Summary
The recent association of Zika virus with cases of microcephaly has sparked a global health crisis and highlighted the need for mechanisms to combat the Zika vector, Aedes aegypti mosquitoes. Wolbachia pipientis, a bacterial endosymbiont of insect, has recently garnered attention as a mechanism for arbovirus control. Here we report that Aedes aegypti harboring Wolbachia are highly resistant to infection with two currently circulating Zika virus isolates from the recent Brazilian epidemic. Wolbachia-harboring mosquitoes displayed lower viral prevalence and intensity and decreased disseminated infection and, critically, did not carry infectious virus in the saliva, suggesting that viral transmission was blocked. Our data indicate that the use of Wolbachia-harboring mosquitoes could represent an effective mechanism to reduce Zika virus transmission and should be included as part of Zika control strategies.
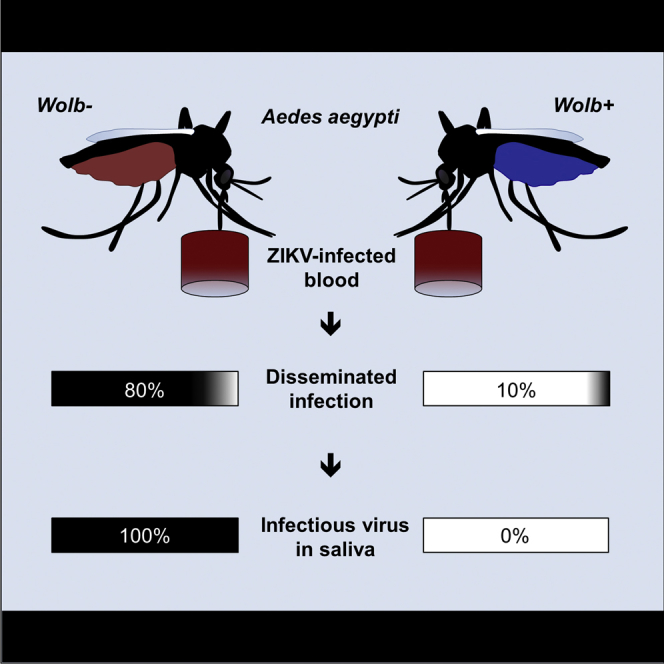
Graphical Abstract
Highlights
-
•
Mosquitoes harboring Wolbachia were resistant to current circulating Zika virus isolates
-
•
Zika virus prevalence, intensity, and disseminated infection were reduced
-
•
Saliva from Wolbachia-harboring mosquitoes did not contain infectious Zika virus
Strategies to combat Zika virus (ZIKV) and its mosquito vector are urgently needed. Dutra et al. report that Wolbachia-carrying mosquitoes are highly resistant to ZIKV and display reduced virus prevalence and intensity. Saliva from Wolbachia-carrying mosquitoes did not contain infectious virus, suggesting the possibility to block ZIKV transmission.
Main Text
The mosquito Aedes aegypti, typically linked with dengue (Flaviviridae) (Kyle and Harris, 2008) and chikungunya (Togaviridae) (Morrison, 2014) transmission, is also associated with the alarming spread of Zika virus (ZIKV) (Flaviviridae), a previously obscure arbovirus that has recently gone global (Enserink, 2015). Since 2007, ZIKV infection has been reported in 39 countries worldwide (Martínez de Salazar et al., 2016), including Brazil, where infection was first linked to cases of microcephaly during a large outbreak in 2015 (Mlakar et al., 2016, Oliveira Melo et al., 2016). Combined with the implication of the virus in cases of the autoimmune disorder Guillain-Barré syndrome (Araujo et al., 2016), ZIKV has ballooned into a public health crisis.
In the absence of a vaccine, current effective control options are limited to reducing the abundance of mosquito vector populations (Heintze et al., 2007). However, there is a clear need for novel efficacious approaches, given that existing strategies such as insecticides (Maciel-de-Freitas et al., 2014) and larval biological control (Vu et al., 2005) have proven unsustainable and ineffective at halting disease spread (Kyle and Harris, 2008).
