Abstract
Aims
Left ventricular (LV) systolic elastance (Ees) and diastolic elastance (Eed) correlate with arterial elastance (Ea), but it is unknown how chronic changes in arterial compliance and resistance, which determine Ea, might differentially affect cardiac properties with aging. We sought to characterize chronic changes in pulsatile and resistive arterial load and correlate them with longitudinal changes in LV structure and function in a prospective, community-based study.
Methods and results
Comprehensive echocardiography was performed in 722 subjects participating in a randomly-selected community-based study at two examinations separated by 4 years, allowing for assessment of LV Ees, Eed, end diastolic volume (EDV), Ea, total arterial compliance and systemic vascular resistance at both examinations. Chronic changes in resistance and heart rate were the dominant contributors to change in Ea. Changes in arterial compliance had little impact on changes in Ea, but were strongly associated with changes in Ees. The combination of increased resistance and decreased compliance was associated with the largest increase in LV diastolic stiffness, an effect that was mediated by decrease in LVEDV. In contrast, subjects with both improved arterial compliance and decreased resistance displayed an increase in LVEDV over time, with no increase in LV Eed.
Conclusion
Increases in pulsatile arterial load with aging contribute more to LV systolic stiffening, while combined pulsatile and resistive loading changes are associated with positive and negative chamber remodeling and diastolic stiffness. Therapies designed to improve arterial resistance particularly enhance aortic compliance may hold promise to deter or reverse cardiac aging and its sequelae.
Keywords: arterial compliance, arterial resistance, left ventricular stiffness, aging, heart failure, heart failure with preserved ejection fraction
INTRODUCTION
Interactions between the left ventricle (LV) and the arterial system are key determinants of cardiovascular function. Ventricular-arterial interaction refers to the coupling between the LV during contraction (end-systolic elastance, Ees) and the load imposed by the systemic vasculature, effective arterial elastance (Ea). Ea is a lumped parameter or “net” arterial stiffness that is related to mean systematic vascular resistance, heart rate, aortic characteristic impedance and total arterial compliance.1 Acute and chronic changes in Ea are correlated with changes in Ees.2 However, different combinations of resistance and compliance can yield the same Ea, but impose different loads on the LV due to differences in pulsatile versus resistive loading.3 Acute changes in pulsatile arterial loading are known to exert negative effects on LV relaxation,4 but it remains unclear how chronic changes in mean vascular resistance and arterial compliance might differentially modulate LV structure and function.
Left ventricular Ees and end-diastolic elastance (Eed) increase with senescence.2 Recent studies suggest LV chamber volume may decrease in normal aging,5 coupled with a leftward-shifted diastolic pressure volume relationship.6 It is possible that chronic changes in systemic arterial resistance and compliance might differentially contribute to changes in LV properties with aging. Demonstration of any such difference would be important to inform future efforts to prevent or attenuate age-related LV stiffening and remodeling and the secondary diseases associated with it, such as heart failure with preserved ejection fraction (HFpEF).
We hypothesized that deconvolution of arterial load into resistive and oscillatory components may provide additional insight into age-related changes in ventricular function and remodelling. To test this hypothesis, we compared the impact of chronic changes in arterial compliance and resistance on LV structure and function over 4 years’ time in a longitudinal, population-based study.
METHODS
Study Population
A random sample of Olmsted County residents ≥45 years of age was identified in 1997 by applying a sampling fraction of 7% within each sex- and age-specific (5years) stratum. 4203 persons were invited and 2042 (47%) participated in Exam 1 (1997–2000). Four years later all participants were invited to return and 1402 participated in Exam 2 (2001–2004). Examination at both exams consisted of physical examination, echocardiography, and medical record abstraction. Diabetes history was based on physician diagnosis and treatment. Myocardial infarction and hypertension were diagnosed according to criteria from the World Health Organization and the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, respectively. Some clinical and echocardiographic data from this population have been previously reported,2, 7–9 but the current study findings regarding the impact of chronic changes in arterial compliance and resistance on end-systolic and end-diastolic elastance over time have not been reported. The study was approved by the Institutional Review Boards of Mayo Clinic and Olmsted Medical Center and complied with the Declaration of Helsinki. Participants provided written informed consent for evaluation and medical record follow-up.
Assessment of LV Structure and Function
Comprehensive echocardiographic assessment was performed by registered diagnostic cardiac sonographers using standardized instruments and techniques as previously described.7, 10 Exam 1 and 2 echocardiograms were performed by the same three sonographers and reviewed by two cardiologists who were masked to clinical and Exam 1 echocardiogram findings. All parameters were measured in triplicate and averaged. LV stroke volume (SV) was calculated from pulse wave Doppler of the LV outflow tract and LV outflow tract diameter. Cardiac output (CO) was determined as the product of stroke volume and heart rate. Brachial blood pressure (BP) was measured by automated device at the time of echocardiographic examination. End-systolic pressure (ESP) was determined from the product of 0.9*systolic BP.
