Abstract
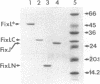
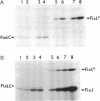
Transcription of nitrogen fixation (nif and fix) genes in Rhizobium meliloti is induced by a decrease in oxygen concentration. The products of two genes, fixL and fixJ, are responsible for sensing and transmitting the low-oxygen signal. The proteins encoded by fixL and fixJ (FixL and FixJ, respectively) are homologous to a family of bacterial proteins that transduce environmental signals through a common phosphotransfer mechanism [David, M., Daveran, M., Batut, J., Dedieu, A., Domergue, O., Ghai, J., Hertig, C., Boistard, P. & Khan, D. (1988) Cell 54, 671-683]. FixL, the oxygen sensor, is a membrane protein. It has previously been shown that a soluble derivative of FixL, FixL*, is an oxygen-binding hemoprotein and a kinase that autophosphorylates and also phosphorylates FixJ [Gilles-Gonzalez, M. A., Ditta, G. S. & Helinski, D. R. (1991) Nature (London) 350, 170-172]. In this work, deletion derivatives of fixL* were constructed and overexpressed in Escherichia coli, and the truncated proteins were purified. We show that a fragment of FixL from amino acid residue 127 to residue 260 binds heme, retains the ability to bind oxygen, and has no detectable kinase activity. A C-terminal fragment of FixL, beginning at residue 260, fails to bind heme but is active as a kinase. We also demonstrate that anaerobiosis results in an enhancement of FixL* autophosphorylation and FixJ phosphorylation activities in vitro. Finally, we show that the heme-binding region of FixL is required in vitro for oxygen regulation of its kinase activities.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar O. M., Reiländer H., Arnold W., Pühler A. Rhizobium meliloti nifN (fixF) gene is part of an operon regulated by a nifA-dependent promoter and codes for a polypeptide homologous to the nifK gene product. J Bacteriol. 1987 Dec;169(12):5393–5400. doi: 10.1128/jb.169.12.5393-5400.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Mizuno T., Mizushima S. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of the ompF and ompC genes in Escherichia coli. J Biol Chem. 1989 May 25;264(15):8563–8567. [PubMed] [Google Scholar]
- Albright L. M., Huala E., Ausubel F. M. Prokaryotic signal transduction mediated by sensor and regulator protein pairs. Annu Rev Genet. 1989;23:311–336. doi: 10.1146/annurev.ge.23.120189.001523. [DOI] [PubMed] [Google Scholar]
- Anthamatten D., Hennecke H. The regulatory status of the fixL- and fixJ-like genes in Bradyrhizobium japonicum may be different from that in Rhizobium meliloti. Mol Gen Genet. 1991 Jan;225(1):38–48. doi: 10.1007/BF00282640. [DOI] [PubMed] [Google Scholar]
- Batut J., Daveran-Mingot M. L., David M., Jacobs J., Garnerone A. M., Kahn D. fixK, a gene homologous with fnr and crp from Escherichia coli, regulates nitrogen fixation genes both positively and negatively in Rhizobium meliloti. EMBO J. 1989 Apr;8(4):1279–1286. doi: 10.1002/j.1460-2075.1989.tb03502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter P., Bedouelle H., Winter G. Improved oligonucleotide site-directed mutagenesis using M13 vectors. Nucleic Acids Res. 1985 Jun 25;13(12):4431–4443. doi: 10.1093/nar/13.12.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- David M., Daveran M. L., Batut J., Dedieu A., Domergue O., Ghai J., Hertig C., Boistard P., Kahn D. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell. 1988 Aug 26;54(5):671–683. doi: 10.1016/s0092-8674(88)80012-6. [DOI] [PubMed] [Google Scholar]
- Di Iorio E. E. Preparation of derivatives of ferrous and ferric hemoglobin. Methods Enzymol. 1981;76:57–72. doi: 10.1016/0076-6879(81)76114-7. [DOI] [PubMed] [Google Scholar]
- Ditta G., Virts E., Palomares A., Kim C. H. The nifA gene of Rhizobium meliloti is oxygen regulated. J Bacteriol. 1987 Jul;169(7):3217–3223. doi: 10.1128/jb.169.7.3217-3223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forst S., Delgado J., Inouye M. Phosphorylation of OmpR by the osmosensor EnvZ modulates expression of the ompF and ompC genes in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6052–6056. doi: 10.1073/pnas.86.16.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R. T., Jr, Becker R. R. Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal Biochem. 1984 Feb;136(2):509–514. doi: 10.1016/0003-2697(84)90253-7. [DOI] [PubMed] [Google Scholar]
- Gilles-Gonzalez M. A., Ditta G. S., Helinski D. R. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature. 1991 Mar 14;350(6314):170–172. doi: 10.1038/350170a0. [DOI] [PubMed] [Google Scholar]