After decades of being proposed as a potential means of vector control, the endosymbiotic bacterium Wolbachia, present in an estimated 40% of all known terrestrial insect species (Zug and Hammerstein, 2012), is currently being utilized around the world as part of an innovative approach to control the transmission of dengue (http://www.eliminatedengue.com) and other pathogens (Bourtzis et al., 2014). This is possible because the reproductive parasitism associated with Wolbachia infection, typified by cytoplasmic incompatibility (Werren et al., 2008), gives the bacterium the ability to quickly and stably invade host populations (Hoffmann et al., 2011). Critically, the bacterium also blocks the transmission of many important human pathogens in mosquitoes, including Plasmodium and chikungunya (Bian et al., 2013, Caragata et al., 2016, Moreira et al., 2009), giving it great utility as a control agent.
As many different strains of the bacterium cause this inhibition, we hypothesized that the wMel Wolbachia strain (wMel_Br), currently being utilized as part of dengue control efforts in Brazil, might be able to restrict ZIKV infection and transmission in Ae. aegypti. To that end, we performed experimental infections with two currently circulating ZIKV isolates and used a qRT-PCR-based assay to a quantify ZIKV levels in mosquito tissues and saliva, in order to assess whether Wolbachia could potentially be used to combat the emerging Zika pandemic.
Through experimental infection and transmission assays using two currently circulating Brazilian ZIKV isolates (BRPE243/2015 [BRPE] and SPH/2015 [SPH]) (Faria et al., 2016), we compared ZIKV infection in wMel-infected mosquitoes (wMel_Br) with Wolbachia-uninfected mosquitoes collected in Urca, Rio de Janeiro, Brazil in early 2016 (Br). Due to the regular introduction of F1 Br males (the eggs of field-collected Br mosquitoes) in wMel_Br colony cages over 2 years, both lines had a similar genetic background (see Supplemental Experimental Procedures).
The ZIKVs were isolated in the field in late 2015 and maintained in cell culture, and viral titers were quantified via plaque-forming assay prior to experimental infection (Table 1). In two separate experiments, fresh ZIKV-infected supernatant was harvested from culture, mixed with human blood, and used to orally infect wMel_Br and Br mosquitoes. ZIKV levels were quantified in mosquito heads/thoraces and in abdomens at 7 and 14 days post-infection (dpi) using a TaqMan-based qRT-PCR assay (Figure 1).
Table 1.
Effects of Wolbachia on ZIKV Prevalence
| Isolate | ZIKV Titer (PFU/mL) | Days Post-infection |
wMel_Br |
Br |
wMel_Br |
Br |
wMel_Br |
Br |
|---|---|---|---|---|---|---|---|---|
| Head/Thorax Infection Rate | Abdomen Infection Rate | Saliva Infection Rate | ||||||
| BRPE | 5.0 × 106 | 7 | 0 | 65 | 55 | 85 | – | – |
| 14 | 10 | 100 | 35 | 100 | 45 | 100 | ||
| SPH | 8.7 × 103 | 7 | 5 | 95 | 30 | 90 | – | – |
| 14 | 25 | 95 | 30 | 95 | – | – | ||
Ae. aegypti were orally infected with fresh, low-passage ZIKV. Initial viral titer was determined by plaque-forming assay. Saliva infection was only examined for mosquitoes at 14 days post-infection with the BRPE isolate. Infection rates are given as percentages. n = 20 per group unless specified. ZIKV, Zika virus; PFU, plaque-forming units; BRPE, ZIKV/H. sapiens/Brazil/BRPE243/2015; SPH, ZIKV/H. sapiens/Brazil/SPH/2015; wMel_Br, Wolbachia-infected; Br, Wolbachia-uninfected.
