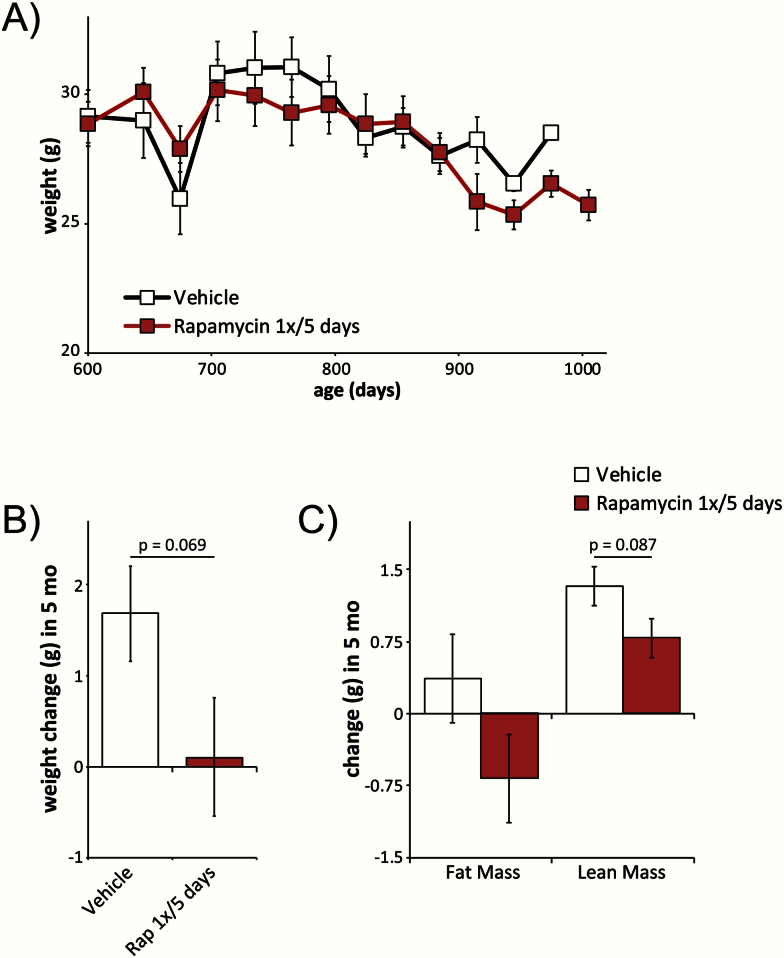
The effect of rapamycin on weight and body composition is unclear, with continuous rapamycin administration associated with increased body weight and adiposity when given to mice fed a high-fat diet (17) and intermittent rapamycin administration associated with decreased body weight and beneficial metabolic effects in other recent studies (34,35). We measured weight and body composition in all mice before commencing the experiment; in a subset of mice, we tracked the change in body weight on a monthly basis thereafter (Figure 3A). We saw no significant change in body weight at any time point. We determined the weight and body composition of all surviving mice at 750 days of age and calculated the change in weight, fat mass, and lean mass in mice over the first 5 months of the experiment. To avoid the confounding effects of weight loss prior to death, we excluded all mice that died in the following 8 weeks from our analysis. We observed a trend (p = .069) toward decreased weight gain in rapamycin-treated mice; consistent with this, we observed a trend (p = .087) toward reduced lean mass gain in mice treated with rapamycin (Figure 3B and C).
Figure 3.
Intermittent administration of rapamycin impacts body weight and composition. (A) Weight tracking of mice receiving vehicle or rapamycin (2mg/kg) once every 5 days (starting n = 10 vehicle-treated mice, 10 rapamycin-treated mice at 615 days of age). (B, C) Changes in weight and body composition after 5 months of intermittent administration of either vehicle or rapamycin (n = 15 vehicle-treated mice, 13 rapamycin-treated mice; p value shown for values between 0.05 and 0.1, two-tailed t test). Error bars represent standard error.
