Abstract
Purpose
To evaluate the results of indocyanine green angiography (ICGA)-guided verteporfin photodynamic therapy (PDT) with half-fluence rate combined with intravitreal application of anti-VEGF in treating choroidal neovascularization (CNV) in chronic central serous chorioretinopathy (CSCR).
Patients and methods
In this retrospective cohort study 17 consecutive patients with secondary CNV due to chronic CSCR had their diagnosis verified with fluorescein angiography (FA) and ICGA at baseline. All eyes received either intravitreal ranibizumab (IVR) or bevacizumab (IVB). On the consecutive day following the initial IVR/IVB treatment, ICGA-guided verteporfin (6 mg/m2) PDT with half-fluence rate (25 J/cm2) was performed on every patient. IVR or IVB was rescheduled on a pro re nata regimen. Main outcome measures were changes in visual acuity (VA) according to the ETDRS letter score and changes in the central foveal thickness (CFT).
Results
Best-corrected VA at baseline was 65.6 letters (±6.7; n=17) according to the ETDRS letter score. At 12 months, mean ETDRS letter score improved to 71.2 letters (P=0.34). CFT was 309 μm and decreased to 216 μm at month 12 control (P=0.0004). Nine eyes (52.9%) received additional treatment with IVR/IVB due to recurrence of subretinal fluid, with an overall mean number of IVR/IVB treatment of 1.8±3.6 per patient with no systemic side effects during 12 months' follow-up.
Conclusions
IVR or IVB combined with ICGA-guided half-fluence PDT with verteporfin is effective in treating CNV in chronic CSCR, with choroidal hyperpermeability in ICGA, resulting in stable vision and significant reduction of CFT.
Introduction
The diagnosis of central serous chorioretinopathy (CSCR) is characterized by the accumulation of subretinal fluid (SRF), inducing a focal serous detachment of the neurosensory retina. Patients are predominantly male, with a history of recent stress, and commonly associated with a type A personality.1 Symptoms vary from a variety of visual disturbances such as decreased visual acuity (VA), micropsia, metamorphopsia, and relative central scotoma.1, 2
The majority of patients with CSCR have a good visual prognosis, however, if secondary CNV develops, it is a crucial cause of visual impairment and even more threatening, as patients are of a younger and therefore working age.3 Currently, there is no gold-standard therapy for CNV in CSCR described in literature.
During the past decade, intravitreal bevacizumab (IVB) and ranibizumab (IVR) have been established as treatment for choroidal neovascularization (CNV) secondary to age-related macular degeneration.4, 5, 6, 7, 8, 9, 10 The ratio of treating CNV with anti-vascular endothelial growth factor (anti-VEGF) is hindering angiogenesis factor being produced by retinal pigment epithelium (RPE) and retinal photoreceptors, and hence avoiding initiating and supporting the growth of abnormal CNV.11, 12
The use of indocyanine green angiography (ICGA) in diagnosing CSCR patients has improved our understanding in the pathogenesis of CSCR. It has been proven that CSCR primarily affects the choroidal circulation, resulting in multifocal areas of choroidal vascular hyperpermeability. As a result of an increased leakage of the choroidal vessels persisting SRF and hence serous retinal detachment mainly accumulate, allowing an exudation from the choroidal vasculature to pass into the subretinal space.13, 14, 15
The presumed therapeutic mechanism of action of photodynamic therapy (PDT) with verteporfin (Visudyne, Novartis Pharma AG, Basel, Switzerland) for treating CSCR is closure of vascular channels in the choriocapillaris leading to hypoperfusion and long-term choroidal vascular remodeling.14
Promising results of previous studies on acute and chronic CSCR conducted at our tertiary center allow us to follow a PDT regimen, using half-dose fluence rate (25 J/cm2), while not changing the dose of verteporfin (6 mg/m2).16, 17, 18 Selecting an appropriate fluence rate avoids collateral damage such as atrophy of the RPE, choroidal ischemia, and development of secondary CNV due to less choriocapillaris damage.19, 20, 21
This retrospective study targets on a treatment regimen for CNV in chronic CSCR that has not been described in literature before: a combination of IVR/IVB and ICGA-guided PDT with half-fluence rate.