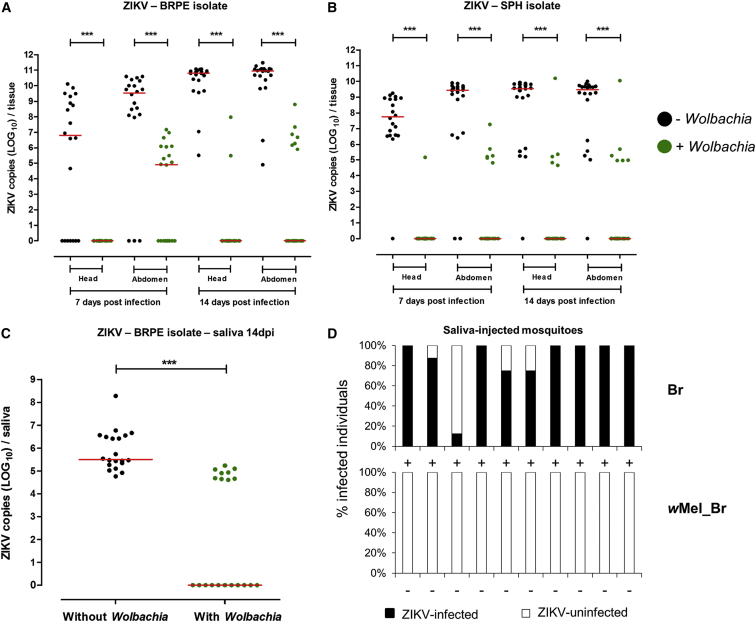
Figure 1.
Wolbachia Infection Restricts ZIKV Infection in Ae. aegypti Mosquitoes
(A–C) Wolbachia-infected (green circles) and -uninfected (black circles) mosquitoes were orally challenged with either (A) the BRPE or (B) the SPH ZIKV isolates. Wolbachia infection reduced both prevalence and intensity of ZIKV infection in mosquito heads/thoraces and abdomens at 7 and 14 dpi. Saliva was then collected for mosquitoes infected with the BRPE ZIKV isolate at 14 dpi infection (C), and we observed that saliva from Wolbachia-infected mosquitoes had a significantly lower rate of saliva infection and median viral load.
(D) When these saliva samples were injected into ZIKV-uninfected Br mosquitoes, all of the Br saliva samples contained infectious virus, while no wMel_Br saliva produced a subsequent infection (columns: black, percentage infected; white, percentage uninfected; +, saliva contained infectious virus, −, saliva did not contain infectious virus). Absolute ZIKV copy numbers were quantified via qRT-PCR.
In (A)–(C), each circle represents tissue or saliva from a single adult female (n = 20 per group). Red lines indicate the median ZIKV copies. ∗∗∗, p < 0.0001; analysis by Mann-Whitney U test. In (D), each column represents mosquitoes injected with a single saliva sample.
The prevalence of ZIKV infection was significantly reduced among Wolbachia-infected mosquitoes (Table 1, analysis via Fisher’s exact test, p < 0.0001 unless stated). For the BRPE isolate (Figure 1A), Wolbachia decreased ZIKV prevalence by 35% in abdomens, although there was no significant difference for this tissue (p > 0.05), by 100% in head/thoraces at 7 dpi, and by 65% and 90% at 14 dpi, respectively. For the SPH isolate (Figure 1B), Wolbachia reduced prevalence by 95% and 67% in head/thoraces and abdomens (p = 0.0002), respectively, at 7 dpi, and by 74% and 68% in head/thoraces and abdomens, respectively, at 14 dpi.
Likewise, the intensity of ZIKV infection was greatly reduced in wMel_Br mosquitoes for both tissues and time points (Mann-Whitney U tests, p < 0.0001). Additionally, we observed that median ZIKV titers in the head/thoraces of Br mosquitoes increased over time for both isolates (Mann-Whitney U test; BRPE, p < 0.0001; SPH, p = 0.0094), while there was no such effect in wMel_Br mosquitoes.
Saliva was collected from Br and wMel_Br mosquitoes at 14 dpi, after the 5- to 10-day ZIKV extrinsic incubation period was likely completed (Li et al., 2012), in order to determine if Wolbachia infection also inhibited ZIKV transmission (Figure 1C). We used mosquitoes infected with the BRPE isolate as it had a higher titer in culture (Table 1). ZIKV levels were quantified directly for individual saliva samples using the same qRT-PCR assay. We observed that Wolbachia infection reduced ZIKV prevalence in individual saliva samples by 55% (Fisher’s exact test, p < 0.0001) and median ZIKV copies by approximately 5 logs (Mann-Whitney U test, p < 0.0001).