The ratio of echo-Doppler and tissue-Doppler early diastolic velocities (E/e’) was used to estimate LV end diastolic pressure (LVEDP) as previously validated (=11.96+0.596*E/e’).11 Operating LV end diastolic chamber stiffness (Eed) was estimated by the ratio of LVEDP and end diastolic volume.12 LV end systolic elastance (Ees) was determined by the single beat technique of Chen and colleagues based upon measured SV, EF, BP and systolic preejection and ejection time (ET) intervals as previously reported.7, 10
Assessment of Arterial Function and Ventricular-Arterial Interaction
Effective arterial elastance (Ea), a measure of total arterial load that incorporates both mean and pulsatile components, was determined as ESP/SV. Systemic vascular resistance (SVR) was calculated as =mean BP*80/CO and total arterial compliance (TAC) as =SV/pulse pressure. The ratio Ea/Ees was used to assess ventricular-arterial coupling.10 Ventricular and arterial elastances represent static measures, and in order to evaluate time-related measurements, longitudinal changes in ET were evaluated.
Statistical methods
Continuous values are expressed as mean ± SD or estimated marginal mean (95% confidence interval). Proportions are expressed as percentages. In graphs estimated marginal mean and 95% confidence interval is plotted. Longitudinal changes in categorical variables were assessed with McNemar test and continuous variables by paired t-test or Wilcoxon signed-rank test, as appropriate. Stepwise linear regression with adjustment for age, sex and baseline values was used to assess factors influencing Ea, Ees, compliance and resistance change over time. Semi-partial R2 is reported, which represents proportion of explained variability by each variable. Due to significant correlation between compliance and resistance (r= −0.52, p<0.0001) that may have effect on results of the linear regression analysis (despite the tolerance factor assessing multicollinearity was not <0.1), in order to further evaluate the independent influence of chronic changes in arterial compliance and resistance on Ea, Ees and Eed changes over time, we created four groups according to compliance and resistance change. We used univariate general linear model to compare differences in Ea, Ees, Eed changes over time with adjustment for age, sex and baseline values of Ea, Ees and Eed, respectively. Bonferroni correction was used to correct for multiple testing. ANOVA and χ2 tests were used to compare differences between groups, as appropriate. Also results of these tests were corrected using the Bonferroni correction. Calculations were done using SPSS 19 (IBM Corporation, NY, USA). A two-sided p-value <0.05 was considered statistically significant.
RESULTS
Of 1402 Olmsted County residents participating in examination 2, 772 had complete echocardiographic data to allow for assessment of ventricular elastance and arterial compliance, elastance and resistance at both examinations. Subjects with complete echocardiographic data were slightly younger (60±9 vs. 61±10 years, p=0.03) but did not differ in sex proportion from individuals with incomplete paired data. As previously reported,2 over 4 years, systolic, diastolic, and mean BPs decreased by 4.6±18.3, 4.3±9.7, and 4.4±11.3 mmHg, respectively, while pulse pressure did not change (+0±15 mmHg; p=0.4; Table 1). LV mass decreased, while chamber size and LVEF remained stable on average. Ea decreased by 3% (p=0.003), while Ees and Eed increased by 14% and 8%, respectively (both p<0.0001). Longitudinal changes in Ea were correlated with changes in Ees (r=0.59, p<0.0001) and changes in Eed (r=0.56, p<0.0001).
Table 1.
Patient characteristic at study entry and after 4 years
| Examination 1 (1997–2000) |
Examination 2 (2001–2004) |
p | |
|---|---|---|---|
| Age, years | 60±9 | 64±9 | <0.001 |
| Body mass index, kg/m2 | 27.9±4.9 | 28.1±4.8 | 0.001 |
| Heart rate, bmp | 64.8±10.4 | 65.6±12.0 | 0.04 |
|
| |||
| Systolic BP, mm Hg | 130.3±19.9 | 125.7±19.3 | <0.001 |
| Diastolic BP, mm Hg | 73.2±9.8 | 69.0±9.6 | <0.001 |
| Pulse pressure, mm Hg | 57.1±15.9 | 56.7±16.3 | 0.57 |
| Mean BP, mm Hg | 92.3±11.8 | 87.9±11.3 | <0.001 |
|
| |||
| SVR, dyne/second*cm5 | 1391±335 | 1309±341 | <0.001 |
| TAC, mL/mm Hg | 1.63±0.57 | 1.65±0.59 | 0.25 |
| Ea, mm Hg/mL | 1.39±0.34 | 1.36±0.36 | 0.003 |
| Ees, mm Hg/mL | 2.09±0.66 | 2.26±0.70 | <0.001 |
| Eed, mm Hg/mL | 0.13±0.03 | 0.14±0.04 | <0.001 |
| Ea/Ees | 0.69±0.15 | 0.62±0.14 | <0.001 |
|
| |||
| LVEDV, mL | 136±32 | 139±37 | 0.54 |
| LV mass index, g/m2 | 95±19 | 91±20 | <0.001 |
| LVM/EDV | 1.34±0.34 | 1.30±0.34 | 0.19 |
| LV ejection fraction, % | 64±7 | 64±7 | 0.9 |
BP – blood pressure; SVR – systematic vascular resistance; TAC – total arterial compliance; Ea – arterial elastance; Ees – end-systolic elastance; Eed – end-diastolic elastance; LVEDV – left ventricular end-diastolic volume; LV – left ventricular
Systemic vascular resistance decreased by 83±354 dyne/second*cm5 (p<0.0001), while total arterial compliance did not change in the overall population (+0.02±0.55 mL/mmHg, p=0.25). Systemic resistance and compliance displayed a hyperbolic relationship with one another at both examinations (Figure 1), and compared to Examination 1, the resistance-compliance curve shifted slightly leftward over 4 years (p=0.01, ANCOVA after log-log transform).
Figure 1.
