Abstract
Microbial rhodopsins are remarkable for the diversity of their functional mechanisms based on the same protein scaffold. A class of rhodopsins from cryptophyte algae show close sequence homology with haloarchaeal rhodopsin proton pumps rather than with previously known channelrhodopsins from chlorophyte (green) algae. In particular, both aspartate residues that occupy the positions of the chromophore Schiff base proton acceptor and donor, a hallmark of rhodopsin proton pumps, are conserved in these cryptophyte proteins. We expressed the corresponding polynucleotides in human embryonic kidney (HEK293) cells and studied electrogenic properties of the encoded proteins with whole-cell patch-clamp recording. Despite their lack of residues characteristic of the chlorophyte cation channels, these proteins are cation-conducting channelrhodopsins that carry out light-gated passive transport of Na+ and H+. These findings show that channel function in rhodopsins has evolved via multiple routes.
Main Text
Phototaxis receptors that depolarize the membranes of green (chlorophyte) algae (1) act as light-gated cation channels when expressed in animal cells (2, 3). This unique property earned them the name “channelrhodopsins” and made them molecules of choice for optogenetic depolarization of the cell membrane and neuronal activation (4). In the phylogenetically distant cryptophyte algae, another family of channelrhodopsins with strictly anion selectivity was found and used to hyperpolarize the membrane and neuronal inhibition (5). Although these latter proteins, called anion channelrhodopsins, share some sequence homology with cation channelrhodopsins (CCRs) from green algae, their conduction mechanisms are clearly different (6, 7, 8).
Guillardia theta, the only cryptophyte organism the genome of which has been completely sequenced (9), is predicted to encode at least 53 microbial (type 1) rhodopsins (5). Except for the anion channelrhodopsins, few other rhodopsins from this organism have been investigated by heterologous expression (10). One cluster of G. theta rhodopsin protein models contains nine sequences, the closest homologs of which, besides similar proteins from other cryptophytes and uncharacterized fungal proteins, are haloarchaeal rhodopsins (Fig. S1 in the Supporting Material). Here we show that these proteins (which we designate as cryptophyte CCRs) are light-gated cation channels with distinctly different structures than those of previously known chlorophyte CCRs from green algae.
We synthesized human codon-adapted versions of three polynucleotides corresponding to the G. theta predicted transcripts 99928 (GenBank: KU761992), 120390 (GenBank: KU761994) and 162755 (GenBank: KU761993). The encoded polypeptides extend 150–200 residues beyond the seven-transmembrane-helix (rhodopsin) domain, but no other putative domains could be detected. We also included in our analysis a highly homologous protein from the cryptophyte alga Proteomonas sulcata (GenBank: KF992056) previously shown to generate photocurrents in neurons (11). We also synthesized a construct (GenBank: KU761991) corresponding to G. theta model 135937, but it was nonfunctional in our system.
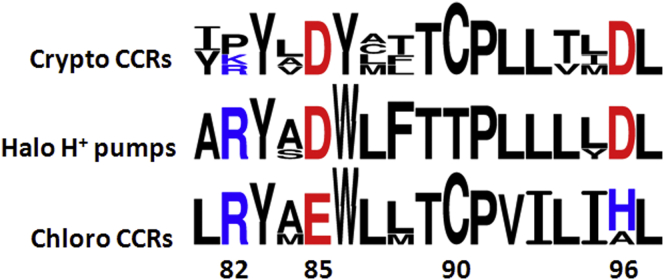
An alignment of the rhodopsin domains showed a closer match of cryptophyte CCRs with haloarchaeal proton pumps than with chlorophyte CCRs (Fig. S2). Asp residues in the positions of the retinylidene Schiff base proton acceptor and donor (Asp-85 and Asp-96 in Halobacterium salinarum bacteriorhodopsin (HsBR), respectively) are conserved in all four studied cryptophyte CCRs, whereas in the majority of chlorophyte CCRs Asp-85 is replaced with Glu, and Asp-96, with His (Fig. 1). Proton transport activity was not observed in a previously studied rhodopsin from the same organism, GtR1, which also has Asp residues in both proton acceptor and donor positions, and a rhodopsin from the fungus Neurospora, NR, in which Asp-85 is conserved and Asp-96 is found as Glu (10, 12).