To determine if the virus in these samples was infectious, a further ten wMel_Br and ten Br saliva samples, from the samples described above, were intrathoracically injected into 8–14 naive Br mosquitoes each (Figure 1D), using a previously described method (Ferguson et al., 2015). The overall mortality rate among injected mosquitoes was 11.93%. The presence or absence of ZIKV infection was determined at 5 dpi in eight mosquitoes injected with each saliva, amounting to a mean proportion sampled of 0.68. Of the 80 mosquitoes injected with Br saliva, 68 (85%) became infected with ZIKV, with all Br saliva samples producing at least one infected mosquito. In contrast, none of the 80 mosquitoes injected with wMel_Br saliva became infected (Fisher’s exact test, p < 0.0001; odds ratio 882.3, 95% CI, 51.3–15187), indicating that while some of the wMel_Br saliva samples did contain detectable ZIKV, we saw no evidence that the saliva contained infectious virus.
There is a clear correlation between the inhibition of pathogens by Wolbachia and bacterial density in insect tissues (Joubert et al., 2016, Martinez et al., 2014). In order to determine if there was a link between Wolbachia density and ZIKV prevalence and intensity, we measured total Wolbachia RNA levels in the wMel_Br mosquitoes used in the ZIKV infection assays, using qRT-PCR as described above. We saw that ZIKV infection explained less than 5% of the variance in Wolbachia density that was observed between ZIKV-infected and -uninfected wMel_Br mosquitoes at either 7 dpi or 14 dpi and was not a significant predictor (PERMANOVA; p > 0.05). Furthermore, we observed no relationship between Wolbachia density and ZIKV load among wMel_Br mosquitoes that became infected with the virus (Spearman correlation; heads/thoraces, r = 0.5952, p = 0.1323; abdomens, r = −0.01891, p = 0.9210). This suggests that there may not be a direct link between Wolbachia density in individual mosquitoes and ZIKV infection, indicating that the inhibition of ZIKV may arise through other means, indirectly due to the presence of the bacterium (Caragata et al., 2013, Moreira et al., 2009, Pan et al., 2012, Rancès et al., 2012).
Our results indicate that the ability of Wolbachia infection to greatly reduce the capacity of mosquitoes to harbor and transmit a range of medically important pathogens, including the dengue and chikungunya viruses (Caragata et al., 2016, Moreira et al., 2009, Walker et al., 2011) also extends to ZIKV. While wMel did not completely inhibit ZIKV infection, we observed a similar decrease in prevalence and intensity of infection to that of wMel-infected Ae. aegypti challenged with viremic blood from dengue patients, which was considered sufficient to drastically decrease viral transmission (Ferguson et al., 2015). Additionally, the fact that we did not observe an increase in disseminated ZIKV infection over time, and that ZIKV prevalence and infectivity in wMel_Br mosquito saliva was significantly decreased, may indicate that, as for dengue, wMel extends the ZIKV extrinsic incubation period (Ye et al., 2015). This in turn would likely further decrease overall ZIKV transmission rates, given the small decrease in lifespan associated with wMel infection (Walker et al., 2011).
We observed that the wMel Wolbachia infection in Ae. aegypti greatly inhibited ZIKV infection in mosquito abdomens, and it reduced disseminated infection in heads and thoraces and ZIKV prevalence in mosquito saliva. Most critically, our results suggest that saliva from wMel-infected mosquitoes did not contain infectious virus. That this inhibition occurred for two ZIKV isolates that circulated in Brazil during the 2015 epidemic, and for mosquitoes with a wild-type genetic background, suggests that wMel could greatly reduce ZIKV transmission in field populations of Ae. aegypti, which in turn would likely reduce the frequency of Zika-associated pathology in humans.
Wolbachia can invade and persist in wild mosquito populations (Hoffmann et al., 2014) and represents a relatively low-cost, self-sustaining form of mosquito control that is already being trialed in countries where ZIKV outbreaks have been reported and has recently been recommended by the World Health Organization as a suitable tool to control ZIKV transmission (http://migre.me/tDWVe). It is important to point out that extensive public engagement will be required before releases of Wolbachia-infected mosquitoes can be scaled up for use in other areas. However, the results presented here indicate that wMel-infected Ae. aegypti represent a realistic and effective option to combat the ZIKV burden in Brazil and potentially in other countries and should be considered as an integral part of future control efforts.
The work reported in this paper was performed under the oversight of the Committee for Ethics in Research (CEP)/FIOCRUZ (License CEP 732.621).