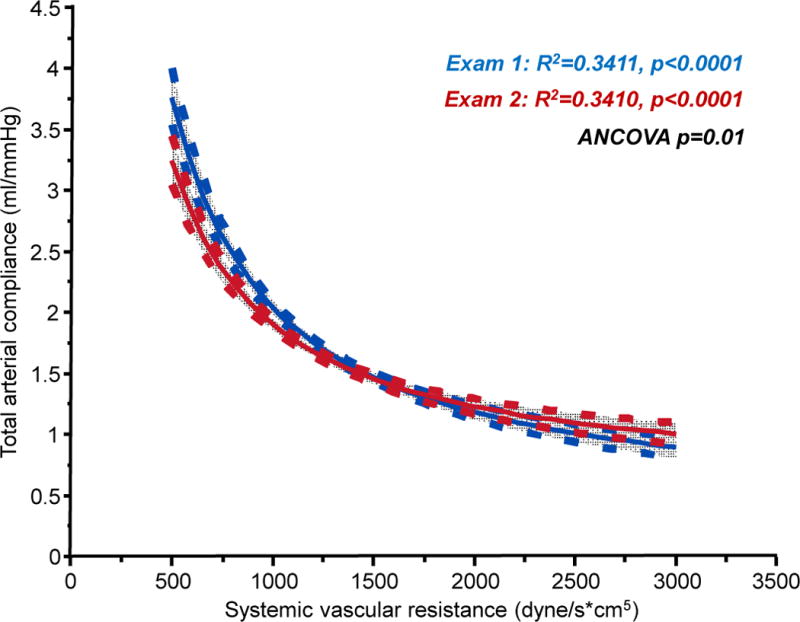
Plot of total arterial compliance versus systemic vascular resistance at examination 1 (blue) and examination 2 (red) showing mean regression lines and 95% CI of the mean. P value reflects ANCOVA comparing the two relations after log-log transformation.
In multivariable regression analysis, the change in Ea from Examination 1 to 2 was largely mediated by changes in resistance, which explained 42% of the variability in ΔEa, followed by changes in HR that explained 22% of ΔEa (Table 2). Changes in arterial compliance explained only 3% of the variability in ΔEa.
Table 2.
Correlations between changes in Resistance, Compliance and Heart Rate changes on Ventricular-arterial Stiffness
| Model | Variables | Beta | R2 | p |
|---|---|---|---|---|
| ΔEa | ||||
| adj R2=86.7%, p<0.0001 | ΔSVR | 0.001 | 41.9% | <0.0001 |
| ΔHR | 0.013 | 21.8% | <0.0001 | |
| ΔTAC | −0.130 | 3.0% | <0.0001 | |
|
| ||||
| ΔEes | ||||
| adj R2=46.2%, p<0.0001 | ΔTAC | −0.233 | 13.2% | <0.0001 |
| ΔSVR | 0.001 | 2.5% | <0.0001 | |
| ΔHR | 0.02 | 4.1% | <0.0001 | |
|
| ||||
| ΔEed | ||||
| adj R2=34.7%, p<0.0001 | ΔSVR | 0.001 | 13.0% | <0.0001 |
| ΔHR | 0.001 | 6.8% | <0.0001 | |
| ΔTAC | −0.001 | 0% | 0.67 | |
Changes in Ees were correlated with changes in Ea (r=0.59, p=0.001). However, while changes in compliance explained little of the variability in ΔEa, reductions in arterial compliance were the strongest correlate of the age-related ΔEes, explaining 13% of its variability (Table 2). In contrast, changes in resistance and HR explained only 3% and 4% of the variability in the change in Ees. Changes in resistance and HR explained 13% and 7% of Eed variability, while change in arterial compliance was not associated with ΔEed.
Multivariable linear regression (Table 2) is susceptible to co-linearity between explanatory variables, which may falsely decrease the strength of (or even mask) the association between one explanatory variable and the dependent variable. Because TAC and SVR are strongly correlated with one another (Figure 1), we next evaluated the influence of changes in arterial compliance and resistance on cardiovascular structure and function by creating four groups that were sorted according to the proposed effect of changes in pulsatile (TAC) and resistive (SVR) load. Comparison across these groups is not influenced by co-linearity, thus providing a more unbiased insight than the regression analysis. Groups 1 to 4 were ordered by progressively increasing arterial load. In groups 1 and 4, both pulsatile and resistive load either decreased (Group 1: ↑TAC and ↓SVR) or increased (Group 4: ↓TAC and ↑SVR). In Groups 2 and 3, pulsatile load decreased (↑TAC but ↑SVR; Group 2) or increased (↓TAC but ↓SVR; Group 3) with discordant changes in resistive load.
There were no differences in age, sex, baseline medication use or changes in medication use between the 4 groups (Table 3). After adjusting for age, sex, and baseline values, ΔEa, and ΔEes increased with progressive increases in pulsatile and/or resistive load across groups (p for linear trend <0.001, Figure 2). There was more reduction in Ea in Group 1 (pulsatile and resistive load decrease) compared to Group 2 (only pulsatile load decrease, p<0.001; Figure 2A), yet there was no difference in ΔEes between these groups that both shared a reduction in pulsatile load (p=0.3) (Figure 2B). Conversely, there was no statistically significant difference in ΔEa between Groups 2 and 3 (with opposing changes in pulsatile and resistive load), yet there were marked differences in ΔEes, which increased more in subjects with compliance reduction as compared to those with resistance increase (p=0.005).
