Abstract
Tumor heterogeneity in hepatocellular carcinoma (HCC), such as that found in second primary tumors after curative treatment, synchronous multifocal tumors of different clonality, or intratumor heterogeneity, poses severe challenges for the development and administration of systemic molecular targeted therapies. Various methodologies, including historical DNA ploidy analysis, integrated hepatitis B virus DNA analysis, DNA fingerprinting, and next-generation sequencing technologies, are used to explore tumor heterogeneity in HCC. It is estimated that 30%-60% of recurrent or metastatic tumors harbor clones different from the primary tumor, 22%-79% of synchronous tumors vary clonally, and 12%-66% of single tumors contain intratumor heterogeneity. Substantial intertumor and intratumor heterogeneity renders biomarker identification, which is critical for the development and administration of molecular targeted therapy, challenging when applied to a single tumor biopsy specimen. The use of circulating tumor cells or circulating tumor DNA to evaluate overall tumor heterogeneity may help resolve this problem. This article reviews previous studies of tumor heterogeneity and discusses the implications and future opportunities regarding tumor heterogeneity in HCC.
Keywords: Circulating tumor cell, Circulating tumor DNA, Clonality, Hepatocellular carcinoma, Tumor heterogeneity
Introduction
In the era of molecular targeted therapy, identifying predictive biomarkers is crucial for the successful implementation of personalized medicine. However, the tumor heterogeneity demonstrated by recent genomic studies may pose challenges for delivering precision medicine [1,2]. For example, Gerlinger et al. performed exome sequencing on various regions of primary renal cell carcinomas and associated metastatic sites. A total of 128 non-synonymous mutations were identified, and more than 60% of them were not detected at all sequenced sites [3]. Taking a single tumor biopsy, which is currently common practice, may underestimate the mutational burden in a cancer patient and in a single tumor. For lung adenocarcinoma, a type of malignancy with well-established oncogene addiction loops and effective molecular targeted agents, recent studies have demonstrated substantial intratumor heterogeneity by using multiregion whole exome or genome sequencing [4,5]. An ongoing prospective study of patients with lung cancer aims to determine the clinical significance of this intratumor heterogeneity and clonal evolution by using multiregion and longitudinal tumor sampling and genetic analysis [6].
Hepatocellular carcinoma (HCC) is a complex malignancy caused by various etiologies, including hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol consumption, and metabolic diseases. Although different etiologies may induce various oncogenic pathways, most HCCs are preceded by a history of chronic hepatitis and liver cirrhosis, which provide a pro-oncogenic microenvironment. Early-stage HCC frequently recurs after curative treatment. The recurrent tumor may arise from the intrahepatic metastasis of the primary tumor or may be a second primary HCC. In addition, it is common to have synchronous and multifocal HCCs at presentation because of the “field cancerization” effect. Furthermore, recent data indicate that intratumor heterogeneity at the phenotypic and genetic levels occurs frequently in HCC.
Tumor heterogeneity is not a trivial concern because it greatly complicates the development of molecular targeted agents for HCC. Despite constant efforts, no targeted agents have been successfully approved for HCC other than sorafenib, a multikinase inhibitor targeting Raf kinase and vascular endothelial growth factor receptor. Tumor tissue-based studies have not yet identified useful biomarkers for predicting sorafenib efficacy in HCC, probably because of the lack of representative HCC tumor tissues. However, if tumor heterogeneity between recurrent and primary tumors, among different synchronous tumors, and among various regions of a single tumor does play a crucial role in HCC, our current practice based on a single biopsy or archived tissues would be seriously inadequate for identifying valid biomarkers for HCC.
Herein, we systematically review the literature on tumor heterogeneity in HCC, focusing on the clonal aspects of primary and recurrent tumors, intertumor heterogeneity, and intra-tumor heterogeneity. Furthermore, we discuss the opportunities and implications of using new technologies, such as next-generation sequencing and circulating tumor cells (CTCs) or circulating tumor DNA (ctDNA), to address the problems of tumor heterogeneity.