Main objectives were assessments of changes in best-corrected ETDRS letter score, as well as changes in central foveal thickness (CFT) at final follow-up. Secondary objectives included assessment of adverse events and mean number of treatments.
Materials and methods
Patients
We retrospectively reviewed the charts of 17 eyes of 17 consecutive phakic Caucasian patients referred to our tertiary care center (Medical Retina Unit, Department of Ophthalmology, Rudolf Foundation Hospital, Vienna, Austria) treated with a combined therapy of IVR or IVB and ICGA-guided verteporfin (6 mg/m2) PDT with half-fluence rate (25 J/cm2) for secondary CNV in chronic CSCR between February 2009 and January 2014. Informed consent was obtained from all patients before treatment, and the study was in adherence to the tenets of the Declaration of Helsinki.
Patients included in the study showed CNV in chronic CSC verified at baseline, with active angiographic leakage in fluorescein angiography (FA; HRA+OCT Spectralis, Heidelberg Engineering GmbH, Heidelberg, Germany), abnormal dilated choroidal vasculature with hyperpermeability in ICGA, present SRF involving the fovea on spectral domain optical coherence tomography (SD-OCT; Cirrus, Carl Zeiss Meditec, Dublin, CA, USA) and symptoms of at least 6-month duration.
Patients who had previously received either PDT or IVB/IVR, previous ocular surgery, or iatrogenic CSCR caused by corticosteroids were not included in this study. At baseline, all patients underwent a complete ophthalmic examination, including determination of best-corrected Early Treatment Diabetic Retinopathy Study (ETDRS) letter score, indirect biomicroscopy, FA, ICGA, and SD-OCT. All were assessed before treatment and followed up at 1, 3, 6, 9, and 12 months after PDT performing ETDRS VA examination and OCT. FA and ICGA were performed at baseline for diagnostic purposes. Re-treatment was administered in case of persisting or recurrent SRF findings in SD-OCT at months 1, 3, 6, 9, and 12.
Photodynamic therapy-spot size determination
The area of choroidal vascular abnormality on ICGA (30° image-field setting) that was connected to the area of serous macular detachment was measured with the built-in measurement software (Heidelberg Eye Explorer version 1.6.4.0; HRA+OCT Spectralis, Heidelberg Engineering).
Then a calibrated Opal PDT-Laser (Coherent Inc., Santa Clara, CA, USA) and an indirect condensing laser lens (Mainster Wide Field) by Ocular Instruments (Bellevue, Washington, USA) for PDT procedure were used. This combination approximates to the most exact spot size without having to add 1000 μm to the greatest linear dimension of the CSCR lesion as a safety margin around the whole lesion preventing over- or undertreatment of the choroidal vasculature. This has led to promising results in a previous study conducted in our tertiary care center.16, 17, 18, 22, 23
Intravitreal anti-VEGF
Patients received 0.5/1.25 mg of IVR/IVB under sterile conditions. Fourteen (82.3%) eyes received IVR and three (17.7%) eyes received IVB on a pro re nata regimen. Topical anesthesia was applied, and povidone-iodine 5% was used to scrub eyelids and lashes. Povidone-iodine eye drops 5% were applied several times and a sterile speculum was placed between the lids, then ranibizumab (0.5 mg) or bevacizumab (1.25 mg) with a 30-gauge needle was injected through the pars plana into the vitreous cavity 3.5 mm posterior to the limbus. The injection site was pressed with a sterile cotton swab for 1 min in order to prevent leakage and to lower the intraocular pressure. After injection, the patient received instructions to apply antibiotic drops to the study eye three times a day for 1 week.
Half-fluence PDT
The day following the IVR/IVB treatment, the safety-enhanced PDT protocol for CSCR was performed on each patient, using the standard dose of verteporfin (Visudyne, Novartis Pharma AG) that is 6 mg/m2 verteporfin.