Author Contributions
Conceptualization, H.L.C.D., M.N.R., and L.A.M.; Methodology, H.L.C.D. F.B.S.D., E.P.C., and L.A.M.; Formal analysis, H.L.C.D. and E.P.C.; Investigation, H.L.C.D.; M.N.R., F.B.S.D., S.B.M., and E.P.C.; Writing—Original Draft, H.L.C.D.; Writing—Review & Editing, H.L.C.D., E.P.C., and L.A.M.; Funding Acquisition, L.A.M; Resources, L.A.M.; Supervision, L.A.M.
Acknowledgments
We are grateful to all members of the Mosquitos Vetores Group (MV—CPqRR/FIOCRUZ), particularly Jéssica Silva, who helped to develop the salivation assay. We thank Dr. Luis Villegas for helpful discussion on statistics and Dr. Alexandre Machado for assistance with viral cultures. The Zika virus isolates were kindly provided by the Department of Virology and Experimental Therapy (Aggeu Magalhães Research Center/FIOCRUZ) and by the Laboratory of Viral Isolation (Evandro Chagas Institute). We thank INCT-EM for the Real-Time PCR machine, and the Brazilian and Australian teams of the Eliminate Dengue program, particularly Prof. Scott L. O’Neill for donating the original wMel line and the Entomology team for providing field mosquito eggs. This work was supported by FAPEMIG, CNPq, CAPES, the Brazilian Ministry of Health (DECIT/SVS), and a grant to Monash University from the Foundation for the National Institutes of Health through the Vector-Based Transmission of Control: Discovery Research (VCTR) program of the Grand Challenges in Global Health Initiatives of the Bill and Melinda Gates Foundation.
Published: May 4, 2016
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.chom.2016.04.021.
Supplemental Information
References
- Araujo L.M., Ferreira M.L., Nascimento O.J. Guillain-Barré syndrome associated with the Zika virus outbreak in Brazil. Arq. Neuropsiquiatr. 2016;74:253–255. doi: 10.1590/0004-282X20160035. [DOI] [PubMed] [Google Scholar]
- Bian G., Joshi D., Dong Y., Lu P., Zhou G., Pan X., Xu Y., Dimopoulos G., Xi Z. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013;340:748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- Bourtzis K., Dobson S.L., Xi Z., Rasgon J.L., Calvitti M., Moreira L.A., Bossin H.C., Moretti R., Baton L.A., Hughes G.L. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014;132(Suppl):S150–S163. doi: 10.1016/j.actatropica.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Caragata E.P., Rancès E., Hedges L.M., Gofton A.W., Johnson K.N., O’Neill S.L., McGraw E.A. Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog. 2013;9:e1003459. doi: 10.1371/journal.ppat.1003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caragata E.P., Dutra H.L., Moreira L.A. Exploiting intimate relationships: Controlling mosquito-transmitted disease with Wolbachia. Trends Parasitol. 2016;32:207–218. doi: 10.1016/j.pt.2015.10.011. [DOI] [PubMed] [Google Scholar]
- Enserink M. INFECTIOUS DISEASES. An obscure mosquito-borne disease goes global. Science. 2015;350:1012–1013. doi: 10.1126/science.350.6264.1012. [DOI] [PubMed] [Google Scholar]
- Faria N.R., Azevedo Rdo.S., Kraemer M.U., Souza R., Cunha M.S., Hill S.C., Thézé J., Bonsall M.B., Bowden T.A., Rissanen I. Zika virus in the Americas: Early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N.M., Kien D.T., Clapham H., Aguas R., Trung V.T., Chau T.N., Popovici J., Ryan P.A., O’Neill S.L., McGraw E.A. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci. Transl. Med. 2015;7:279ra37. doi: 10.1126/scitranslmed.3010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintze C., Velasco Garrido M., Kroeger A. What do community-based dengue control programmes achieve? A systematic review of published evaluations. Trans. R. Soc. Trop. Med. Hyg. 2007;101:317–325. doi: 10.1016/j.trstmh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Hoffmann A.A., Montgomery B.L., Popovici J., Iturbe-Ormaetxe I., Johnson P.H., Muzzi F., Greenfield M., Durkan M., Leong Y.S., Dong Y. