Abstract
Guillain–Barré syndrome (GBS) is an acute demyelinating polyneuropathy, usually evoked by antecedent infection. Sarcoidosis is a multisystem chronic granulomatous disorder with neurological involvement occurring in a minority. We present a case of a 43-year-old Caucasian man who presented with acute ascending polyradiculoneuropathy with a recent diagnosis of pulmonary sarcoidosis. The absence of acute flaccid paralysis excluded a clinical diagnosis of GBS in the first instance. Subsequently, a rapid onset of proximal weakness with multi-organ failure led to the diagnosis of GBS, which necessitated intravenous immunoglobulin and plasmapheresis to which the patient responded adequately, and he was subsequently discharged home. Neurosarcoidosis often masquerades as other disorders, leading to a diagnostic dilemma; also, the occurrence of a GBS-like clinical phenotype secondary to neurosarcoidosis may make diagnosing coexisting GBS a therapeutic challenge. This article not only serves to exemplify the rare association of neurosarcoidosis with GBS but also highlights the need for a high index of clinical suspicion for GBS and accurate history taking in any patient who may present with rapidly progressing weakness to an Intensive Care Unit.
Keywords: Diagnosis, Guillain–Barrι syndrome, neurosarcoidosis
Introduction
Guillain-Barré syndrome (GBS) is an acute immune-mediated polyneuropathy, typically presenting with rapid ascending paralysis caused by an antecedent infection. Acute inflammatory demyelinating polyradiculoneuropathy (AIDP) is the most frequently encountered variant. Sarcoidosis is a multi-system noncaseating granulomatous disorder of unknown etiology presenting with bilateral hilar lymphadenopathy, pulmonary reticulonodular opacities, and skin, joint, or ocular lesions. A minority of patients with known sarcoidosis develop neurological complications, making neurosarcoidosis a diagnostic consideration.[1,2,3]
Case Report
A 43-year-old Australian presented with an acute progressive distal limb weakness and sensory alteration of both hands and feet. This occurred on a recent diagnosis of pulmonary sarcoidosis. This was accompanied by 3 months of blurred vision and significant weight loss. In addition, during this period, he was administered influenza vaccine. This information was subsequently unearthed during his readmission to the Intensive Care Unit (ICU). Inpatient investigations revealed elevated serum corrected calcium of 2.36 mmol/L (N: 1.10-1.30 mmol/L) and an angiotensin-converting enzyme (ACE) level of 108 u/L (N: 8-52 u/L), confirming advanced sarcoidosis.
Chest X-rays and high-resolution computed tomography scan of the chest showed extensive fine nodular patterns on both lungs. Sarcoidosis was confirmed by transbronchial biopsy results showing inflammation consistent with noncaseating granuloma. The patient's symptoms, apart from the blurred vision, improved with 50 mg of daily oral prednisolone. Two weeks postdischarge, he re-presented to the hospital with acute progressive limb weakness which started distally with gradual proximal involvement over 2 days. Differential diagnoses have been listed [Table 1].
Table 1.
Differential diagnoses for acute flaccid paralysis relevant to an Intensive Care Unit patient

Neurological examination of the upper and lower limbs showed global areflexia. Magnetic resonance imaging (MRI) of the brain and spine demonstrated abnormal enhancement of the cauda equina nerve roots and patchy white matter signal change representing inflammatory demyelination extending up to the T3 level of the thoracic cord, preferentially involving the ventral nerve roots [Figures 1 and 2]. Nerve conduction studies revealed demyelination with severe axonal polyneuropathy. Cerebrospinal fluid (CSF) findings showed acellular fluid with a mildly elevated protein level of 0.67 g/L (0.10-0.65 g/L) and normal ACE levels.
Figure 1.
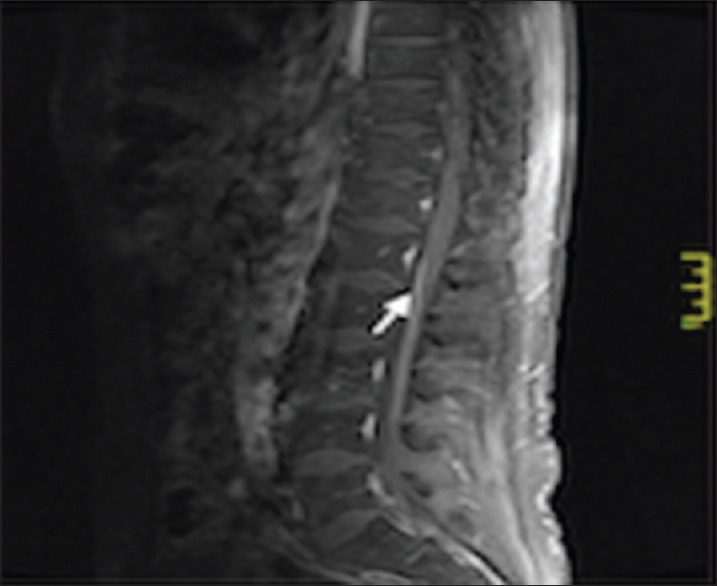
Magnetic resonance imaging of the spine showing high signal and linear contrast enhancement of the cauda equina
Figure 2.