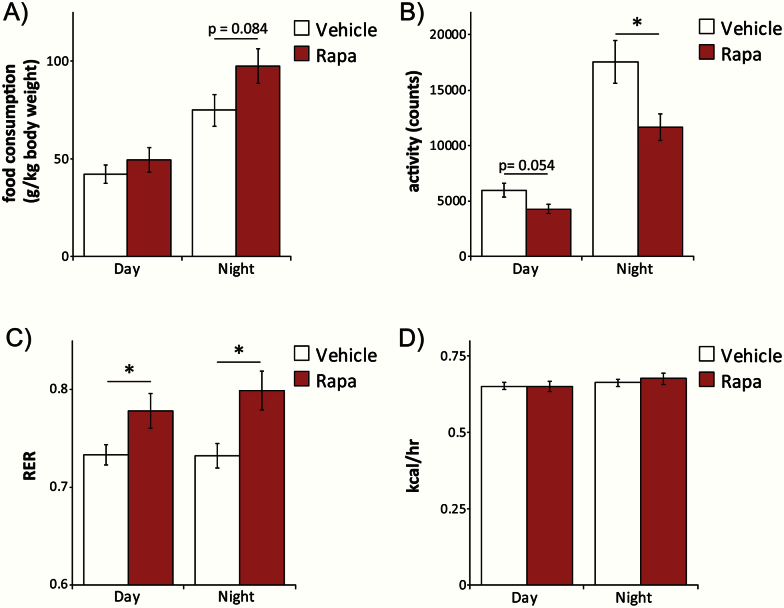
We placed mice at 25 months of age in metabolic chambers in order to assess food consumption, spontaneous activity, respiration, and energy expenditure. Intermittent rapamycin administration tended to increase food consumption during the dark cycle (Figure 4A), despite a decrease in spontaneous locomotor activity (Figure 4B). The respiratory exchange ratio (RER) indicates the predominant carbon source for ATP synthesis. Interestingly, RER was higher in mice intermittently receiving rapamycin than in vehicle-treated mice (Figure 4C), indicating preferential use of carbohydrates. However, we observed no significant difference in energy expenditure (Figure 4D).
Figure 4.
Intermittent administration of rapamycin alters food consumption, spontaneous activity, and respiratory exchange ratio (RER). Metabolic chambers were used to assess (A) food consumption, (B) spontaneous activity, (C) RER, and (D) calculated energy expenditure in mice administered either vehicle or rapamycin intermittently for 5 months. (n = 15 vehicle-treated mice, 12 rapamycin-treated mice; *p < .05, p value shown for values between 0.05 and 0.1, two-tailed t test). Error bars represent standard error.
Discussion
The discovery that continuous rapamycin treatment extends the life span of model organisms, including mice, has spurred significant interest in understanding the mechanistic basis for this effect as a means to avoid the many negative side effects associated with rapamycin in humans (12,14). We and others have shown that rapamycin disrupts not only mTORC1, the canonical target of rapamycin, but also mTORC2 in most tissues (20,24,27). Disruption of mTORC2 is likely responsible for many of the negative impacts of rapamycin on metabolism and the immune system, as well as decreased benefits shown in male mice treated with rapamycin (12,26).
Intermittent treatment with rapamycin has been suggested as a strategy that might reduce negative side effects while still extending life span (12,36,37), and indeed administration of rapamycin 2 weeks out of every 4 significantly extends the life span of female 129/Sv mice (16). Unfortunately, subsequent work suggests that the “washout period” for the metabolic side effects of rapamycin treatment is 2 weeks or greater (17,18), and therefore this type of intermittent regimen will likely not significantly reduce metabolic side effects. The persistence of the negative immunological effects of rapamycin has not been similarly established in mice, but a recent study found that short-term low-dose everolimus (a rapamycin analog) ameliorated immunosenescence in elderly humans and boosted the response to a vaccine given 2 weeks after the last dose of rapamycin (38), suggesting a “washout period” similar to the metabolic side effects. More recently, two groups have reported generally beneficial results from weekly administration of rapamycin, with Leontieva and colleagues finding that weekly administration of rapamycin can increase the median survival of C57BL/6NCr mice fed a 60% high-fat diet (34,35). While demonstrating the potential benefits of alternative rapamycin treatment regimens, the effect of weekly rapamycin administration on the longevity of animals on a healthy diet has yet to be determined.
In the present study, we tested intermittent administration of rapamycin as a pro-longevity intervention on aged C57BL/6J female mice, the life span of which can be extended by chronic dietary rapamycin when begun either early or late in life (1,39). As expected, administration of rapamycin once every 5 days had minimal negative effects on metabolism. We find that administration of rapamycin once every 5 days significantly extends life span as well as maximum life span. Fascinatingly, the increase in life span we observed (Figure 1A) was greater than that observed in mice of the same sex and genetic background fed rapamycin chronically from 19 months of age (1). It remains to be determined whether this difference was due to differences in route of administration or the reduced side effects of intermittent administration; in our previous study of this regimen (28), we determined that the blood level of rapamycin 3 days after intraperitoneal administration of 2mg/kg rapamycin was very similar to the blood level of rapamycin observed in C57BL/6J female mice that received 14ppm rapamycin in the diet (39). The mice in the present study were exposed to a higher peak level (and a lower trough level) of rapamycin compared with mice receiving chronic dietary rapamycin. The smaller number of mice used in our study, and the exclusion of animals with dermatological issues from our principle analysis, may have also contributed to this observation.