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- Hoffmann A.A., Iturbe-Ormaetxe I., Callahan A.G., Phillips B.L., Billington K., Axford J.K., Montgomery B., Turley A.P., O’Neill S.L. Stability of the wMel Wolbachia Infection following invasion into Aedes aegypti populations. PLoS Negl. Trop. Dis. 2014;8:e3115. doi: 10.1371/journal.pntd.0003115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert D.A., Walker T., Carrington L.B., De Bruyne J.T., Kien D.H., Hoang Nle.T., Chau N.V., Iturbe-Ormaetxe I., Simmons C.P., O’Neill S.L. Establishment of a Wolbachia Superinfection in Aedes aegypti Mosquitoes as a Potential Approach for Future Resistance Management. PLoS Pathog. 2016;12:e1005434. doi: 10.1371/journal.ppat.1005434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle J.L., Harris E. Global spread and persistence of dengue. Annu. Rev. Microbiol. 2008;62:71–92. doi: 10.1146/annurev.micro.62.081307.163005. [DOI] [PubMed] [Google Scholar]
- Li M.I., Wong P.S., Ng L.C., Tan C.H. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl. Trop. Dis. 2012;6:e1792. doi: 10.1371/journal.pntd.0001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel-de-Freitas R., Avendanho F.C., Santos R., Sylvestre G., Araújo S.C., Lima J.B., Martins A.J., Coelho G.E., Valle D. Undesirable consequences of insecticide resistance following Aedes aegypti control activities due to a dengue outbreak. PLoS ONE. 2014;9:e92424. doi: 10.1371/journal.pone.0092424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Longdon B., Bauer S., Chan Y.S., Miller W.J., Bourtzis K., Teixeira L., Jiggins F.M. Symbionts commonly provide broad spectrum resistance to viruses in insects: a comparative analysis of Wolbachia strains. PLoS Pathog. 2014;10:e1004369. doi: 10.1371/journal.ppat.1004369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez de Salazar P., Suy A., Sánchez-Montalvá A., Rodó C., Salvador F., Molina I. Zika fever. Enferm. Infecc. Microbiol. Clin. 2016;34:247–252. doi: 10.1016/j.eimc.2016.02.016. [DOI] [PubMed] [Google Scholar]
- Mlakar J., Korva M., Tul N., Popović M., Poljšak-Prijatelj M., Mraz J., Kolenc M., Resman Rus K., Vesnaver Vipotnik T., Fabjan Vodušek V. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Moreira L.A., Iturbe-Ormaetxe I., Jeffery J.A., Lu G., Pyke A.T., Hedges L.M., Rocha B.C., Hall-Mendelin S., Day A., Riegler M. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- Morrison T.E. Reemergence of chikungunya virus. J. Virol. 2014;88:11644–11647. doi: 10.1128/JVI.01432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira Melo A.S., Malinger G., Ximenes R., Szejnfeld P.O., Alves Sampaio S., Bispo de Filippis A.M. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet. Gynecol. 2016;47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- Pan X., Zhou G., Wu J., Bian G., Lu P., Raikhel A.S., Xi Z. Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA. 2012;109:E23–E31. doi: 10.1073/pnas.1116932108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rancès E., Ye Y.H., Woolfit M., McGraw E.A., O’Neill S.L. The relative importance of innate immune priming in Wolbachia-mediated dengue interference. PLoS Pathog. 2012;8:e1002548. doi: 10.1371/journal.ppat.1002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu S.N., Nguyen T.Y., Tran V.P., Truong U.N., Le Q.M., Le V.L., Le T.N., Bektas A., Briscombe A., Aaskov J.G. Elimination of dengue by community programs using Mesocyclops(Copepoda) against Aedes aegypti in central Vietnam. Am. J. Trop. Med. Hyg. 2005;72:67–73. [PubMed] [Google Scholar]
- Walker T., Johnson P.H., Moreira L.A., Iturbe-Ormaetxe I., Frentiu F.D., Mcmeniman C.J., Leong Y.S., Dong Y., Axford J., Kriesner P. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–455. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- Werren J.H., Baldo L., Clark M.E. Wolbachia: master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Ye Y.H., Carrasco A.M., Frentiu F.D., Chenoweth S.F., Beebe N.W., van den Hurk A.F., Simmons C.P., O’Neill S.L., McGraw E.A. Wolbachia reduces the transmission potential of dengue-infected Aedes aegypti. PLoS Negl. Trop. Dis. 2015;9:e0003894. doi: 10.1371/journal.pntd.0003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug R., Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.