Clonality of Primary and Recurrent Tumors
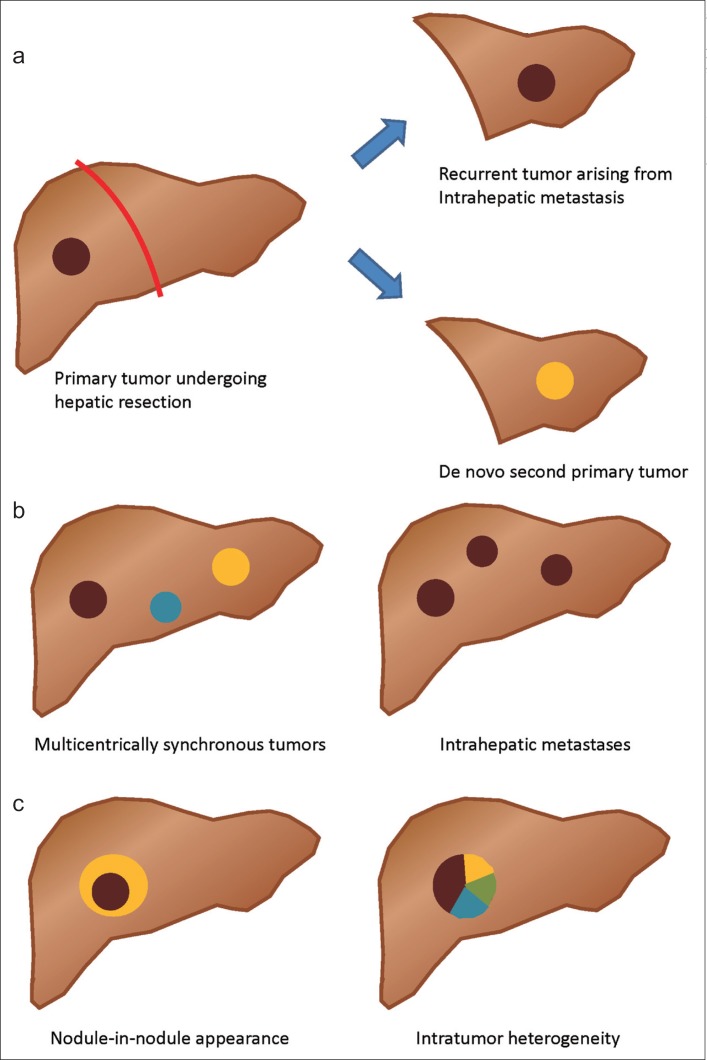
Curative resection is the indicated therapy for early localized HCC. However, following curative resection, approximately 70% of patients with localized HCC develop recurrence [7,8]. The incidence of HCC recurrence generally peaks during the first year after resection, and then declines, but gradually increases again 2 years after curative treatment [9,10]. Previous studies have attributed early recurrence (within 2 years of curative treatment) to residual tumor or intrahepatic micrometastasis, whereas late recurrence (more than 2 years after curative treatment) has been attributed to de novo development of a second primary tumor (fig. 1a) [9,11]. However, these hypotheses have not been completely validated.
Fig. 1.
Tumor heterogeneity of HCC. a Recurrent HCC after hepatic resection may arise from intrahepatic metastasis of the primary tumor or may be a second de novo primary tumor. b Multiple HCCs may occur multicentrically (intertumor heterogeneity) or may arise from intrahepatic metastasis. c Intratumor heterogeneity may arise from a dedifferentiation process (nodule-in-nodule appearance, left) or be clonal evolution within a single HCC tumor (right).
A previous study used integrated HBV DNA as the determinant of clonality for recurrent tumors and found that 60% (3/5) were de novo second primary tumors [12]. Furthermore, by using markers such as DNA ploidy, comparative genomic hybridization (CGH), and the loss of heterozygosity (LOH) of certain microsatellite markers, some studies have identified 42%-53% of recurrent or metastatic tumors with clones different from the primary tumors [13,14,15]. A recent Chinese study with a large patient cohort of HBV-related HCC determined the clonality of primary and recurrent HCC by analyzing the LOH of 10 microsatellite markers. Thirty percent (48/160) of patients with recurrent HCC were considered to have multicentric occurrence (i.e., tumors of different clonality). The median relapse time was 15.6 months for patients considered to have true recurrence but was approximately 2 years for those with multicentric occurrence (p=0.001) [16]. Table 1 summarizes the key findings of studies that assessed the clonality of primary and recurrent HCC.
Table 1.
Studies on the clonality of primary and recurrent HCCs
| Study/year | Methods | Ratio of different clonality in recurrent HCC | Etiology |
|---|---|---|---|
| Chen et al., Taiwan/1989 [12] | Integrated HBV DNA (Southern blot analysis) | 3/5 (60%) | HBV |
| Yoshida et al., Japan/1992 [13] | DNA ploidy (microspectrophotometry) | 11/25 (44%); lung metastasis | HCV predominant |
| Chen et al., Taiwan/2000 [14] | CGH + integrated HBV DNA | 13/31 (42%) | HBV in 19 |
| Morimoto et al., Japan/2003 [15] | LOH | 10/19 (53%) | HCV predominant |
| Li et al., China/2008 [16] | LOH | 48/160 (30%) | HBV |
Intertumor Heterogeneity
The question whether multiple hepatic tumors in HCC patients develop multicentrically from different clones or arise from a single original tumor via intrahepatic metastasis has been investigated for decades (fig. 1b). Multiple HCCs arising by these two different mechanisms are likely to have different effects on patient prognosis and theoretically should be treated differently. However, the implications of these differences for clinical practice have not been systematically addressed.