The infusion of verteporfin was performed over 10 min; 15 min after the start of the infusion, a laser light at 689 nm delivered 25 J/cm2 with an intensity of 300 mW/cm2 over 83 s to both the area of choroidal hyperperfusion from which the subretinal macular fluid seemed to originate as observed in ICGA and the area of CNV as shown in FA. The leakage site was treated based on FA, as well as ICGA results because the area of abnormal choroidal vasculature with hyperpermeability can be visualized better with ICGA.24
All PDT treatments were performed by the same medical retina specialist (SA-S). The half-fluence (25 J/cm2) rate was selected based on two rationales: first the axial length having an effect of the actual effective amount of PDT light fluence, and second using half-fluence rate being sufficiently effective with minimizing choroidal hypoperfusion and hence possibly being safer and minimizing side effects as shown in the Visudyne in Minimally Classic Choroidal Neovascularization Study Group (VIM) study.25, 26, 27
After treatment, all patients were given protective spectacles and were instructed to avoid strong light for 48 h.
Baseline and follow-up examinations
A complete ophthalmic examination, including indirect slit-lamp biomicroscopy and determination of best-corrected ETDRS letter score at baseline and at 1, 3, 6, 9, and 12 months' follow-up using ETDRS charts by counting every correctly read letter in a darkened room in 4-m distance was performed. The ETDRS letter score was calculated by adding 30 to the number of letters correctly read at 4 m.28
The SD-OCT examination was performed at baseline and at every control visit. Before examination the pupil of the study eye was dilated to at least 6 mm diameter, with drops containing 0.5% tropicamide and 2.5% phenylnephrine (Mydriaticum, Agepha Pharmaceuticals, Vienna, Austria). Cirrus SD-OCT achieves an axial resolution of ~5 μm. Scanning speed is ~27 000 A-scans per second. For registration, scans are registered to a scanning laser ophthalmoscope image, which is performed with the scan. The scanning area measured 6x6mm. In this study we used the 512 × 128 × 1024 scan to image the foveal region. Scans performed by Cirrus OCT had to meet the quality limits reaching at least 5 out of 10 points at the quality score.
Statistical analysis
Patients' visits were classified into visit windows, defined as follows: 1 month (4–8 weeks), 3 months (2–4 months), 6 months (5–7 months), 9 months (8–10 months), and 12 months (11–13 months). If more than one visit occurred within a visit window, the one closest to the defined follow-up date was selected. To analyze the course of best-corrected ETDRS letter score and CFT between baseline and each timepoint (1, 3, 6, 9, and 12 months), linear mixed effect models for ETDRS and CFT were calculated, assuming heterogeneous variances at different timepoints and a common correlation between observations from the same patient. These models at least partially compensate for bias due to missing values and were used to estimate means and variances of ETDRS and CFT at the different timepoints. The models were also used to test the null hypotheses of no mean change between baseline and a given timepoint. Adjusted P-values for these tests were calculated using the Dunnett–Hsu correction for many-to-one comparisons. All calculations were done using the MIXED procedure in SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Graphs to visualize the changes in mean ETDRS and CFT were drawn with R version 3.1.1 (The R Foundation for Statistical Computing, Vienna, Austria). For all analyses, the significance level has been set to 0.05.
Results
A total of 17 eyes of 17 patients presenting CNV due to chronic CSCR received IVB/IVR combined with half-fluence PDT. The mean age±SD of the patients was 53±6.1 years (range: 43–62), and 12 (71%) were male. Mean best-corrected ETDRS letter score was 65.6 letters (±6.7; n=17; range: 10–96) at baseline. The mean PDT laser spot size was 2274 μm (±773; range: 1500–3900 μm). Patients' characteristics are shown in Table 1.
Table 1. Baseline demographics of 17 eyes of 17 patients with choroidal neovascularization due to chronic central serous chorioretinopathy.
| All eyes (n=17) | |
|---|---|
| Mean age±SD (years) | 53±6.1 |
| Gender | |
| Male | 12 (70.5%) |
| Female | 5 (29.5%) |
| Mean±SD baseline best-corrected ETDRS letter score | 65.6±6.7 |
| Mean±SD baseline central foveal thickness (μm) | 308.7±80.6 |
Abbreviation: ETDRS, Early Treatment Direct Retinopathic Study.