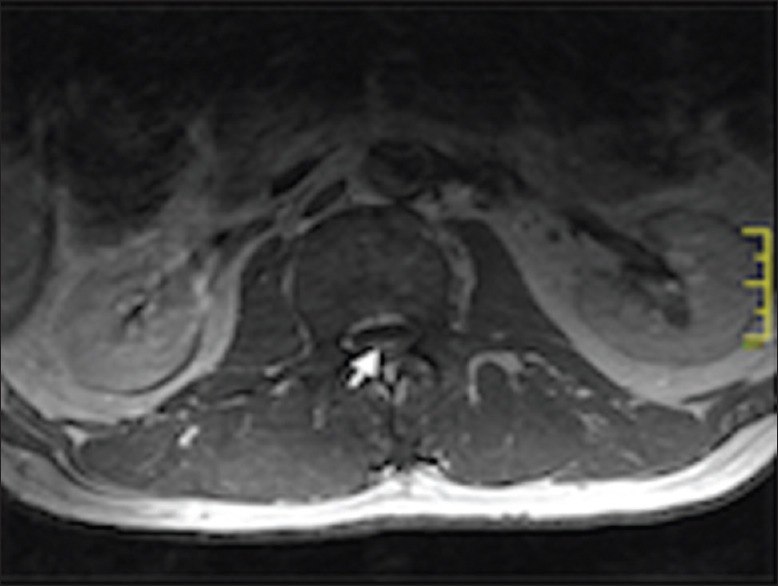
Magnetic resonance imaging of the spine showing enhancement preferentially of the ventral nerve roots
Based on these findings, a diagnosis of GBS was made, and the patient was started on a 5-day course of immunoglobulin. Over the next 24 h, he clinically deteriorated to MRC power 0/5 in all his limbs, with respiratory failure requiring ventilatory support. His stay was complicated by ventilator-associated pneumonia and he underwent a tracheostomy. As he failed to improve after a full dose of immunoglobulin, plasmapheresis was initiated. He showed progressive improvement and was transferred to a ward. He subsequently underwent plasma exchange every fortnight for six cycles.
Discussion
Sarcoidosis is a multisystem inflammatory disorder of unexplained etiology. In a prospective study from Australia, Allen et al., reported neurological involvement in 26% of patients with diagnosed sarcoidosis.[3] Other studies suggest 5-15%.[1,2] Neurological manifestations in a patient with known sarcoidosis should always prompt a possible diagnosis of neurosarcoidosis. The neuroimaging procedure of choice is contrast-enhanced MRI. The most common brain MRI finding of neurosarcoidosis is a basilar leptomeningeal involvement (in about 30-40%), which is usually seen as a thickening and diffuse or nodular enhancement.[4]
Nonspecific anomalies that support a diagnosis of neurosarcoidosis are elevated ACE, pleocytosis, reduced or normal glucose, high protein count (>0.5 g/L), elevated beta-2 microglobulin and increased IgG index with possible oligoclonal bands. High CSF values strongly support neurosarcoidosis. Axonal neuropathy is the most common finding.[2] Evidence of patchy demyelination with conduction block has been seen, which is most likely as a result of mechanical neural compression by granulomata.[5]
A risk estimate of one additional GBS case per million people vaccinated is found in the advisory committee on immunization practices recommendation and the vaccine information statement for influenza vaccine.[6] The period between vaccination and first symptom onset ranged could potentially range from as short as 3-5 days to 6-18 weeks, and up to a few months and even years.[7] Our patient received the influenza vaccine approximately 2 weeks before the onset of weakness. This correlated with the peak incidence of vaccine-associated neuromuscular weakness as differential diagnoses, even though it was hard to prove that the vaccine had definitely caused the axonal neuropathy. The other speculation with regards to the vaccination as a precipitant of the inflammatory polyradiculoneuropathy was the axonal pattern on the nerve conduction study as opposed to the typical demyelinating pattern with conduction blockade and reduced amplitude which occurs with AIDP. Furthermore, the H response and F wave were not particularly abnormal keeping in with a typical GBS, which happens following an infectious prodrome.
Neuroimaging studies and CSF analysis results were also consistent with GBS as opposed to neurosarcoidosis. The classical CSF findings in GBS would be acellularity with elevated CSF protein. Peripheral nerve conduction studies hold a prognostic value and frequently show classical demyelination but can show axonopathy and inexcitability in certain subgroups of GBS.[5,8]
MRI findings of GBS report marked enhancement of the thickened nerve roots in the conus medullaris and cauda equina, with no abnormalities on precontrast images. The marked enhancement indicates a breakdown of the blood-nerve barrier. It is thought to correlate with the inflammatory infiltration of GBS.[9,10] These MRI features were in contradistinction to the pachymenigeal and leptomeningeal enhancement which is typical of neurosarcoidosis along with involvement of the perivascular spaces, the circle of willis, and the cranial nerves.