Previous studies with small patient cohorts, mostly from Taiwan and Hong Kong, have analyzed DNA ploidy, integrated HBV DNA patterns with or without other markers, or the LOH of specific microsatellite markers to determine the clonality of multiple HCCs, and observed that 22%-61% of patients had synchronous tumors of different clonality [15,17,18,19,20,21,22]. A study conducted in the UK used the arbitrarily primed polymerase chain reaction (AP-PCR) technique to compare the DNA fingerprints of 55 HCCs from 13 cirrhotic liver explants. The results unexpectedly showed that no two tumors had identical electrophoretic patterns [23]. A recent Japanese study that evaluated the promoter methylation status of multiple tumor suppressor genes as clonal markers also identified frequent (79%, 15/19 of patients) multi-centric and synchronous tumors [24]. Table 2 summarizes studies that explored the clonality of multiple HCCs by using various methods.
Table 2.
Studies exploring the clonality of multiple HCCs
| Study/year | Methods | Ratio of different clonality in patients with multiple HCC | Etiology |
|---|---|---|---|
| Kuo et al., Taiwan/1987 [17] | DNA ploidy (Feulgen method) | 4/14 (29%) | HBV predominant |
| Hsu et al., Taiwan/1991 [18] | Integrated HBV DNA (Southern blot analysis) | 16/28 (57%) | HBV |
| Sheu et al., Taiwan/1993 [19] | DNA fingerprinting + integrated HBV DNA | 11/18 (61%) | HBV in 9 |
| Sirivatanauksorn et al., UK/1999 [23] | DNA fingerprinting (AP-PCR) | 13/13 (100%) | Alcohol predominant |
| Cheung et al., Hong Kong/2002 [20] | cDNA microarray + p53 status + integrated HBV DNA | 2/6 (33%) | HBV |
| Ng et al., Hong Kong/2003 [21] | LOH + CGH + integrated HBV DNA | 4/11 (36%) | HBV in 10 |
| Morimoto et al., Japan/2003 [15] | LOH | 2/9 (22%) | HCV predominant |
| Lin et al., Taiwan/2005 [22] | LOH | 8/16 (50%) | HBV in 11 |
| Nomoto et al., Japan/2007 [24] | Promoter hypermethylation | 15/19 (79%) | HCV in 13 |
High-throughput molecular analyses, such as next-generation sequencing, have been used to reveal the landscape of genetic alterations in numerous malignant diseases, including HCC [25]. One study employed multiomics analyses, including genomics and proteomics data, to investigate the clonality of multiple tumors in two patients with HBV-related HCC. The HBV DNA integration pattern, genomic mutations such as indels and substitutions, copy number variations, and chromosomal structure were similar in different tumors from one patient who was believed to have intrahepatic metastasis and died of recurrent disease shortly after curative resection. By contrast, the multiple tumors of the other patient had distinct HBV DNA integrations and genomic alterations, and the patient showed no recurrence for more than 2 years after the resection of multifocal HCC [26]. This study proved that comprehensive multiomics analyses can be used to characterize multifocal tumors, facilitate clinical decision making, and help implement personalized medicine.
Intratumor Heterogeneity
Intratumor heterogeneity is a characteristic of many solid tumors [3,27,28,29]. Tumor cells undergo evolutionary processes and natural selection that lead to diverse clones in a single tumor. Intratumor heterogeneity is believed to play a crucial role in tumor resistance to cancer therapy, including molecular targeted agents. Intratumor heterogeneity may be more pronounced in HCC because the “nodule-in-nodule” appearance of HCC is commonly detected using imaging studies [30,31,32,33]. This characteristic growth pattern reflects a dedifferentiation process in a differentiated HCC and further contributes to the heterogeneity within a single tumor (fig. 1c).
Previous studies with small sample sizes have analyzed DNA ploidy, LOH of microsatellite markers, and other DNA fingerprints to evaluate the clonality within specific HCC tumors and identified that 12%-66% had intratumor heterogeneity [15,17,34,35,36,37]. A Japanese study analyzed the histological differentiation of 41 small HCCs (less than 3 cm in diameter) and observed that 14 of them (34%) showed heterogeneous histological differentiation [38]. Another recently published study combined histology, immunohistochemical staining (β-catenin, glutamine synthetase, CK7, CD44, AFP, and EpCAM), and mutations of TP53 and CTNNB1 to determine the intratumor heterogeneity of HCC. The majority of patients (20/23, 87%) showed intratumor heterogeneity based on at least one of the aforementioned histological, immunophenotypic, or genetic factors. Among the 23 patients, 5 (22%) showed intratumor heterogeneity with regard to all the tested factors [39]. These findings challenge the previous knowledge and classifications of HCC based on phenotypes and molecular changes [40,41]. Table 3 summarizes the findings of studies on the intratumor heterogeneity of HCC.
Table 3.