Table 3.
Hemodynamics and Ventricular-Arterial Mechanics Grouped by Resistance and Compliance Change
| ↓Pulsatile Load | ↑Pulsatile Load | ||||
|---|---|---|---|---|---|
| ↓Resistive Load | ↑Resistive Load | ↓Resistive Load | ↑Resistive Load | ||
| Group 1 (n=308) TAC↑, SVR↓ | Group 2 (n=80) TAC↑, SVR↑ | Group 3 (n=152) TAC↓, SVR↓ | Group 4 (n=232) TAC↓, SVR↑ | p | |
| Age at Examination 1, years | 60.7±9.0 | 59.0±8.9 | 59.2±9.3 | 60.7±9.9 | 0.2 |
| Male sex, n (%) | 148 (48%) | 34 (43%) | 73 (48%) | 144 (49%) | 0.7 |
| Anthropometrics | |||||
| Body mass index Ex1, kg/m2 | 28.3±4.9 | 28.1±4.7 | 27.9±5.0 | 27.2±4.8 | 0.045 |
| ΔWeight, kg | −0.4±5.1 | +0.5±6.1 | +0.5±5.0 | +1.5±4.1* | 0.001 |
| Hemodynamics | |||||
| HR Ex1, bpm | 64.1±10.3 | 68.7±11.2* | 62.3±9.3¶ | 66.0±10.4† | <0.0001 |
| ΔHR, bpm | +1.7±9.7 | −7.9±10.3* | +7.7±12.9*¶ | −2.0±9.4*¶† | <0.0001 |
| ET Ex 1, msec | 337±29 | 342±31 | 340±28 | 343±31 | 0.09 |
| ΔET, msec | 28±38 | 21±37 | 27±41 | 17±38* | 0.008 |
| SBP Ex1, mm Hg | 135.7±19.8 | 133.4±19.4 | 124.7±16.7*¶ | 125.8±20.2*¶ | <0.0001 |
| ΔSBP, mm Hg | −14.5±16.0 | −12.0±14.7 | +3.3±15.1*¶ | +5.0±16.0*¶ | 0.001 |
| DBP Ex1, mm Hg | 74.4±9.6 | 71.4±11.2 | 74.2±9.3 | 71.7±9.5* | 0.002 |
| ΔDBP, mm Hg | −6.4±8.6 | +0.1±10.8* | −7.5±9.5¶ | −0.8±9.2*† | <0.0001 |
| PP Ex1, mm Hg | 61.3±15.6 | 62.1±14.9 | 50.5±12.8*¶ | 54.2±16.5*¶ | <0.0001 |
| ΔPP, mm Hg | −8.0±11.4 | −12.1±10.7* | +10.8±11.5*¶ | +6.6±11.9*¶† | <0.0001 |
| MBP Ex 1, mm Hg | 94.8±11.8 | 92.0±12.6 | 91.1±10.7* | 89.7±11.6* | <0.0001 |
| ΔMBP, mm Hg | −9.1±10.3 | −3.9±11.1* | −3.9±10.4* | +1.4±10.5*† | <0.0001 |
| Medications | |||||
| Vasodilator Ex 1, n (%) | 49 (16) | 9 (12) | 23 (16) | 45 (20) | 0.4 |
| Vasodilator Ex 2, n (%) | 44 (14) | 13 (16) | 21 (14) | 28 (12) | 0.8 |
| ACEI Ex 1, n (%) | 28 (9) | 5 (7) | 12 (8) | 25 (11) | 0.6 |
| ACEI Ex 2, n (%) | 26 (8) | 3 (4) | 15 (10) | 19 (8) | 0.4 |
| ARB Ex 1, n (%) | 8 (3) | 0 (0) | 5 (3) | 3 (1) | 0.3 |
| ARB Ex 2, n (%) | 11 (4) | 4 (5) | 4 (3) | 9 (4) | 0.8 |
| Beta-blocker Ex 1, n (%) | 44 (15) | 16 (22) | 22 (15) | 38 (17) | 0.5 |
| Beta-blocker Ex 2, n (%) | 30 (10) | 6 (7) | 21 (14) | 32 (14) | 0.2 |
| Diuretic Ex 1, n (%) | 54 (18) | 16 (22) | 23 (16) | 43 (19) | 0.7 |
| Diuretic Ex 2, n (%) | 32 (10) | 11 (14) | 15 (10) | 22 (10) | 0.8 |
| Arterial function | |||||
| SVR Ex 1 dyne/second*cm5 | 1530±340 | 1290±240* | 1420±310*¶ | 1230±300*† | <0.0001 |
| ΔSVR dyne/second*cm5 | −350±250 | +150±160* | −230±180*¶ | +280±220*¶† | <0.0001 |
| TAC Ex 1, mL/mm Hg | 1.40±0.40 | 1.46±0.43 | 1.83±0.62*¶ | 1.86±0.64*¶ | <0.0001 |
| ΔTAC, mL/mm Hg | +0.46±0.36 | +0.33±0.31* | −0.29±0.30*¶ | −0.47±0.39*¶† | <0.0001 |
| Ea Ex1, mm Hg/mL | 1.51±0.31 | 1.41±0.30 | 1.34±0.35* | 1.26±0.32*¶ | <0.0001 |
| ΔEa, mm Hg/mL | −0.29±.21 | −0.06±.17* | +0.02±.19*† | +0.29±.24*¶† | <0.0001 |
| VA coupling | |||||
| Ees Ex 1, mm Hg/mL | 2.23±0.65 | 2.14±0.73 | 2.01±0.64* | 1.92±0.61* | <0.0001 |
| ΔEes, mm Hg/mL | −0.18±0.60 | +0.01±0.69 | +0.32±0.59*¶ | +0.61±0.65*¶† | <0.0001 |