Figure 1.
Sequence logos of the transmembrane helix 3 generated with WebLogo 3 (17) from the alignment shown in Fig. S2. The numbering is according to the HsBR sequence.
None of the five conserved Glu residues in helix 2 of chlorophyte CCRs (highlighted green in Fig. S2) that contribute to their cation translocation pathway (13) is conserved in the tested cryptophyte proteins. However, cryptophyte CCRs share with CCRs from green algae a Cys residue in the position of Thr-90 in HsBR (Fig. 1). Arg-82 (HsBR numbering), conserved in most microbial rhodopsins, is replaced with Pro or Lys in G. theta CCRs. Modification of the Arg-82 homolog in an H+-pumping rhodopsin from the green alga Coccomyxa converted it into an operational H+ channel (14).
We expressed the constructs encoding the rhodopsin domains of four cryptophyte CCRs fused to enhanced yellow fluorescent protein in human embryonic kidney (HEK293) cells and tested their electrogenic function with whole-cell patch-clamp. Under our standard conditions (the holding potential (Eh) −60 mV, 150 mM NaCl in the bath, 126 mM KCl in the pipette, pH 7.4; for other details, see the Supporting Material), all four proteins generated inward currents in response to a light pulse. The mean amplitudes and half-decay rates of the photocurrents are shown in Fig. S3. Protein 99928 generated the largest currents, whereas the currents from the other three proteins were considerably smaller.
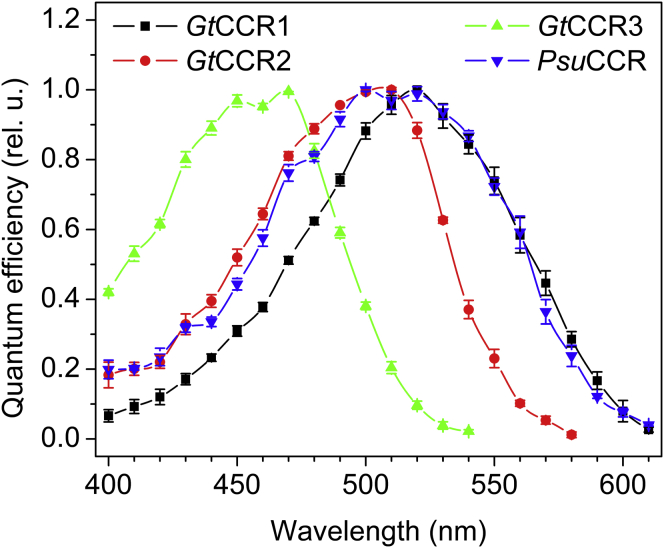
The action spectra of the photocurrents are shown in Fig. 2. In the genomes of most green algae, only two channelrhodopsin homologs have been found. According to a historical convention, the more red-shifted rhodopsin from the same organism is assigned the number 1, and the more blue-shifted is assigned the number 2 (1).
Figure 2.
The action spectra of photocurrents generated by cryptophyte CCRs in HEK293 cells.
As shown below, all three G. theta rhodopsins tested in this study are nonselective light-gated cation channels. Therefore, we assigned the abbreviation GtCCR1 to the most red-shifted one (120390) with the maximal sensitivity at 520 nm; GtCCR2, to the more blue-shifted rhodopsin (99928) with the peak at 505 nm; and GtCCR3, to the most blue-shifted rhodopsin (162755) with peaks at ∼460 nm. The spectral maximum of this latter protein corresponds to that of the G. theta photoaccumulation response (10).
Initially, when no information regarding its ionic selectivity was available, the homologous protein from P. sulcata was referred to as “PsChR2” (11). To avoid confusion with CCRs from the chlorophyte alga Platymonas subcordiformis (15), we will call it PsuCCR in this study. No number can be assigned to this rhodopsin as yet, because the spectral properties of its several other homologs from the same organism (Fig. S1) have not yet been determined.