Studies evaluating the intratumor heterogeneity of HCC
| Study/year | Methods | Ratio of HCC tumors with intratumor heterogeneity | Etiology |
|---|---|---|---|
| Kuo et al., Taiwan/1987 [17] | DNA ploidy (Feulgen method) | 2/17 (12%) | HBV predominant |
| Okada et al., Japan/1995 [34] | DNA ploidy (flow cytometry) | 7/28 (25%) | HCV predominant |
| Oriyama et al., Japan/1998 [35] | DNA ploidy (flow cytometry) | 9/20 (45%) | HCV in 12 |
| Sirivatanauksorn et al., UK/1999 [36] | DNA fingerprinting (AP-PCR) | 13/31 (42%) | Alcohol predominant |
| Saeki et al., Japan/2000 [37] | Restriction landmark genomic scanning | 4/6 (66%) | HCV in 5 |
| An et al., Japan/2001 [38] | Histology | 14/41 (34%) | HCV predominant |
| Morimoto et al., Japan/2003 [15] | LOH | 1/7 (14%) | HCV predominant |
| Friemel et al., Switzerland/ 2014 [39] | Histology + IHC + TP53/CTNNB1 mutations | 5/23 (22%) | Mixed |
IHC=immunohistochemistry.
Recently, Tao et al. reported mutation profiles from multiple regions of a primary HCC and recurrent tumors by using whole genome and exome sequencing in a single patient. The study dissected the tumor progression patterns by identifying different clones of the primary tumor and additional mutations (foreground mutations) that led to intrahepatic metastasis [42]. The findings confirmed that tumor heterogeneity and evolution can be analyzed with high resolution at the nucleotide level. Additional studies on large HCC patient cohorts are warranted.
Exploiting CTCs or DNA to Evaluate Tumor Heterogeneity in HCC
Various methods using cell density gradients, cell size differences, and specific surface markers have been developed to isolate CTCs in patients with solid tumors. Two studies have evaluated circulating EpCAM-positive cells as CTCs in patients with HCC and demonstrated that the presence of such cells in the blood stream was associated with poor prognosis [43,44]. However, during the epithelial-mesenchymal transition, a process that is required for invasion and metastasis, epithelial markers such as EpCAM could be lost. Using EpCAM-based CTC-isolation methods may result in a substantial loss of CTCs. Recently, an asialoglycoprotein receptor-ligand-based separation method was developed to identify CTCs in HCC patients, but this method requires further validation [45,46].
The clinical applications of CTC or ctDNA isolation may include the early detection of recurrence, the monitoring of treatment efficacy, and predicting prognosis. In the era of molecular targeting therapy, “liquid biopsies” are being actively investigated for surrogate bio-markers of the primary tumor [47]. For example, epidermal growth factor receptor (EGFR) mutations, which are associated with the efficacy of EGFR tyrosine kinase inhibitors, can be detected using various methods involving CTCs or ctDNA in patients with non-small cell lung cancer [48,49]. Therefore, assessing the molecular heterogeneity of primary and metastatic tumors by using CTCs or ctDNA may be a rational approach, because circulating samples are derived from multiple tumor sites in a patient. Thus, based on the assumption that different clones have a similar tendency to disseminate or shed DNA into the circulation, CTC and ctDNA isolation could potentially reveal a complete picture of the genetic landscape in a longitudinal and dynamic manner. However, this type of study remains relatively unexplored for HCC.
Clinical Implications
Establishing the tumor heterogeneity of HCC may impact clinical decisions and patient management. For patients with early-stage HCC, curative treatments are indicated. If such a patient shows intrahepatic metastasis-related multiple HCC, adjuvant treatment may be beneficial because of the high risk of recurrence. In contrast, for patients with intermediate-stage HCC and multicentric tumors of different clonality, aggressive locoregional therapy may be beneficial. Additional clinical studies are warranted to validate the significance of these hypothetical approaches.
For patients with advanced HCC, several clinical trials of molecular targeted therapy have accepted archived tumor tissues for biomarker testing. In many cases, archived tissues were obtained from primary tumors many years before the development of recurrent and advanced HCC when patients were indicated for systemic therapy. Such “recurrent” tumors could have developed from a clone of the previous primary tumor or could have arisen as a de novo second primary tumor. Therefore, using biomarker profiles obtained from archived tissues for predicting the treatment response of a second primary tumor is unjustified. Even if a fresh biopsy is taken for molecular profiling, the inter- and intratumor heterogeneity of HCC may still result in the failure to identify appropriate biomarkers for molecular targeted therapy. With an improved understanding of the significance of tumor heterogeneity in HCC, clinical trials adopting biomarker-enrichment and adaptive-design strategies might constitute a route leading to the successful development of personalized targeted therapy for HCC.
