Abstract
Background and purpose:
The ability of the National Institutes of Health Stroke Scale (NIHSS) score to predict functional outcome in minor stroke is controversial. In this study, we examined the association of itemized NIHSS score with discharge outcome.
Methods:
We included all patients with final diagnosis of stroke with an NIHSS score of 0 to 5 untreated with thrombolysis enrolled in the “Stroke Warning Information and Faster Treatment” trial. Individual components of the NIHSS score were the primary predictors. Poor outcome was defined as not being discharged home. Logistic regression was used to identify predictors of outcome.
Results:
A total of 861 patients met the inclusion criteria; 162 (19%) were not discharged home. In multivariable regression, predictors of discharge other than home were age (odds ratio [OR] = 1.02 per year increase, P < .001) and total NIHSS score (OR per unit increase in the NIHSS = 1.51, P < .001). Motor (OR = 2.32, P < .001), level of consciousness (LOC; OR = 6.62, P = .004), and ataxia (OR = 3.10, P < .001) were also associated with not being discharged home. Motor (area under the curve [AUC] 0.623) appeared to be more predictive of poor outcome than ataxia (AUC 0.569) and LOC (AUC 0.517). The total NIHSS had a fair correlation with discharge outcome (AUC 0.683).
Conclusion:
Total and itemized NIHSS components have a fair correlation with outcome in minor stroke highlighting the importance of other measures of stroke severity for clinical trials.
Keywords: minor stroke, outcome, NIHSS score subsets, NIHSS score, treatment, stroke, cerebrovascular disorders, ischemic attack, transient, outcomes, techniques
Background
Approximately two-third of patients with ischemic stroke are found to have mild deficits, with a National Institutes of Health Stroke Scale (NIHSS) ≤5 across a variety of study designs.1,2 One-third of patients are excluded from thrombolysis because of mild deficits, making it a very common exclusion criterion.3,4 Approximately 30% of patients with minor strokes who are excluded from thrombolytic therapy for mild symptoms have poor outcomes: disability at 90 days (modified Rankin scale [mRS] 2-6),5,6 discharge other than to home,4,7 and lack of independent ambulation at discharge.4 It is not clear, however, whether treating those patients with thrombolysis improves their clinical outcome.4
A consistent predictor of outcome in patients with minor deficits is evidence of large-vessel occlusion on imaging.8,9 Limiting hyperacute treatment to only patients with large artery occlusion may lead to poor outcome in patients with potentially disabling deficits but no large-vessel occlusion. Physicians tend to rely on the type of deficits to define minor stroke,5 but the correlation between the neurological deficit and the outcome is incomplete. While one study showed that the types of deficits do not predict outcome in minor stroke,10 other studies showed that distal hand weakness,11 gait disorder,11 language,11 leg weakness,12 and neglect12 predict poor outcome in patients with mild deficits. Thrombolytic therapy in minor stroke may depend on the treating physician’s subjective opinion of a “nondisabling” deficit.13 Subjective assessments introduce wide variability in the treatment of mild strokes such that the efficacy of treatment cannot be determined with current studies. Identifying whether and to what degree the itemized NIHSS (used on the majority of patients with stroke in routine clinical practice) predicts outcomes would identify which patients are best suited for new clinical trials. In the present study, we examined the association between itemized components of the NIHSS with discharge outcome among a sample of participants in a clinical trial with mild stroke untreated with thrombolysis.
We examined the association between the itemized NIHSS components with discharge outcome and the ability of the itemized NIHSS to predict outcome. We hypothesized that the itemized NIHSS would provide improved prediction of outcomes in minor stroke compared to the total NIHSS.
Methods
We retrospectively analyzed data from the Stroke Warning Information and Faster Treatment (SWIFT) trial that randomized 1635 adults with stroke or transient ischemic attack to an intensive stroke-specific education intervention versus standard of care. The primary outcome in SWIFT was time from symptom onset to arrival to the emergency department if the participant had a second stroke.14 The trial was conducted from February 2006 to February 2010. In our analysis, we included only participants with (1) final diagnosis by the attending vascular neurologist of ischemic stroke, (2) initial NIHSS score of 0 to 5 inclusive, and (3) we excluded patients with transient ischemic attack based on the most recent tissue definition15 and patients treated with thrombolytic therapy to eliminate potential confounding on outcome from thrombolysis itself.16 Participants were divided into 2 groups based on discharge outcome (home vs not home), and poor outcome was defined as nondischarge home.7 Each itemized NIHSS component on admission was dichotomized into 2 groups (0 for no score and 1 for a score of 1 or more). For simplicity and due to the expected low number of patients having minor stroke with deficits in level of consciousness (LOC)12, the items 1a, 1b, and 1c were combined into 1 category.
Clinical characteristics were compared between the categorical discharge outcome using t test for continuous variables and chi-square for categorical variables.