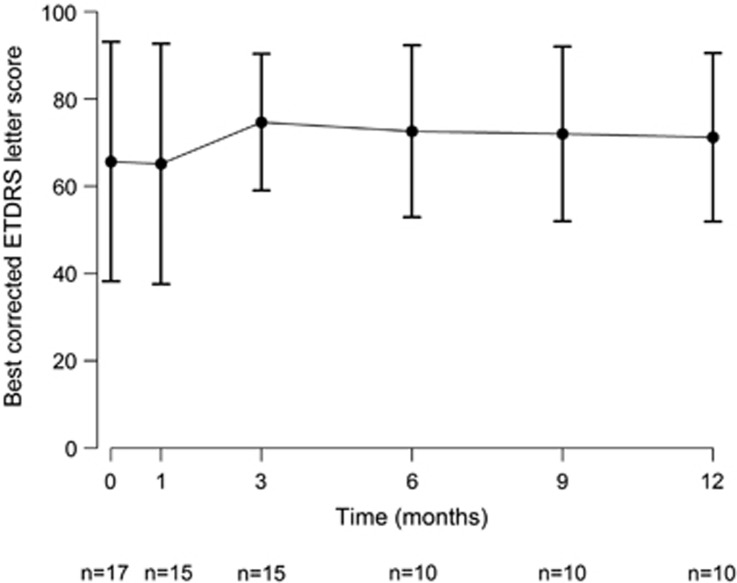
The mean ETDRS letter score initially worsened by a mean of 0.5 letters (P=0.99) to a mean of 65.1 (±27.6; range: 7–92; n=15) letters at 1 month.
A total of 15 eyes were evaluated at month 3, the mean ETDRS letter score improved, compared with baseline, by a mean of 9.0 letters (P=0.07) to 74.7 letters (±15.7; range: 54–95; n=15). Six months after PDT, 10 eyes were evaluated, mean ETDRS letter score showed an improvement compared with baseline by a mean of 7.0 letters (P=0.17) to 72.6 letters (±19.7; range: 51–97; n=10). Ten eyes were evaluated at month 9, the mean ETDRS letter score changed by a mean of 6.4 letters (P=0.23) to 72.0 letters (±20.0; range: 56–98; n=10).
Twelve months after PDT, 10 eyes were evaluated, and mean ETDRS letter score has changed by a mean of 5.6 letters (P=0.34) to 71.2 letters (±19.3; range: 55–94; n=10) compared with baseline, respectively.
In all, 7 (70%) out of 10 eyes evaluated at 12 months' control had improved or stable vision, but three had decreased vision.
The declining number of patients evaluated at the different follow-up visits is due to omitted follow-up visits. The changes in ETDRS letter score during the follow-up period are shown in Figure 1.
Figure 1.
Median change from baseline in best-corrected ETDRS letter score measured with the Early Diabetic Retinopathy Study chart at each study visit over time for eyes (n) treated with ICGA-guided half-fluence (25 J/cm2) PDT with verteporfin (6 mg/m2) combined with IVB/IVR. Error bars represent SEM.