| Eed Ex 1, mm Hg/mL | 0.14±0.03 | 0.13±0.03 | 0.13±0.03 | 0.13±0.03* | 0.001 |
| ΔEed, mm Hg/mL | +0.001±0.04 | +0.01±0.02 | +0.01±0.03* | +0.03±0.03*¶† | <0.0001 |
| Ea/Ees Ex 1 | 0.71±0.16 | 0.70±0.18 | 0.69±0.14 | 0.68±0.14 | 0.2 |
| ΔEa/Ees | −0.10±0.17 | −0.05±0.21 | −0.08±0.18 | −0.05±0.17* | 0.01 |
| LV structure | |||||
| LVEDV Ex 1, mL | 130±32 | 133±25 | 139±35 | 144±32* | <0.001 |
| ΔLVEDV, mL | +15±33 | −1±23* | +2±27.81* | −18±27*¶† | <0.0001 |
| LV mass index Ex 1, g/m2 | 96±20 | 94±22 | 93±17 | 94±17 | 0.3 |
| ΔLV mass index, g/m2 | −4±19 | −4±21 | −2±18 | −1±17 | 0.3 |
| LVM/EDV Ex 1, g/ml | 1.5±0.4 | 1.4±0.3 | 1.3±0.3* | 1.2±0.3* | <0.0001 |
| ΔLVM/EDV, g/ml | −0.20±0.42 | −0.03±0.34 | −0.01±0.32* | +0.22±0.41*¶† | <0.0001 |
| LV Diastolic function | |||||
| E/E’ Ex 1 | 8.39±2.90 | 8.06±2.57 | 8.79±3.10 | 8.27±2.65 | 0.31 |
| Δ E/E’ | 2.31±3.95 | 2.22±3.56 | 2.26±7.70 | 2.42±3.42 | 0.99 |
p<0.05 vs. Group 1
p<0.05 vs. Group 2
p<0.05 vs. Group 3
Δ – Change between exam 1 and exam 2; BP – blood pressure; ACEI – angiotensin-converting enzyme inhibitor; ARB - angiotensin receptor blockers; SVR – systematic vascular resistance; TAC – total arterial compliance; Ea – arterial elastance; Ees – end-systolic elastance; Eed – end-diastolic elastance; LVEDV – left ventricular end-diastolic volume; LV – left ventricular.
Figure 2.
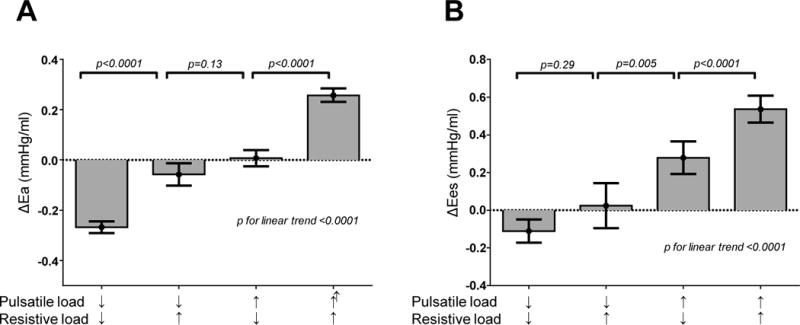
[A,B] Longitudinal changes over 4 years in effective arterial elastance (ΔEa) and left ventricular end systolic elastance (ΔEes) according to groupings based upon longitudinal changes in total arterial compliance (TAC) and mean systemic vascular resistance (SVR), showing estimated marginal means ± 95% CI. Data are adjusted for age, sex and baseline elastance values. Bonferroni correction for multiple comparisons was used.
There was no difference in ΔEed between groups 1 and 2 (p=0.10) or 2 and 3 (p=0.99) after adjustments. However, the combination of both pulsatile and resistive load increase (Group 4) was associated with greater increases in Eed as compared to all other groups (Figure 3A). Left ventricular end-diastolic volume (LVEDV) significantly increased in subjects with pulsatile and resistive load decrease (Group 1) as compared to all other groups (all p<0.05), while LVEDV decreased in subjects with pulsatile and resistive load increase (Group 4) as compared to all other groups (all p<0.01) (Figure 3B) in the adjusted model. There was no difference in changes in left ventricular mass index decrease between groups (p=0.3).
Figure 3.
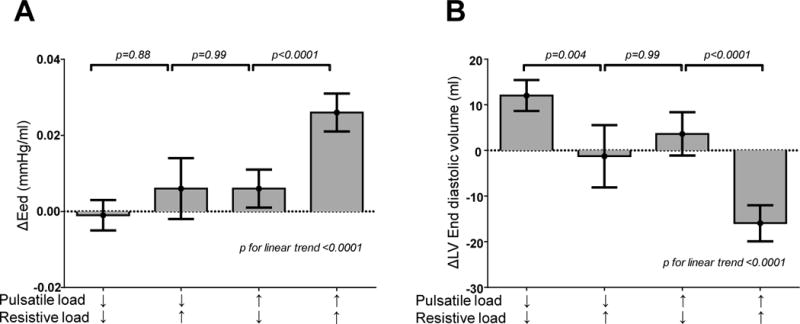
[A,B] Longitudinal changes over 4 years in left ventricular end diastolic elastance (ΔEed) and left ventricular end diastolic volume according to groupings based upon longitudinal changes in total arterial compliance (TAC) and mean systemic vascular resistance (SVR), showing estimated marginal means ± 95% CI. Data are adjusted for age, sex and baseline Eed and end diastolic volumes, respectively. Bonferroni correction for multiple comparisons was used.