Future Directions and Challenges
Previous studies based on the simple analysis of DNA ploidy, HBV DNA integration, or other DNA fingerprinting method have revealed substantial intertumor and intratumor clonal heterogeneity in HCC. Advances in deep-sequencing and cutting-edge technologies have facilitated understanding of the genetic alterations found in HCC with high resolution, multiple dimensions, and pathway-driven insights. Future studies using these novel technologies should address the following issues related to the tumor heterogeneity of HCC.
Although HCC is caused by clear etiologies, and some studies have proposed the classification of HCC based on phenotypes, molecular changes, or pathway activation [25,41], no driver event corresponding to targeted therapy has been identified. The intertumor and intratumor heterogeneity of HCC makes the identification and verification of such driver genetic alterations challenging. Future studies need to evaluate in detail genetic alterations geographically across different regions, within and across different tumors in individual patients, and chronologically along the tumor progression. This information will clarify the evolutionary genetic changes involved in hepatocarcinogenesis and cancer progression, and thus might facilitate the identification of potential key genetic drivers of HCC.
Deep sequencing technologies, however, are still based on “snapshot” tumor biopsies. Although multiregion sequencing reveals higher mutational burdens and possible evolutionary processes, it is unclear how many biopsies are needed to display all subclones of a single tumor, or even multiple tumors; in addition, multiregional sampling is difficult in the clinical setting. Studying CTCs and ctDNA could potentially identify more tumor clones and could potentially build up a complete picture of tumor heterogeneity in a longitudinal and dynamic manner. Further studies are warranted to standardize and validate the methods for collecting CTCs and ctDNA from patients with HCC.
In conclusion, understanding tumor heterogeneity highlights the limitations of current methods for exploring biomarkers in HCC. As high-throughput methods and bioinformatics technologies develop and improve, highly comprehensive genomic studies on large patient cohorts will be facilitated, and such studies will produce new strategies to tackle the challenges of developing novel molecular targeted agents for HCC.
Conflicts of Interest
The authors do not have any conflicts of interest to declare.
Acknowledgments
This study was supported by grants from National Taiwan University Hospital, Taipei, Taiwan (NTUH.103-M2526) and the Ministry of Science and Technology, Taiwan (MOST-103-2314-B-002-090).
References
- 1.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, Carter SL, Stewart C, Mermel CH, Roberts SA, Kiezun A, Hammerman PS, McKenna A, Drier Y, Zou L, Ramos AH, Pugh TJ, Stransky N, Helman E, Kim J, Sougnez C, Ambrogio L, Nickerson E, Shefler E, Cortés ML, Auclair D, Saksena G, Voet D, Noble M, DiCara D, Lin P, Lichtenstein L, Heiman DI, Fennell T, Imielinski M, Hernandez B, Hodis E, Baca S, Dulak AM, Lohr J, Landau DA, Wu CJ, Melendez-Zajgla J, Hidalgo-Miranda A, Koren A, McCarroll SA, Mora J, Lee RS, Crompton B, Onofrio R, Parkin M, Winckler W, Ardlie K, Gabriel SB, Roberts CW, Biegel JA, Stegmaier K, Bass AJ, Garraway LA, Meyerson M, Golub TR, Gordenin DA, Sunyaev S, Lander ES, Getz G. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell. 2015;27:15–26. doi: 10.1016/j.ccell.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Bruin EC, McGranahan N, Mitter R, Salm M, Wedge DC, Yates L, Jamal-Hanjani M, Shafi S, Murugaesu N, Rowan AJ, Grönroos E, Muhammad MA, Horswell S, Gerlinger M, Varela I, Jones D, Marshall J, Voet T, Van Loo P, Rassl DM, Rintoul RC, Janes SM, Lee SM, Forster M, Ahmad T, Lawrence D, Falzon M, Capitanio A, Harkins TT, Lee CC, Tom W, Teefe E, Chen SC, Begum S, Rabinowitz A, Phillimore B, Spencer-Dene B, Stamp G, Szallasi Z, Matthews N, Stewart A, Campbell P, Swanton C. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science. 