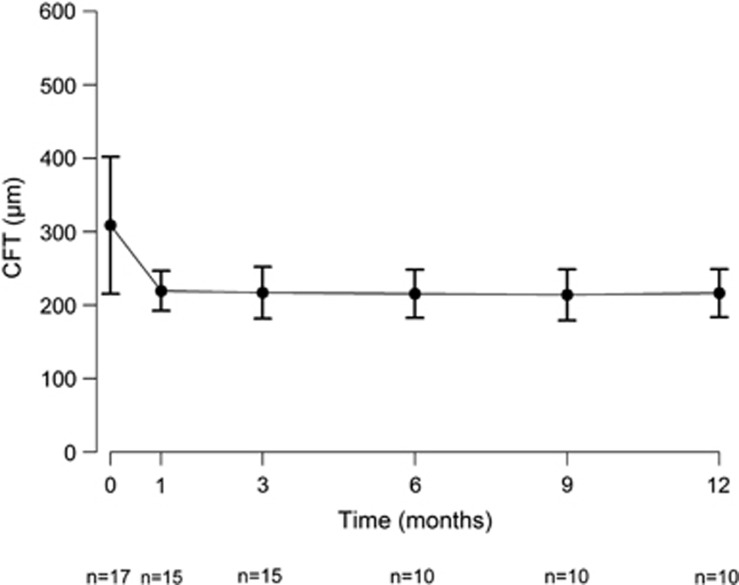
Pre-treatment CFT was 309 μm (±93.4; range: 185–517 μm; n=17) and declined to 220 μm (±27.2; range: 169–278 μm; n=15) at month 1 (P=0.0005), changed to 217 μm (±35.4; range: 167–285 μm; n=15) at 3 months' control (P=0.0003), to 216 μm (±32.8; range: 155–286 μm; n=10) at month 6 (P=0.0004), to 214 μm (±34.9; range: 150–275 μm; n=10) at month 9 (P=0.0003), and to 216 μm (±32.6; range: 166–282 μm; n=10) at month 12 (P=0.0004). Changes in CFT during the follow-up period are shown in Figure 2.
Figure 2.
Median change from baseline in CFT measured with the OCT (Cirrus) at each study visit over time for eyes (n) treated with ICGA-guided half-fluence (25 J/cm2) PDT with verteporfin (6 mg/m2) combined with IVB/IVR. Error bars represent SEM.
Because two patients did not attend any follow-up visits and five patients did not complete the entire 12 months' follow-up schedule, another calculation with those 10 patients who completed the follow-up period of 12 months was additionally performed. Mean ETDRS letter score at baseline was 77 (±12; median: 76), changed to 74.6 (±12.9; median: 73.5; P=0.49) at 1 month, to 76.9 (±12.8; median: 13.8; P=1) at 3 months, to 78.3 (±13.9; median: 82.5; P=0.5) at 6 months, to 77.8 (±14.2; median: 81; P=0.7) at 9 months, and finally to 76.8 (±13.7; median: 81.5; P=0.97) at 12 months' control visit. CFT changed from a baseline mean of 321 μm (±85.5; median: 326) to 228 μm (±29.7; median: 228; P=0.003) at 1 month, to 221 μm (±41.5; median: 229; P=0.01) at 3 months, to 216 μm (±40.7; median: 206; P=0.02) at 6 months; to 217 μm (±41; median: 218; P=0.008) at 9 months, and to 219 μm (±38.9; median: 218; P=0.007) at 12 months.
Baseline FA in all 17 (100%) eyes showed juxtafoveal fluorescein leakage, and ICGA of all 17 (100%) eyes demonstrated dilated choroidal vasculature with late extravascular leakage at the macula consistent with CNV in chronic CSCR.
The SD-OCT results at month 1 demonstrated resolution of SRF in all 15 examined eyes (100%).
None of the patients underwent a second half-dose PDT, eight patients (47%) received further IVR/IVB treatment, following the pro re nata regimen, summing up to a mean number of IVR/IVB treatments during follow-up period of 1.8±3.6 per patient. Re-treatment led to a complete resolution of exudation (SRF) in SC-OCT in 10 (100%) of 10 eyes, who completed the 12 months' follow-up period. Overall mean follow-up time was 7.9 months (±5.3; range: 0–12; median 12).
None of the treated eyes developed any systemic adverse event during verteporfin infusion or IVB/IVR treatment or during follow-up.
Discussion
In this retrospective study we evaluated the efficacy and the rate of side effects of half-fluence (25 J/cm2) PDT with verteporfin (6 mg/m2) combined with IVR/IVB in treatment of secondary CNV in chronic CSCR.
To our knowledge, results of a combined IVR/IVB pro re nata regimen with half-fluence PDT in treating CNV due to chronic CSCR have not been reported before.