Heart rate normalized ejection time change (ΔET) was inversely associated with ΔSVR (r= −0.20, p<0.0001), and directly associated with ΔTAC (r=0.12, p<0.001). The ET increased over time in group 1 as compared to group 4 (Table 3). In multivariable analysis with SVR, TAC and heart rate change, ΔET was not associated with Eed or Ea change. When Ea and Eed comparisons across groups were adjusted for ET, differences among groups did not change.
DISCUSSION
In this large population-based, longitudinal study we have shown differential effects of chronic changes in systemic arterial compliance and resistance on age-related changes in left ventricular structure and function. We confirm previous studies showing that systemic resistance and heart rate are the dominant contributors to arterial elastance. However, while changes in arterial compliance had little impact on Ea, they were strongly correlated with the age-related increase in Ees. Differential changes in pulsatile and mean resistive load were not evident from inspection of Ea change alone. The combination of increased resistance and decreased compliance (Group 4) was associated with the largest increase in LV systolic and diastolic stiffness, with the latter being related to reduction in LV chamber volume rather than estimated LV filling pressure (E/e′ ratio). In contrast, subjects with improved arterial compliance and drop in systemic vascular resistance over 4 years (Group 1) displayed an increase in LV chamber volume, with no progression in LV Eed. These data suggest the chronic changes in systemic arterial resistance and compliance play differing but additive roles in promoting LV stiffening and chamber remodeling. Interventions that improve arterial compliance may be more effective to prevent age-related increases in systolic elastance, while combined improvements in compliance and resistance may be needed to prevent remodeling and diastolic stiffening.
Total arterial compliance, a parameter of pulsatile load, expresses the ability of the arterial system to store blood in systole without excessive pressure rise. A decrease in compliance is associated with increased pulse wave velocity and wave reflection, which contributes to increased pulsatile load. Reduced compliance is associated with excessive myocardial oxygen consumption during exercise and worsening ischemia.13, 14 Compliance is determined by vessel tone, endothelial function, and vascular structure and composition. On the other hand, resistance represents the steady load on LV, which is dependent on vascular tone of arterioles and precapillary sphincters. Prior cross sectional studies have shown that while total arterial compliance decreases with aging, mean systemic vascular resistance tends to remain stable.15 The importance of pulsatile load, particularly the late systolic load, on LV structure-function relationships has been demonstrated in healthy volunteers and heart failure patients,4, 16–21 and late-systolic arterial loading is determined largely by wave reflections and arterial compliance.22
This is the first longitudinal, population-based study to examine how arterial resistance and compliance change over time, and how these changes correlate with changes in LV structure and function. We have previously demonstrated in this sample that LV stiffness increases even as Ea decreases on average, suggesting that age-related LV stiffening is mediated in part by afterload-independent factors.2 Despite these load-independent components, LV Ees and Eed remain intimately related to Ea (r=0.5–0.6, p<0.0001), and we now demonstrate that this relationship is mediated more by pulsatile components than resistive load. Multivariable linear regression and Group analyses revealed a strong relationship between compliance and Ees, but changes in compliance were not independently related to Eed change in linear regression. Because of the collinearity between compliance and resistance (Figure 1), we felt that regression analysis was not the best approach to separate the effects of resistance and compliance.
To overcome this problem, subjects were separated by compliance and resistance change over time in a group analysis. This group analysis revealed that in subjects with compliance increase and resistance increase, Eed did not change significantly from baseline and was significantly lower as compared to subjects with compliance decrease but resistance increase. This suggests that compliance change had an effect on Eed progression that was underestimated by multivariable regression, likely due to correlation between compliance and resistance. Collectively these data point suggest that loss of arterial compliance plays an important role in LV stiffening during both systole and diastole.
As compared to baseline data, in subjects with afterload increase, left ventricular end-diastolic volume decreased. These findings are in line with recently published cross sectional studies suggesting age-related shrinkage of the left ventricle.5, 6, 23 Recently, Cheng et al.5 showed that LV volume decreased out of proportion to LV mass with increasing age, thus increasing the mass/volume ratio. In another cross-sectional study,6 Fujimoto and colleagues observed reductions in LV volume above the age of 65 years, with a leftward shifted diastolic pressure-volume relationship. In a cross-sectional study by Roman,24 pressure-independent stiffness index β was negatively associated with end-diastolic diameter and positively associated with relative wall thickness, while no association with left ventricular mass was observed. The current longitudinal data confirms and extends upon these previous cross sectional studies, and suggests that the age-related reductions in arterial compliance and changes in arteriolar tone might contribute to LV stiffening through a novel mechanism of ‘volume loss’.