The effect of intermittent rapamycin on the RER, food consumption, and spontaneous activity was quite interesting. In contrast to many rapamycin studies that have observed decreased food intake (30), we observed a trend toward increased food intake. At the same time, we observed a decrease in spontaneous activity in mice receiving intermittent rapamycin (Figure 4B), which is in line with similar effects previously observed in aged female rapamycin-treated C57BL/6J.Nia mice (1). Inhibition of mTORC2 either by chronic rapamycin administration or by treatment with an mTOR kinase inhibitor decreases the RER (40,41), and thus the increase in RER we observed (Figure 4C) is in accordance with our current and previous data suggesting that mTORC2 is not inhibited by intermittent rapamycin administration. The increase in RER indicates a shift toward carbohydrate utilization and away from lipid oxidation. A similar increase in RER has previously been reported in aged male mice treated with rapamycin as resembling the RER seen in young mice (42). Interestingly, we did not observe a change in energy expenditure (Figure 4D), which suggests that the mice may be expending energy in other ways to offset their reduced activity, such as shivering thermogenesis due to an inhibition of white adipose beiging (43). Understanding the physiological and molecular basis for these effects on activity and metabolism is an area ripe for additional research.
Our data support our initial hypothesis that this alternative treatment regimen efficiently extends life span while avoiding the negative effects of chronic rapamycin on glucose tolerance and insulin sensitivity. The lack of effect of this regimen on glucose homeostasis, combined with a generally positive trend on body composition and weight, supports the suggestion that intermittent rapamycin administration could be useful for the treatment of metabolic diseases (34,35). An important unanswered question is the immunological consequences of prolonged intermittent rapamycin administration in either aged mice or humans; despite the potent immunosuppressive effects of short-term rapamycin treatment, a recent study suggests that long-term rapamycin treatment may extend mouse life span in part by boosting certain aspects of the immune response in the aged (44). In conclusion, our results suggest that an intermittent treatment regimen could be a translatable strategy for the safer use of rapamycin in humans for age-related diseases.
Supplementary Material
Please visit the article online at http://gerontologist.oxfordjournals.org/ to view supplementary material.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (R00 AG041765 to D.W.L.); the UW-Madison School of Medicine and Public Health; and the UW-Madison Department of Medicine. S.I.A.A is supported by a fellowship from the American Diabetes Association (1-16-PMF-001). This work was supported using facilities and resources at the William S. Middleton Memorial Veterans Hospital. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
Supplementary Material
Acknowledgments
We thank all the members of the Lamming laboratory for their assistance and insight, and the Davis, Kimple and Merrins laboratories for their continual support.
References
- 1. Zhang Y, Bokov A, Gelfond J, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69:119–130. doi:10.1093/gerona/glt056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi:10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Majumder S, Caccamo A, Medina DX, et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1beta and enhancing NMDA signaling. Aging Cell. 2012;11:326–335. doi:10.1111/j.1474-9726.2011.00791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979. doi:10.1371/journal.pone.0009979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dai DF, Karunadharma PP, Chiao YA, et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell. 2014;13:529–539. doi:10.1111/acel.12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ozcelik S, Fraser G, Castets P, et al. Rapamycin attenuates the progression of tau pathology in P301S tau transgenic mice. PLoS One. 2013;8:e62459. doi:10.1371/journal.pone.0062459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi:10.1111/j.1474-9726.2012.00832.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin AL, Jahrling JB, Zhang W, et al. Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with pre-symptomatic Alzheimer’s disease. J Cereb Blood Flow Metab. 2015. doi:10.1177/0271678X15621575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halloran J, Hussong SA, Burbank R, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113. doi:10.1016/j.neuroscience.2012.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lin AL, Zheng W, Halloran JJ, et al. Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33:1412–1421. doi:10.1038/jcbfm.2013.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramos FJ, Chen SC, Garelick MG, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. doi:10.1126/scitranslmed.3003802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest. 2013;123:980–989. doi:10.1172/JCI64099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trelinska J, Dachowska I, Kotulska K, Fendler W, Jozwiak S, Mlynarski W. Complications of mammalian target of rapamycin inhibitor anticancer treatment among patients with tuberous sclerosis complex are common and occasionally life-threatening. Anticancer Drugs. 2015;26:437–442. doi:10.1097/CAD.0000000000000207 [DOI] [PubMed] [Google Scholar]