It is typically thought that CSCR is not accompanied by CNV and that patients have a good visual prognosis, yet for those affected by CNV due to CSCR imminent help for saving sight is needed, and various studies were performed to better understand the nature of the disease, as well as aiming for a suitable but not aggressive treatment.1, 2, 3, 15, 16, 17, 18, 19, 20, 21
In a retrospective study, Mudvari et al29 found that none of the 340 consecutive CSCR patients developed CNV during an approximate 4-year follow-up period (mean of 49 months). However, Spaide et al30 reported that older patients with CSCR had a lower VA, and were more likely to have diffuse retinal pigment epitheliopathy and secondary CNV than their younger counterparts. Subsequent reports have suggested that classic CNV (mainly type 2) and polypoidal lesions are possible complications of CSCR and may contribute to visual loss in these eyes.31, 32, 33, 34
There are even possible challenges of correctly diagnosing CNV in CSCR.
Fung et al35 recently described 9 eyes with longstanding CSCR that went on to develop type 1 CNV. They concluded that a portion of these eyes was given a diagnosis of neovascular AMD, but should have been given a diagnosis of CNV secondary to CSCR instead, hence CNV masquerading as AMD.
Once the diagnosis of CNV in CSCR is secured, a safe and effective treatment is required. In this study, experience due to previous studies conducted at our tertiary center on acute and chronic CSCR enabled us to follow a PDT regimen, using half-dose fluence rate (25 J/cm2), while not changing the dose of verteporfin (6 mg/m2).16, 17, 18 Selecting an appropriate fluence rate helps avoiding collateral damage as atrophy of the RPE, choroidal ischemia, and development of secondary CNV due to less choriocapillaris damage.18, 19, 20 Said avoidance of negative treatment effect of RPE's metabolic activity was shown in a study of Hagen et al,36 where the focus was set on RPE alterations in eyes with acute CSCR treated with half-fluence PDT observed via short-wavelength fundus autofluorescence.
We focused on the exact calculation of the spot size, as this reduces risk of collateral damage such as choroidal ischemia and retinal atrophy. An excessive spot size may increase the risk of choriocapillaris closure and hypoxia while treatment with a spot size, which is too small, may not lead to the desired effect.23
In terms of safety profile, the use of the regimen applied in our study of pro re nata IVR/IVB combined with half-fluence ICGA-guided PDT seems to be promising.
Taking each patients' final follow-up visit into consideration (which was not always at 12 months due to omitted follow-up visits) 10 patients (58.8%) showed improved vision and 2 (11.7%) patients had stable vision. Those 3 patients (17.6%) whose vision had decreased at their last follow-up visit all received further IVR/IVB treatment.
One shortcoming of this study is its retrospective character. Of 17 consecutive patients treated for CNV in chronic CSCR, 10 completed the 12 months' follow-up period. Of these 10 patients, 7 (70%) had improved or stable vision and 3 (30%) had decreased vision at the 12 months' control visit. Five patients did not attend follow-up visits after month 3. All of them showed improved vision at 3 months' follow-up.
The long-term stability of half-fluence PDT combined with IVR/IVB, as well as the long-term effect of decreased choroidal perfusion on visual function need further investigations in order to assess the value of ICGA-guided half-fluence (25 J/cm2) PDT with verteporfin (6 mg/m2) combined with IVB/IVR in CNV due to chronic CSCR.
In conclusion, this 1-year retrospective review demonstrates ICGA-guided half-fluence PDT with verteporfin combined with IVR/IVB to be a safe treatment option for secondary CNV in chronic CSCR. Satisfying outcomes in VA and a significant reduction of CFT were observed. No adverse events attributable to the treatment were detected.
The authors declare no conflict of interest.