Conversely, decreased afterload (pulsatile and resistive) was associated with LV end-diastolic volume increase. Eccentric LV remodeling has traditionally considered to be deleterious, but it is known that endurance trained athletes develop physiologic hypertrophy, and recently Brinker et al. observed that even in healthy middle-aged adults, fitness level was directly correlated with LVEDV and inversely correlated with estimated LV filling pressures.25 Indeed, when scaled to body size, some studies have observed that EDV is lower in HFpEF than age-matched controls,26 and in an animal model of HFpEF,27 improvements in arterial compliance were associated with increases in LV end-diastolic volume. In aged humans, Chantler and colleagues have demonstrated that subjects with reduction in Ea during exercise display higher LV end-diastolic volumes compared to subjects with Ea increase during exercise.28 Thus unlike HFrEF, where afterload reduction decreases LV volumes,29 it is possible that in subjects with normal EF and increased LV stiffness, LV volume increase due to afterload reduction may be a marker of more healthy or ‘successful’ aging compared to the decrease in LV size noted on average with aging in humans.24
In this study, difference in heart rate and SBP change explained differences in Ees among subjects with the same change in Ea caused by different combination of compliance and resistance. Our results show that decrease in heart rate is associated with compliance increase and Ees decrease. This finding is in line with an animal model, in which heart rate reduction using ivabradine improved vascular stiffness and was associated with lower Ees.27 Furthermore, heart-rate reduction by ivabradine increases total arterial compliance and improves ventricular-arterial coupling among subjects with HFrEF.29 In other studies, exercise training was proven to decrease total arterial compliance in sedentary adults as in patients with heart failure.30, 31 Collectively, these observations suggest that heart rate decrease and exercise training may be effective to prevent or mitigate the age-related LV stiffening. We did not observe differences in anti-hypertensive medication use between groups, suggesting that the blood pressure decrease may not have been caused by therapy, but rather by secular trends in population due to life-style changes.
Limitations
The noninvasive methods used to assess ventricular and arterial stiffness are each validated against gold-standard invasive methods, but echo-Doppler data inherently have greater variability compared with invasive measurements. The study cohort was almost exclusively white, and these results may apply to other ethnic groups. Longitudinal changes in ventricular-arterial mechanics in this study reflect not only aging but environmental factors and medication usage. Indeed, the reductions in blood pressure and LV mass likely reflect effects of medical intervention rather than cardiac aging. Because we did not record pressure and flow waves directly in the study, we could not assess the effects of aortic impedance, wave reflection and aortic pulse wave velocity on ventricular structure and function. The linear estimate of Eed based upon the ratio of estimated diastolic LV pressure to LVEDV is a simplification of the curvilinear end diastolic pressure-volume relationship as measured directly in the catheterization laboratory. Anti-hypertensive drug use was recorded but dosage was not in this study, and this may explain the different blood pressure changes in the 4 groups of resistance-compliance changes despite similar medication use. This study examined the relationships between static measures of elastance, resistance and compliance. Future studies identifying chronic changes in pulse waveform analysis and wave reflections will provide greater insight into the nature of ventricular aging. Despite our finding that arterial compliance was most strongly correlated with LV systolic stiffening, we cannot exclude parallel stiffening that may reflect shared exposure to risk factors that are not causally linked. The major strength of this study is its longitudinal design, which allowed us to separate the effect of pulsatile and resistive afterload on LV stiffness.
Conclusions
In men and women greater aged 45 years and above, chronic changes in systemic arterial compliance and resistance are differentially correlated with age-related LV stiffening and chamber remodeling. Increases in pulsatile load contribute more to LV systolic stiffening while combined pulsatile and resistive loading changes are associated with positive of negative chamber remodeling. Therapies designed to improve resistance and in particular aortic compliance may hold promise to deter or reverse cardiac aging and the diseases associated with it, including HFpEF.
Acknowledgments
none
Funding Source: This work was supported by National Heart, Lung, and Blood Institute (grant number RO1-55502), by the European Regional Development Fund, project FNUSA-ICRC (grant number Z.1.05/1.1.00/02.0123) and Czech Ministry of Health (grant number NT13434-4/2012).