2014;346:251–256. doi: 10.1126/science.1253462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Fujimoto J, Zhang J, Wedge DC, Song X, Zhang J, Seth S, Chow CW, Cao Y, Gumbs C, Gold KA, Kalhor N, Little L, Mahadeshwar H, Moran C, Protopopov A, Sun H, Tang J, Wu X, Ye Y, William WN, Lee JJ, Heymach JV, Hong WK, Swisher S, Wistuba II, Futreal PA. Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing. Science. 2014;346:256–259. doi: 10.1126/science.1256930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamal-Hanjani M, Hackshaw A, Ngai Y, Shaw J, Dive C, Quezada S, Middleton G, de Bruin E, Le Quesne J, Shafi S, Falzon M, Horswell S, Blackhall F, Khan I, Janes S, Nicolson M, Lawrence D, Forster M, Fennell D, Lee SM, Lester J, Kerr K, Muller S, Iles N, Smith S, Murugaesu N, Mitter R, Salm M, Stuart A, Matthews N, Adams H, Ahmad T, Attanoos R, Bennett J, Birkbak NJ, Booton R, Brady G, Buchan K, Capitano A, Chetty M, Cobbold M, Crosbie P, Davies H, Denison A, Djearman M, Goldman J, Haswell T, Joseph L, Kornaszewska M, Krebs M, Langman G, MacKenzie M, Millar J, Morgan B, Naidu B, Nonaka D, Peggs K, Pritchard C, Remmen H, Rowan A, Shah R, Smith E, Summers Y, Taylor M, Veeriah S, Waller D, Wilcox B, Wilcox M, Woolhouse I, McGranahan N, Swanton C. Tracking genomic cancer evolution for precision medicine: the lung TRACERx study. PLoS Biol. 2014;12:e1001906. doi: 10.1371/journal.pbio.1001906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 8.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 9.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 10.Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G, Tagger A, Colombo M, Bonino F, Majno P, Llovet JM. HCC Italian Task Force: Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 11.Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, Huo TI, Sheen IJ, Lee SD, Lui WY. Risk factors for early and late recurrence in hepatitis B-related hepatocellular carcinoma. J Hepatol. 2009;51:890–897. doi: 10.1016/j.jhep.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Chen PJ, Chen DS, Lai MY, Chang MH, Huang GT, Yang PM, Sheu JC, Lee SC, Hsu HC, Sung JL. Clonal origin of recurrent hepatocellular carcinomas. Gastroenterology. 1989;96:527–529. doi: 10.1016/0016-5085(89)91581-3. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida Y, Kanematsu T, Korenaga D, Sonoda T, Sugimachi K. DNA ploidy of primary hepatocellular carcinoma and pulmonary metastases. Clin Exp Metastasis. 1992;10:337–344. doi: 10.1007/BF00058173. [DOI] [PubMed] [Google Scholar]
- 14.Chen YJ, Yeh SH, Chen JT, Wu CC, Hsu MT, Tsai SF, Chen PJ, Lin CH. Chromosomal changes and clonality relationship between primary and recurrent hepatocellular carcinoma. Gastroenterology. 2000;119:431–440. doi: 10.1053/gast.2000.9373. [DOI] [PubMed] [Google Scholar]
- 15.Morimoto O, Nagano H, Sakon M, Fujiwara Y, Yamada T, Nakagawa H, Miyamoto A, Kondo M, Arai I, Yamamoto T, Ota H, Dono K, Umeshita K, Nakamori S, Sasaki Y, Ishikawa O, Imaoka S, Monden M. Diagnosis of intrahepatic metastasis and multicentric carcinogenesis by microsatellite loss of heterozygosity in patients with multiple and recurrent hepatocellular carcinomas. J Hepatol. 2003;39:215–221. doi: 10.1016/s0168-8278(03)00233-2. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Wang J, Juzi JT, Sun Y, Zheng H, Cui Y, Li H, Hao X. Clonality analysis for multicentric origin and intrahepatic metastasis in recurrent and primary hepatocellular carcinoma. J Gastrointest Surg. 2008;12:1540–1547. doi: 10.1007/s11605-008-0591-y. [DOI] [PubMed] [Google Scholar]
- 17.Kuo SH, Sheu JC, Chen DS, Sung JL, Lin CC, Hsu HC. DNA clonal heterogeneity of hepatocellular carcinoma demonstrated by Feulgen-DNA analysis. Liver. 1987;7:359–363. doi: 10.1111/j.1600-0676.1987.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 18.Hsu HC, Chiou TJ, Chen JY, Lee CS, Lee PH, Peng SY. Clonality and clonal evolution of hepatocellular carcinoma with multiple nodules. Hepatology. 1991;13:923–928. [PubMed] [Google Scholar]
- 19.Sheu JC, Huang GT, Chou HC, Lee PH, Wang JT, Lee HS, Chen DS. Multiple hepatocellular carcinomas at the early stage have different clonality. Gastroenterology. 1993;105:1471–1476. doi: 10.1016/0016-5085(93)90153-4. [DOI] [PubMed] [Google Scholar]
- 20.Cheung ST, Chen X, Guan XY, Wong SY, Tai LS, Ng IO, So S, Fan ST. Identify metastasis-associated genes in hepatocellular carcinoma through clonality delineation for multinodular tumor. Cancer Res. 2002;62:4711–4721. [PubMed] [Google Scholar]