References
- Fine HF, Ober MD, Hariprasad SM. Current concepts in managing central serous chorioretinopathy. Ophthalmic Surg Lasers Imaging Retina 2014; 45: 9–13. [DOI] [PubMed] [Google Scholar]
- Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol 1967; 63: 1–139. [PubMed] [Google Scholar]
- Inhoffen W, Ziemssen F, Bartz-Schmidt KU. Chronic central serous chorioretinopathy (cCSC): differential diagnosis to choroidal neovascularisation (CNV) secondary to age-related macular degeneration (AMD). Klin Monbl Augenheilkd 2012; 229: 889–896. [DOI] [PubMed] [Google Scholar]
- Boyer DS, Antoszyk AN, Awh CC, Bhisitkul RB, Shapiro H, Acharya NR, MARINA Study Group. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology 2007; 114: 246–252. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Review Retina 2006; 26: 859–870. [DOI] [PubMed] [Google Scholar]
- Rosenfeld PJ, Schwartz SD, Blumenkranz MS, Miller JW, Haller JA, Reimann JD et al. Maximum tolerated dose of a humanized anti-vascular endothelial growth factor antibody fragment for treating neovascular age-related macular degeneration. Ophthalmology 2005; 112: 1048–1053. [DOI] [PubMed] [Google Scholar]
- Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006; 113: 363–372. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Laud K, Fine HF, Klancnik JM Jr, Meyerle CB, Yannuzzi LA et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age related macular degeneration. Retina 2006; 26: 383–390. [DOI] [PubMed] [Google Scholar]
- Rich RM, Rosenfeld PJ, Puliafito CA, Dubovy SR, Davis JL, Flynn HW Jr et al. Short-term safety and efficacy of intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Retina 2006; 26: 495–511. [DOI] [PubMed] [Google Scholar]
- Bashshur ZF, Bazarbachi A, Schakal A, Haddad ZA, El Haibi CP, Noureddin BN. Intravitreal bevacizumab for the management of choroidal neovascularization in age-related macular degeneration. Am J Ophthalmol 2006; 142: 1–9. [DOI] [PubMed] [Google Scholar]
- Lopez PF, Sippy BD, Lambert HM, Thach AB, Hinton DR. Transdifferentiated retinal pigment epithelial cells are immunore active for vascular endothelial growth factor in surgically excised age-related macular degeneration-related choroidalneovascular membranes. Invest Ophthalmol Vis Sci 1996; 37: 855–868. [PubMed] [Google Scholar]
- Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci 1996; 37: 1929–1934. [PubMed] [Google Scholar]
- Prunte C, Flammer J. Choroidal capillary and venous congestion in central serous chorioretinopathy. Am J Ophthalmol 1996; 121: 26–34. [DOI] [PubMed] [Google Scholar]
- Piccolino FC, Borgia L. Central serous chorioretinopathy and indocyanine green angiography. Retina 1994; 14: 231–242. [DOI] [PubMed] [Google Scholar]
- Chan WM, Lai TY, Lai RY, Tang EW, Liu DT, Lam DS. Safety enhanced photodynamic therapy for chronic central serous chorioretinopathy: one-year results of a prospective study. Retina 2008; 28: 85–93. [DOI] [PubMed] [Google Scholar]
- Smretschnig E, Ansari-Shahrezaei S, Hagen S, Glittenberg C, Krebs I, Binder S. Half-fluence photodynamic therapy in chronic central serous chorioretinopathy. Retina 2013; 33: 316–323. [DOI] [PubMed] [Google Scholar]
- Hagen S, Ansari-Shahrezaei S, Smretschnig E, Glittenberg C, Krebs I, Graf A et al. The effect of photodynamic therapy on macular sensitivity in eyes with acute central serous chorioretinopathy. Graefes Arch Clin Exp Ophthalmol 2013; 251: 1081–1089. [DOI] [PubMed] [Google Scholar]
- Smretschnig E, Ansari-Shahrezaei S, Moussa S, Glittenberg C, Krebs I, Binder S. Half-fluence photodynamic therapy in acute central serous chorioretinopathy. Retina 2012; 32: 2014–2019. [DOI] [PubMed] [Google Scholar]
- Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003; 23: 752–763. [DOI] [PubMed] [Google Scholar]