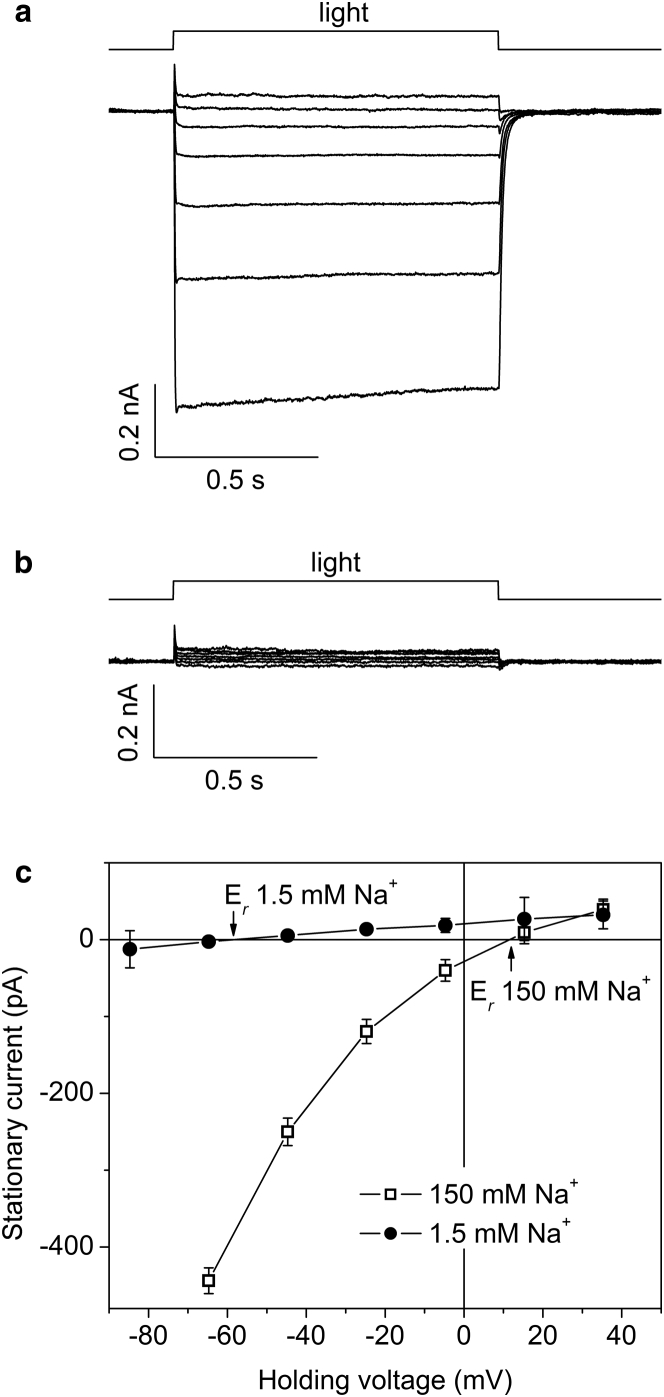
Upon shifting Eh to more positive values, the photocurrents generated by cryptophyte CCRs reversed their direction (Fig. 3 a). The current-voltage dependencies (IE curves) measured under standard conditions exhibited a significant inward rectification (Fig. 3 c, open squares).
Figure 3.
Representative series of photocurrents generated by GtCCR2 in a HEK293 cell at 150 (a) and 1.5 (b) mM Na+ in the bath. Eh was changed in 20-mV steps from −80 to 40 mV at the amplifier output (bottom to top). (c) The current-voltage relationship for the cell shown in (a) and (b). The data are the mean values mean ± SE (n = 3) corrected for liquid junction potentials.
To identify the nature of the transported ions, we determined reversal potentials (Er) upon variation of the ionic composition of the bath solution. When Na+ concentration in the bath was reduced 100-fold by partial replacement with n-methyl-D-glucamine (NMG+), a dramatic inhibition of photocurrents was observed (Fig. 3 b). Moreover, for all tested proteins, Er shifted to more negative values (Figs. 3 c, solid circles, and S4), indicating that they passively transported Na+ across the cell membrane.