- 21.Ng IO, Guan XY, Poon RT, Fan ST, Lee JM. Determination of the molecular relationship between multiple tumour nodules in hepatocellular carcinoma differentiates multicentric origin from intrahepatic metastasis. J Pathol. 2003;199:345–353. doi: 10.1002/path.1287. [DOI] [PubMed] [Google Scholar]
- 22.Lin YW, Lee HS, Chen CH, Huang GT, Lee PH, Sheu JC. Clonality analysis of multiple hepatocellular carcinomas by loss of heterozygosity pattern determined by chromosomes 16q and 13q. J Gastroenterol Hepatol. 2005;20:536–546. doi: 10.1111/j.1440-1746.2005.03609.x. [DOI] [PubMed] [Google Scholar]
- 23.Sirivatanauksorn Y, Sirivatanauksorn V, Bhattacharya S, Davidson BR, Dhillon AP, Kakkar AK, Williamson RC, Lemoine NR. Genomic heterogeneity in synchronous hepatocellular carcinomas. Gut. 1999;45:761–765. doi: 10.1136/gut.45.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nomoto S, Kinoshita T, Kato K, Otani S, Kasuya H, Takeda S, Kanazumi N, Sugimoto H, Nakao A. Hypermethylation of multiple genes as clonal markers in multicentric hepatocellular carcinoma. Br J Cancer. 2007;97:1260–1265. doi: 10.1038/sj.bjc.6604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB, Calderaro J, Bioulac-Sage P, Letexier M, Degos F, Clément B, Balabaud C, Chevet E, Laurent A, Couchy G, Letouzé E, Calvo F, Zucman-Rossi J. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet. 2012;44:694–698. doi: 10.1038/ng.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miao R, Luo H, Zhou H, Li G, Bu D, Yang X, Zhao X, Zhang H, Liu S, Zhong Y, Zou Z, Zhao Y, Yu K, He L, Sang X, Zhong S, Huang J, Wu Y, Miksad RA, Robson SC, Jiang C, Zhao Y, Zhao H. Identification of prognostic biomarkers in hepatitis B virus-related hepatocellular carcinoma and stratification by integrative multiomics analysis. J Hepatol. 2014;61:840–849. doi: 10.1016/j.jhep.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Inukai M, Toyooka S, Ito S, Asano H, Ichihara S, Soh J, Suehisa H, Ouchida M, Aoe K, Aoe M, Kiura K, Shimizu N, Date H. Presence of epidermal growth factor receptor gene T790M mutation as a minor clone in non-small cell lung cancer. Cancer Res. 2006;66:7854–7858. doi: 10.1158/0008-5472.CAN-06-1951. [DOI] [PubMed] [Google Scholar]
- 28.Navin N, Kendall J, Troge J, Andrews P, Rodgers L, McIndoo J, Cook K, Stepansky A, Levy D, Esposito D, Muthuswamy L, Krasnitz A, McCombie WR, Hicks J, Wigler M. Tumour evolution inferred by single-cell sequencing. Nature. 2011;472:90–94. doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almendro V, Marusyk A, Polyak K. Cellular heterogeneity and molecular evolution in cancer. Annu Rev Pathol. 2013;8:277–302. doi: 10.1146/annurev-pathol-020712-163923. [DOI] [PubMed] [Google Scholar]
- 30.Kojiro M. Pathology of early hepatocellular carcinoma: progression from early to advanced. Hepatogastroenterology. 1998;45(Suppl 3):1203–1205. [PubMed] [Google Scholar]
- 31.Ogata R, Majima Y, Tateishi Y, Kuromatsu R, Shimauchi Y, Torimyra T, Tanaka M, Kumashiro R, Kojiro M, Sata M. Bright loop appearance; a characteristic ultrasonography sign of early hepatocellular carcinoma. Oncol Rep. 2000;7:1293–1298. doi: 10.3892/or.7.6.1293. [DOI] [PubMed] [Google Scholar]
- 32.Ishida Y, Nagamatsu H, Koga H, Yoshida H, Kojiro M, Sata M. Hepatocellular carcinoma with a “nodule-in-nodule” appearance reflecting an unusual dilated pseudoglandular structure. Intern Med. 2008;47:1215–1218. doi: 10.2169/internalmedicine.47.0640. [DOI] [PubMed] [Google Scholar]
- 33.Dong A, Yu H, Wang Y, Dong H, Zuo C. FDG PET/CT and enhanced CT imaging of tumor heterogeneity in hepatocellular carcinoma: imaging-pathologic correlation. Clin Nucl Med. 2014;39:808–810. doi: 10.1097/RLU.0b013e3182a75812. [DOI] [PubMed] [Google Scholar]
- 34.Okada S, Ishii H, Nose H, Okusaka T, Kyogoku A, Yoshimori M, Sakamoto M, Hirohashi S. Intratumoral DNA heterogeneity of small hepatocellular carcinoma. Cancer. 1995;75:444–450. doi: 10.1002/1097-0142(19950115)75:2<444::aid-cncr2820750206>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 35.Oriyama T, Yamanaka N, Fujimoto J, Ichikawa N, Okamoto E. Progression of hepatocellular carcinoma as reflected by nuclear DNA ploidy and cellular differentiation. J Hepatol. 1998;28:142–149. doi: 10.1016/s0168-8278(98)80213-4. [DOI] [PubMed] [Google Scholar]