- Levina R, Brucker AJ, Robinson F. Long-term follow up of idiopathic central serous chorioretinopathy by fluorescein angiography. Ophthalmology 1989; 96: 854–859. [DOI] [PubMed] [Google Scholar]
- Colucciello M. Choroidal neovascularization complicating photodynamic therapy for central serous retinopathy. Retina 2006; 26: 239–242. [DOI] [PubMed] [Google Scholar]
- Ansari-Shahrezaei S, Ergun E, Stur M. The effect of digital measurement software on photodynamic therapy. Graefes Arch Clin Exp Ophthalmol 2006; 244: 137–142. [DOI] [PubMed] [Google Scholar]
- Ansari-Shahrezaei S, Binder S, Stur M. The effect of laser unit on photodynamic therapy spot size. Graefes Arch Clin Exp Ophthalmol 2011; 249: 11–14. [DOI] [PubMed] [Google Scholar]
- Inoue R, Sawa M, Tsujikawa M, Gomi F. Association between the efficacy of photodynamic therapy and indocyanine green angiography findings for central serous chorioretinopathy. Am J Ophthalmol 2010; 149: 441–466. [DOI] [PubMed] [Google Scholar]
- Kondo M, Ito Y, Miyata K, Kondo N, Ishikawa K, Terasaki H. Effect of axial length on laser spot size during photodynamic therapy: an experimental study in monkeys. Am J Ophthalmol 2006; 141: 214–215. [DOI] [PubMed] [Google Scholar]
- Ansari-Shahrezaei S, Ergun E, Stur M. The effect of axial length on photodynamic therapy. Am J Ophthalmol 2006; 141: 699–702. [DOI] [PubMed] [Google Scholar]
- Visudyne in Minimally Classic Choroidal Neovascularization Study Group. Verteporfin therapy of subfoveal minimally classic choroidal neovascularization in age-related macular degeneration. 2-year results of a randomized clinical trial. Arch Ophthalmol 2005; 123: 448–457. [DOI] [PubMed] [Google Scholar]
- Ferris FL III, Bailey I. Standardizing the measurement of visual acuity for clinical research studies: guidelines from the Eye Care Technology Forum. Ophthalmology 1996; 103: 181–182. [DOI] [PubMed] [Google Scholar]
- Mudvari SS, Goff MJ, Fu AD, McDonald HR, Johnson RN, Ai E et al. The natural history of pigment epithelial detachment associated with central serous chorioretinopathy. Retina 2007; 27: 1168–1173. [DOI] [PubMed] [Google Scholar]
- Spaide RF, Campeas L, Haas A, Yannuzzi LA, Fisher YL, Guyer DR et al. Central serous chorioretinopathy in younger and older adults. Ophthalmology 1996; 103: 2070–2079. [DOI] [PubMed] [Google Scholar]
- Cooper BA, Thomas MA. Submacular surgery to remove choroidal neovascularization associated with central serous chorioretinopathy. Am J Ophthalmol 2000; 130: 187–191. [DOI] [PubMed] [Google Scholar]
- Chan WM, Lam DS, Lai TY, Yuen KS, Liu DT, Chan CK et al. Treatment of choroidal neovascularization in central serous chorioretinopathy by photodynamic therapy with verteporfin. Am J Ophthalmol 2003; 136: 836–845. [DOI] [PubMed] [Google Scholar]
- Yang CS, Chen KC, Lee SM, Lee FL. Photodynamic therapy in the treatment of choroidal neovascularization complicating central serous chorioretinopathy. J Chin Med Assoc 2009; 71: 501–505. [DOI] [PubMed] [Google Scholar]
- Ahuja RM, Downes SM, Stanga PE, Koh AH, Vingerling JR, Bird AC. Polypoidal choroidal vasculopathy and central serous chorioretinopathy. Ophthalmology 2001; 108: 1009–1010. [DOI] [PubMed] [Google Scholar]
- Fung AT, Yannuzzi LA, Freund KB. Type 1 (sub-retinal pigmentepithelial) neovascularization in central serous chorioretinopathy masquerading as neovascular age-related macular degeneration. Retina 2012; 32: 1829–1837. [DOI] [PubMed] [Google Scholar]
- Hagen S, Ansari-Shahrezaei S, Smretschnig E, Glittenberg C, Krebs I, Steiner I et al. Effect of photodynamic therapy on short-wavelength fundus autofluorescence in eyes with acute central serous chorioretinopathy. Retina 2015; 35: 223–230. [DOI] [PubMed] [Google Scholar]