- Huang Y., Morel P., Powell B., Kado C. I. VirA, a coregulator of Ti-specified virulence genes, is phosphorylated in vitro. J Bacteriol. 1990 Feb;172(2):1142–1144. doi: 10.1128/jb.172.2.1142-1144.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo M. M., Ninfa A. J., Stock J. B., Silhavy T. J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989 Nov;3(11):1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- Igo M. M., Silhavy T. J. EnvZ, a transmembrane environmental sensor of Escherichia coli K-12, is phosphorylated in vitro. J Bacteriol. 1988 Dec;170(12):5971–5973. doi: 10.1128/jb.170.12.5971-5973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin S., Roitsch T., Ankenbauer R. G., Gordon M. P., Nester E. W. The VirA protein of Agrobacterium tumefaciens is autophosphorylated and is essential for vir gene regulation. J Bacteriol. 1990 Feb;172(2):525–530. doi: 10.1128/jb.172.2.525-530.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski P. A., Elmerich C. Involvement of fixLJ in the regulation of nitrogen fixation in Azorhizobium caulinodans. Mol Microbiol. 1991 Mar;5(3):665–673. doi: 10.1111/j.1365-2958.1991.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Kaminski P. A., Mandon K., Arigoni F., Desnoues N., Elmerich C. Regulation of nitrogen fixation in Azorhizobium caulinodans: identification of a fixK-like gene, a positive regulator of nifA. Mol Microbiol. 1991 Aug;5(8):1983–1991. doi: 10.1111/j.1365-2958.1991.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Keener J., Kustu S. Protein kinase and phosphoprotein phosphatase activities of nitrogen regulatory proteins NTRB and NTRC of enteric bacteria: roles of the conserved amino-terminal domain of NTRC. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4976–4980. doi: 10.1073/pnas.85.14.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullik I., Fritsche S., Knobel H., Sanjuan J., Hennecke H., Fischer H. M. Bradyrhizobium japonicum has two differentially regulated, functional homologs of the sigma 54 gene (rpoN). J Bacteriol. 1991 Feb;173(3):1125–1138. doi: 10.1128/jb.173.3.1125-1138.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino K., Shinagawa H., Amemura M., Kawamoto T., Yamada M., Nakata A. Signal transduction in the phosphate regulon of Escherichia coli involves phosphotransfer between PhoR and PhoB proteins. J Mol Biol. 1989 Dec 5;210(3):551–559. doi: 10.1016/0022-2836(89)90131-9. [DOI] [PubMed] [Google Scholar]
- Ninfa A. J., Magasanik B. Covalent modification of the glnG product, NRI, by the glnL product, NRII, regulates the transcription of the glnALG operon in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5909–5913. doi: 10.1073/pnas.83.16.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa A. J. Protein phosphorylation and the regulation of cellular processes by the homologous two-component regulatory systems of bacteria. Genet Eng (N Y) 1991;13:39–72. doi: 10.1007/978-1-4615-3760-1_2. [DOI] [PubMed] [Google Scholar]
- Nixon B. T., Ronson C. W., Ausubel F. M. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7850–7854. doi: 10.1073/pnas.83.20.7850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F. Regulation of oxygen affinity of hemoglobin: influence of structure of the globin on the heme iron. Annu Rev Biochem. 1979;48:327–386. doi: 10.1146/annurev.bi.48.070179.001551. [DOI] [PubMed] [Google Scholar]
- Ronson C. W., Nixon B. T., Ausubel F. M. Conserved domains in bacterial regulatory proteins that respond to environmental stimuli. Cell. 1987 Jun 5;49(5):579–581. doi: 10.1016/0092-8674(87)90530-7. [DOI] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Szeto W. W., Nixon B. T., Ronson C. W., Ausubel F. M. Identification and characterization of the Rhizobium meliloti ntrC gene: R. meliloti has separate regulatory pathways for activation of nitrogen fixation genes in free-living and symbiotic cells. J Bacteriol. 1987 Apr;169(4):1423–1432. doi: 10.1128/jb.169.4.1423-1432.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto W. W., Zimmerman J. L., Sundaresan V., Ausubel F. M. A Rhizobium meliloti symbiotic regulatory gene. Cell. 1984 Apr;36(4):1035–1043. doi: 10.1016/0092-8674(84)90053-9. [DOI] [PubMed] [Google Scholar]
- Virts E. L., Stanfield S. W., Helinski D. R., Ditta G. S. Common regulatory elements control symbiotic and microaerobic induction of nifA in Rhizobium meliloti. Proc Natl Acad Sci U S A. 1988 May;85(9):3062–3065. doi: 10.1073/pnas.85.9.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Philip P., Batut J., Boistard P. Rhizobium meliloti Fix L is an oxygen sensor and regulates R. meliloti nifA and fixK genes differently in Escherichia coli. J Bacteriol. 1990 Aug;172(8):4255–4262. doi: 10.1128/jb.172.8.4255-4262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]