- 14. Lamming DW. Inhibition of the mechanistic target of rapamycin (mTOR) – rapamycin and beyond. In: Olshansky SJ, Martin GM, Kirkland JL, eds. Aging: The Longevity Dividend. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2016:165–178. doi:10.1101/cshperspect.a025924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anisimov VN, Zabezhinski MA, Popovich IG, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176:2092–2097. doi:10.2353/ajpath.2010.091050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anisimov VN, Zabezhinski MA, Popovich IG, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi:10.4161/cc.10.24.18486 [DOI] [PubMed] [Google Scholar]
- 17. Liu Y, Diaz V, Fernandez E, et al. Rapamycin-induced metabolic defects are reversible in both lean and obese mice. Aging. 2014;6:742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang SB, Lee HY, Young DM, et al. Rapamycin induces glucose intolerance in mice by reducing islet mass, insulin content, and insulin sensitivity. J Mol Med. 2012;90:575–585. doi:10.1007/s00109-011-0834-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lamming DW, Ye L, Astle CM, Baur JA, Sabatini DM, Harrison DE. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell. 2013;12:712–718. doi:10.1111/acel.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi:10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sataranatarajan K, Ikeno Y, Bokov A, et al. Rapamycin increases mortality in db/db mice, a mouse model of type 2 diabetes. J Gerontol A Biol Sci Med Sci. 2015. doi:10.1093/gerona/glv170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delgoffe GM, Pollizzi KN, Waickman AT, et al. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nature Immunol. 2011;12:295–303. doi:10.1038/ni.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Festuccia WT, Pouliot P, Bakan I, Sabatini DM, Laplante M. Myeloid-specific Rictor deletion induces M1 macrophage polarization and potentiates in vivo pro-inflammatory response to lipopolysaccharide. PLoS One. 2014;9:e95432. doi:10.1371/journal.pone.0095432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schreiber KH, Ortiz D, Academia EC, Anies AC, Liao CY, Kennedy BK. Rapamycin-mediated mTORC2 inhibition is determined by the relative expression of FK506-binding proteins. Aging Cell. 2015;14:265–273. doi:10.1111/acel.12313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ye L, Varamini B, Lamming DW, Sabatini DM, Baur JA. Rapamycin has a biphasic effect on insulin sensitivity in C2C12 myotubes due to sequential disruption of mTORC1 and mTORC2. Front Genet. 2012;3:177. doi:10.3389/fgene.2012.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamming DW, Mihaylova MM, Katajisto P, et al. Depletion of Rictor, an essential protein component of mTORC2, decreases male lifespan. Aging Cell. 2014;13:911–917. doi:10.1111/acel.12256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sarbassov DD, Ali SM, Sengupta S, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi:10.1016/j.molcel.2006.03.029 [DOI] [PubMed] [Google Scholar]
- 28. Arriola Apelo SI, Neuman JC, Baar EL, et al. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell. 2016;15:28–38. doi:10.1111/acel.12405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev. 2004;125:629–632. doi:10.1016/j.mad.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 30. Houde VP, Brule S, Festuccia WT, et al. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi:10.2337/db09-1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi:10.1038/nature06322 [DOI] [PubMed] [Google Scholar]
- 32. Fang Y, Bartke A. Prolonged rapamycin treatment led to beneficial metabolic switch. Aging. 2013;5:328–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fang Y, Westbrook R, Hill C, et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. doi:10.1016/j.cmet.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell. 2014;13:616–622. doi:10.1111/acel.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Makki K, Taront S, Molendi-Coste O, et al. Beneficial metabolic effects of rapamycin are associated with enhanced regulatory cells in diet-induced obese mice. PLoS One. 2014;9:e92684. doi:10.1371/journal.pone.0092684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging. 2012;4:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Longo VD, Fontana L. Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging. 2011;3:1039–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mannick JB, Del Giudice G, Lattanzi M, et al. mTOR inhibition improves immune function in the elderly. Sci Transl Med. 2014;6:268ra179. doi:10.1126/scitranslmed.3009892 [DOI] [PubMed] [Google Scholar]
- 39. Fok WC, Chen Y, Bokov A, et al. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One. 2014;9:e83988. doi:10.1371/journal.pone.0083988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kleinert M, Sylow L, Fazakerley DJ, et al. Acute mTOR inhibition induces insulin resistance and alters substrate utilization in vivo. Mol Metabol. 2014;3:630–641. doi:10.1016/j.molmet.2014.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deblon N, Bourgoin L, Veyrat-Durebex C, et al. Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol. 2012;165:2325–2340. doi:10.1111/j.1476-5381.2011.01716.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Neff F, Flores-Dominguez D, Ryan DP, et al. Rapamycin extends murine lifespan but has limited effects on aging. J Clin Invest. 2013;123:3272–3291. doi:10.1172/JCI67674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tran CM, Mukherjee S, Ye L, et al. Rapamycin blocks induction of the thermogenic program in white adipose tissue. Diabetes. 2016. doi:10.2337/db15-0502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hurez V, Dao V, Liu A, et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell. 2015;14:945–956. doi:10.1111/acel.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.