The therapeutic cornerstone of management is immunosuppression. Corticosteroids are the first-line agents while other immunomodulatory and biological agents can be used.[1,2] This is in stark contrast to the treatment of GBS in which steroid therapy has no proven benefit. Conversely, intravenous immunoglobulin (IVIG) and plasma exchange have proven a benefit.[11,12,13]
Starting with IVIG or plasmapheresis would be a reasonable option in these patients, especially given the concern about a poor response with steroids for typical GBS. Nevertheless, if there is an unexpectedly high pleocytosis in CSF followed by additional confirmation of sarcoidosis, following up with further steroid treatment for neurosarcoidosis is highly recommended.
Conclusion
Neurosarcoidosis is not a self-fulfilling prophecy in a patient with sarcodiosis. The authors are happy to report this interesting case illustrating the neurological complications of sarcoidosis in a man with rapid onset peripheral neuropathy, which prompted further investigations for neurosarcoidosis and the eventual results that conversely supported a diagnosis of acute GBS. This case also illustrates the importance of accurate history taking in patients with extreme complexity, especially in an ICU environment, wherein the art of history taking is regrettably sidelined due to the vagaries of time, conflicting commitments and the absence of a reliable historian. Our report also highlights the temporal association between influenza vaccine and GBS, even though causality has not been proven.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest
Acknowledgment
We would like to acknowledge Dr. Janakan Ravindran and his contribution toward patient management and follow-up outside ICU.
References
- 1.Hoitsma E, Drent M, Sharma OP. A pragmatic approach to diagnosing and treating neurosarcoidosis in the 21 st century. Curr Opin Pulm Med. 2010;16:472–9. doi: 10.1097/MCP.0b013e32833c86df. [DOI] [PubMed] [Google Scholar]
- 2.Hoitsma E, Faber CG, Drent M, Sharma OP. Neurosarcoidosis: A clinical dilemma. Lancet Neurol. 2004;3:397–407. doi: 10.1016/S1474-4422(04)00805-1. [DOI] [PubMed] [Google Scholar]
- 3.Allen RK, Sellars RE, Sandstrom PA. A prospective study of 32 patients with neurosarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:118–25. [PubMed] [Google Scholar]
- 4.Hebel R, Dubaniewicz-Wybieralska M, Dubaniewicz A. Overview of neurosarcoidosis: Recent advances. J Neurol. 2015;262:258–67. doi: 10.1007/s00415-014-7482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saifee TA, Reilly MM, Ako E, Rugg-Gunn F, Brandner S, Lunn MP, et al. Sarcoidosis presenting as acute inflammatory demyelinating polyradiculoneuropathy. Muscle Nerve. 2011;43:296–8. doi: 10.1002/mus.21890. [DOI] [PubMed] [Google Scholar]
- 6.Vellozzi C, Iqbal S, Broder K. Guillain-Barre syndrome, influenza, and influenza vaccination: The epidemiologic evidence. Clin Infect Dis. 2014;58:1149–55. doi: 10.1093/cid/ciu005. [DOI] [PubMed] [Google Scholar]
- 7.Israeli E, Agmon-Levin N, Blank M, Chapman J, Shoenfeld Y. Guillain-Barré syndrome - A classical autoimmune disease triggered by infection or vaccination. Clin Rev Allergy Immunol. 2012;42:121–30. doi: 10.1007/s12016-010-8213-3. [DOI] [PubMed] [Google Scholar]
- 8.Burns TM, Dyck PJ, Aksamit AJ, Dyck PJ. The natural history and long-term outcome of 57 limb sarcoidosis neuropathy cases. J Neurol Sci. 2006;244:77–87. doi: 10.1016/j.jns.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Byun WM, Park WK, Park BH, Ahn SH, Hwang MS, Chang JC. Guillain-Barré syndrome: MR imaging findings of the spine in eight patients. Radiology. 1998;208:137–41. doi: 10.1148/radiology.208.1.9646804. [DOI] [PubMed] [Google Scholar]
- 10.Baran GA, Sowell MK, Sharp GB, Glasier CM. MR findings in a child with Guillain-Barré syndrome. AJR Am J Roentgenol. 1993;161:161–3. doi: 10.2214/ajr.161.1.8517296. [DOI] [PubMed] [Google Scholar]
- 11.Hughes RA, Swan AV, van Koningsveld R, van Doorn PA. Corticosteroids for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2006;2:CD001446. doi: 10.1002/14651858.CD001446.pub2. [DOI] [PubMed] [Google Scholar]
- 12.Hughes RA, Swan AV, van Doorn PA. Intravenous immunoglobulin for Guillain-Barré syndrome. Cochrane Database Syst Rev. 2012;7:CD002063. doi: 10.1002/14651858.CD002063.pub5. [DOI] [PubMed] [Google Scholar]
- 13.Hoyle JC, Jablonski C, Newton HB. Neurosarcoidosis: Clinical review of a disorder with challenging inpatient presentations and diagnostic considerations. Neurohospitalist. 2014;4:94–101. doi: 10.1177/1941874413519447. [DOI] [PMC free article] [PubMed] [Google Scholar]