Footnotes
Conflict of interest: none declared
References
- 1.Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol. 1983;245:H773–780. doi: 10.1152/ajpheart.1983.245.5.H773. [DOI] [PubMed] [Google Scholar]
- 2.Borlaug BA, Redfield MM, Melenovsky V, Kane GC, Karon BL, Jacobsen SJ, Rodeheffer RJ. Longitudinal changes in left ventricular stiffness: a community-based study. Circ Heart Fail. 2013;6:944–952. doi: 10.1161/CIRCHEARTFAILURE.113.000383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segers P, Stergiopulos N, Westerhof N. Relation of effective arterial elastance to arterial system properties. Am J Physiol. 2002;282:H1041–1046. doi: 10.1152/ajpheart.00764.2001. [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA, Melenovsky V, Redfield MM, Kessler K, Chang HJ, Abraham TP, Kass DA. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. doi: 10.1016/j.jacc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovascular imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujimoto N, Hastings JL, Bhella PS, Shibata S, Gandhi NK, Carrick-Ranson G, Palmer D, Levine BD. Effect of ageing on left ventricular compliance and distensibility in healthy sedentary humans. J Physiol. 2012;590:1871–1880. doi: 10.1113/jphysiol.2011.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circ Heart Fail. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 8.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jr, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ammar KA, Redfield MM, Mahoney DW, Johnson M, Jacobsen SJ, Rodeheffer RJ. Central obesity: association with left ventricular dysfunction and mortality in the community. Am Heart J. 2008;156:975–981. doi: 10.1016/j.ahj.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54:410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circ Heart Fail. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 12.Kass DA. Assessment of diastolic dysfunction: Invasive modalities. Cardiol Clinics. 2000;18:571–586. doi: 10.1016/s0733-8651(05)70162-4. [DOI] [PubMed] [Google Scholar]
- 13.Otsuki T, Maeda S, Kesen Y, Yokoyama N, Tanabe T, Sugawara J, Miyauchi T, Kuno S, Ajisaka R, Matsuda M. Age-Related Reduction of Systemic Arterial Compliance Induces Excessive Myocardial Oxygen Consumption during Sub-Maximal Exercise. Hypertens Res. 2006;29:65–73. doi: 10.1291/hypres.29.65. [DOI] [PubMed] [Google Scholar]
- 14.Haluska BA, Matthys K, Fathi R, Rozis E, Carlier SG, Marwick TH. Influence of arterial compliance on presence and extent of ischaemia during stress echocardiography. Heart. 2006;92:40–43. doi: 10.1136/hrt.2004.052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi S, Yano M, Kohno M, Obayashi M, Hisamatsu Y, Ryoke T, Ohkusa T, Yamakawa K, Matsuzaki M. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation. 1996;94:3362–3368. doi: 10.1161/01.cir.94.12.3362. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto J, Westerhof BE, Westerhof N, Imai Y, O’Rourke MF. Different role of wave reflection magnitude and timing on left ventricular mass reduction during antihypertensive treatment. J Hypertens. 2008;26:1017–1024. doi: 10.1097/HJH.0b013e3282f62a9b. [DOI] [PubMed] [Google Scholar]
- 18.Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: the Asklepios study. Hypertension. 2013;61:296–303. doi: 10.1161/HYPERTENSIONAHA.111.00530. [DOI] [PubMed] [Google Scholar]
- 19.Frank O. Zur Dynamik des Herzmuskels. Z Biol. 1895;32:370–437. [Google Scholar]
- 20.Wiggers C. Studies on the consecutive phases of the cardiac cycle. Am J Physiol. 1921;56:415–459. [Google Scholar]
- 21.Brutsaert DL, Sys SU. Relaxation and diastole of the heart. Physiol Rev. 1989;69:1228–1315. doi: 10.1152/physrev.1989.69.4.1228. [DOI] [PubMed] [Google Scholar]
- 22.Chirinos JA, Segers P, Gillebert TC, Gupta AK, De Buyzere ML, De Bacquer D, St John-Sutton M, Rietzschel ER. Arterial properties as determinants of time-varying myocardial stress in humans. Hypertension. 2012;60:64–70. doi: 10.1161/HYPERTENSIONAHA.112.190710. [DOI] [PubMed] [Google Scholar]
- 23.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roman MJ, Ganau A, Saba PS, Pini R, Pickering TG, Devereux RB. Impact of arterial stiffening on left ventricular structure. Hypertension. 2000;36:489–494. doi: 10.1161/01.hyp.36.4.489. [DOI] [PubMed] [Google Scholar]
- 25.Brinker SK, P A, Pandey A, Ayers CR, Barlow CE, DeFina LF, Willis BL, Radford NB, Farzaneh-Far R, de Lemos JA, Drazner MH, Berry JD. Association of Cardiorespiratory Fitness with left ventricular remodeling and diastolic function: the Cooper Center Longitudinal Study. JACC Heart Fail. 2014;2:238–246. doi: 10.1016/j.jchf.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lam CS, Roger VL, Rodeheffer RJ, Bursi F, Borlaug BA, Ommen SR, Kass DA, Redfield MM. Cardiac structure and ventricular-vascular function in persons with heart failure and preserved ejection fraction from Olmsted County, Minnesota. Circulation. 2007;115:1982–1990. doi: 10.1161/CIRCULATIONAHA.106.659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reil JC, Hohl M, Reil GH, Granzier HL, Kratz MT, Kazakov A, Fries P, Muller A, Lenski M, Custodis F, Graber S, Frohlig G, Steendijk P, Neuberger HR, Bohm M. Heart rate reduction by If-inhibition improves vascular stiffness and left ventricular systolic and diastolic function in a mouse model of heart failure with preserved ejection fraction. Eur Heart J. 2013;34:2839–2849. doi: 10.1093/eurheartj/ehs218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chantler PD, Melenovsky V, Schulman SP, Gerstenblith G, Becker LC, Ferrucci L, Fleg JL, Lakatta EG, Najjar SS. Use of the Frank-Starling mechanism during exercise is linked to exercise-induced changes in arterial load. Am J Physiol. 2012;302:H349–358. doi: 10.1152/ajpheart.00147.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reil JC, Tardif JC, Ford I, Lloyd SM, O’Meara E, Komajda M, Borer JS, Tavazzi L, Swedberg K, Bohm M. Selective heart rate reduction with ivabradine unloads the left ventricle in heart failure patients. J Am Coll Cardiol. 2013;62:1977–1985. doi: 10.1016/j.jacc.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 30.Cameron JD, Dart AM. Exercise training increases total systemic arterial compliance in humans. Am J Physiol. 1994;266:H693–H701. doi: 10.1152/ajpheart.1994.266.2.H693. [DOI] [PubMed] [Google Scholar]
- 31.Parnell MM, Holst DP, Kaye DM. Exercise training increases arterial compliance in patients with congestive heart failure. Clin Sci (Lond) 2002;102:1–7. [PubMed] [Google Scholar]