A 100-fold reduction of the bath H+ concentration (from pH 5.4 to pH 7.4) also led to an Er shift to negative values, although its magnitude was smaller than that for Na+ (Fig. S4). Therefore, we concluded that cryptophyte CCRs transport both Na+ and H+, as do chlorophyte CCRs from green algae. Purely passive H+ influx has also been demonstrated at low extracellular pH and negative membrane potentials for some rhodopsin H+ pumps, such as that from Gloeobacter violaceus (16) i.e., a leaky-pump phenomenon. However, channel currents generated by cryptophyte CCRs cannot be explained by this mechanism, because at physiological conditions they are carried practically only by Na+ ions (Fig. 3). Na+ pumping can be excluded, because the direction of photocurrents depended on the Nernst equilibrium potential for Na+.
Our results indicate that the microbial rhodopsin common ancestor of chlorophyte and cryptophyte CCRs converged on the same function through structurally different paths. Further research is needed to elucidate the mechanisms of cation conductance in cryptophyte CCRs, but it is already clear that their identification expands our current concepts about channelrhodopsins in an unexpected direction.
Acknowledgments
This work was supported by National Institutes of Health grants No. R01GM027750 and No. U01MH109146 and Endowed Chair No. AU-0009 from the Robert A. Welch Foundation.
Editor: Andreas Engel.
Footnotes
Four figures are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(16)30274-0.
Supporting Material
References
- 1.Sineshchekov O.A., Jung K.-H., Spudich J.L. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 2002;99:8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagel G., Ollig D., Hegemann P. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 3.Nagel G., Szellas T., Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyden E.S., Zhang F., Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 5.Govorunova E.G., Sineshchekov O.A., Spudich J.L. NEUROSCIENCE. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science. 2015;349:647–650. doi: 10.1126/science.aaa7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sineshchekov O.A., Govorunova E.G., Spudich J.L. Gating mechanisms of a natural anion channelrhodopsin. Proc. Natl. Acad. Sci. USA. 2015;112:14236–14241. doi: 10.1073/pnas.1513602112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govorunova E.G., Sineshchekov O.A., Spudich J.L. Proteomonas sulcata ACR1: a fast anion channelrhodopsin. Photochem. Photobiol. 2015;92:257–263. doi: 10.1111/php.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sineshchekov O.A., Li H., Spudich J.L. Photochemical reaction cycle transitions during anion channelrhodopsin gating. Proc. Natl. Acad. Sci. USA. 2016:201525269. doi: 10.1073/pnas.1525269113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis B.A., Tanifuji G., Archibald J.M. Algal genomes reveal evolutionary mosaicism and the fate of nucleomorphs. Nature. 2012;492:59–65. doi: 10.1038/nature11681. [DOI] [PubMed] [Google Scholar]
- 10.Sineshchekov O.A., Govorunova E.G., Spudich J.L. Rhodopsin-mediated photoreception in cryptophyte flagellates. Biophys. J. 2005;89:4310–4319. doi: 10.1529/biophysj.105.070920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klapoetke N.C., Murata Y., Boyden E.S. Independent optical excitation of distinct neural populations. Nat. Methods. 2014;11:338–346. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown L.S., Dioumaev A.K., Spudich J.L. Photochemical reaction cycle and proton transfers in Neurospora rhodopsin. J. Biol. Chem. 2001;276:32495–32505. doi: 10.1074/jbc.M102652200. [DOI] [PubMed] [Google Scholar]
- 13.Kato H.E., Zhang F., Nureki O. Crystal structure of the channelrhodopsin light-gated cation channel. Nature. 2012;482:369–374. doi: 10.1038/nature10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogt A., Guo Y., Hegemann P. Conversion of a light-driven proton pump into a light-gated ion channel. Sci. Rep. 2015;5:16450. doi: 10.1038/srep16450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Govorunova E.G., Sineshchekov O.A., Spudich J.L. Characterization of a highly efficient blue-shifted channelrhodopsin from the marine alga Platymonas subcordiformis. J. Biol. Chem. 2013;288:29911–29922. doi: 10.1074/jbc.M113.505495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogt A., Wietek J., Hegemann P. Gloeobacter rhodopsin, limitation of proton pumping at high electrochemical load. Biophys. J. 2013;105:2055–2063. doi: 10.1016/j.bpj.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crooks G.E., Hon G., Brenner S.E. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.