- 36.Sirivatanauksorn Y, Sirivatanauksorn V, Bhattacharya S, Davidson BR, Dhillon AP, Kakkar AK, Williamson RC, Lemoine NR. Evolution of genetic abnormalities in hepatocellular carcinomas demonstrated by DNA fingerprinting. J Pathol. 1999;189:344–350. doi: 10.1002/(SICI)1096-9896(199911)189:3<344::AID-PATH430>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 37.Saeki R, Nagai H, Kaneko S, Unoura M, Yamanaka N, Okamoto E, Kobayashi K, Matsubara K. Intratumoral genomic heterogeneity in human hepatocellular carcinoma detected by restriction landmark genomic scanning. J Hepatol. 2000;33:99–105. doi: 10.1016/s0168-8278(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 38.An FQ, Matsuda M, Fujii H, Tang RF, Amemiya H, Dai YM, Matsumoto Y. Tumor heterogeneity in small hepatocellular carcinoma: analysis of tumor cell proliferation, expression and mutation of p53 AND beta-catenin. Int J Cancer. 2001;93:468–474. doi: 10.1002/ijc.1367. [DOI] [PubMed] [Google Scholar]
- 39.Friemel J, Rechsteiner M, Frick L, Böhm F, Struckmann K, Egger M, Moch H, Heikenwalder M, Weber A. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res. 2015;21:1951–1961. doi: 10.1158/1078-0432.CCR-14-0122. [DOI] [PubMed] [Google Scholar]
- 40.Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Solé M, Tovar V, Alsinet C, Ramos AH, Barretina J, Roayaie S, Schwartz M, Waxman S, Bruix J, Mazzaferro V, Ligon AH, Najfeld V, Friedman SL, Sellers WR, Meyerson M, Llovet JM. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68:6779–6788. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M, Watanabe G, Gabriel S, Friedman SL, Kumada H, Llovet JM, Golub TR. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao Y, Ruan J, Yeh SH, Lu X, Wang Y, Zhai W, Cai J, Ling S, Gong Q, Chong Z, Qu Z, Li Q, Liu J, Yang J, Zheng C, Zeng C, Wang HY, Zhang J, Wang SH, Hao L, Dong L, Li W, Sun M, Zou W, Yu C, Li C, Liu G, Jiang L, Xu J, Huang H, Li C, Mi S, Zhang B, Chen B, Zhao W, Hu S, Zhuang SM, Shen Y, Shi S, Brown C, White KP, Chen DS, Chen PJ, Wu CI. Rapid growth of a hepatocellular carcinoma and the driving mutations revealed by cell-population genetic analysis of whole-genome data. Proc Natl Acad Sci USA. 2011;108:12042–12047. doi: 10.1073/pnas.1108715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, Riethdorf S, Wege H. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer. 2013;133:2165–2171. doi: 10.1002/ijc.28230. [DOI] [PubMed] [Google Scholar]
- 44.Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, Shi RY, Hu B, Zhou J, Fan J. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458–1468. doi: 10.1002/hep.26151. [DOI] [PubMed] [Google Scholar]
- 45.Xu W, Cao L, Chen L, Li J, Zhang XF, Qian HH, Kang XY, Zhang Y, Liao J, Shi LH, Yang YF, Wu MC, Yin ZF. Isolation of circulating tumor cells in patients with hepatocellular carcinoma using a novel cell separation strategy. Clin Cancer Res. 2011;17:3783–3793. doi: 10.1158/1078-0432.CCR-10-0498. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Chen L, Zhang X, Zhang Y, Liu H, Sun B, Zhao L, Ge N, Qian H, Yang Y, Wu M, Yin Z. Detection of circulating tumor cells in hepatocellular carcinoma using antibodies against asialoglycoprotein receptor, carbamoyl phosphate synthetase 1 and pan-cytokeratin. PLoS ONE. 2014;9:e96185. doi: 10.1371/journal.pone.0096185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz LA, Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32:579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchetti A, Del Grammastro M, Felicioni L, Malatesta S, Filice G, Centi I, De Pas T, Santoro A, Chella A, Brandes AA, Venturino P, Cuccurullo F, Crinó L, Buttitta F. Assessment of EGFR mutations in circulating tumor cell preparations from NSCLC patients by next generation sequencing: toward a real-time liquid biopsy for treatment. PLoS ONE. 2014;9:e103883. doi: 10.1371/journal.pone.0103883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qiu M, Wang J, Xu Y, Ding X, Li M, Jiang F, Xu L, Yin R. Circulating tumor DNA is effective for the detection of EGFR mutation in non-small cell lung cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2015;24:206–212. doi: 10.1158/1055-9965.EPI-14-0895. [DOI] [PubMed] [Google Scholar]