Two multivariate logistic regression models were chosen a priori and built to identify predictors of poor outcome in patients with mild deficits. Model 1 included the total NIHSS score adjusting for age, gender, race–ethnicity, hypertension, prior stroke, diabetes, and atrial fibrillation. In model 2, we added all aspects of the itemized NIHSS (LOC questions, best gaze, visual, facial, motor, sensory, ataxia, language, dysarthria, and neglect) to model 1. Since certain social factors can influence discharge disposition, we performed sensitivity analysis adjusting for the variables marital status (married: yes or no) and lives alone (yes or no), which are variables captured by SWIFT. In order to examine whether the itemized and total NIHSS predicted outcomes, receiver–operating curves (ROCs) were built and area under the curve (AUC) was calculated. We also calculated AUC scores for each of itemized NIHSS subsets to determine which ones were more likely associated with discharge disposition than others. Statistical analysis was performed using SAS and P < .05 was considered significant. The study was approved by the institutional board review, and all patients in the SWIFT trial provided written informed consent.
Results
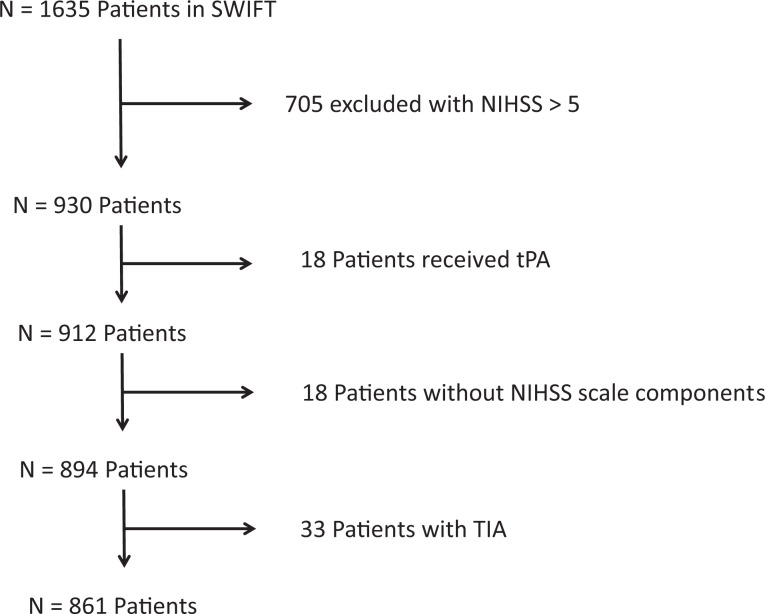
We identified 930 participants in the SWIFT trial who had an initial NIHSS score of 0 to 5, and 49% arrived within 12 hours of symptom onset. Of those 930 patients, 864 (93%) met the inclusion criteria (18 were excluded because they received intravenous tissue plasminogen activator, 18 patients were excluded because they did not have individual components of the NIHSS score recorded, and 33 patients were excluded due to a diagnosis of transient ischemic attack). Figure 1 includes the number of participants excluded in this analysis due to the NIHSS, TIA diagnosis, unknown itemized NIHSS score, and treatment with thrombolysis. Table 1 outlines the baseline demographics of our sample. There were 162 (19%) not discharged home, the majority (84%) of which were discharged to acute rehab, and there were no in-hospital deaths. On univariate analysis, predictors of discharge other than home were older age, diabetes, motor deficits, ataxia, dysarthria, and deficits in LOC (Table 1). We also found that the percentage of patients not discharged home increased with the NIHSS score; NIHSS 0: (22 of 229) 9.6%, NIHSS 1: (23 of 183) 12.5%, NIHSS 2: (28 of 168) 16.7%, NIHSS 3: (31 of 118) 26.3%, NIHSS 4: (30 of 97) 30.9%, and NIHSS 5: (28 of 66) 42.4% (Figure 2).
Figure 1.
Patient flowchart.
Table 1.
Baseline Characteristics of Patients Discharged Home Versus Not Discharged Home in Our Cohort.
| Number of Participants (n) | Discharged Home, n = 699 | Not Discharged Home, n = 162 | P Value |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD), y | 62.6 ± 15.0 | 67.7 ± 14.4 | <.001 |
| Male, n (%) | 340 (48.6) | 83 (51.2) | .601 |
| Ethnicity | |||
| Hispanic, n (%) | 364 (52.1) | 71 (44.1) | .080 |
| Race | |||
| White, n (%) | 210 (30.0) | 53 (32.7) | .509 |
| Black, n (%) | 151 (21.6) | 45 (27.8) | .097 |
| Risk factors and medical history (%) | |||
| Hypertension | 518 (80.6) | 129 (74.7) | .125 |
| Diabetes mellitus | 257 (37.3) | 72 (45.3) | .026 |
| Atrial fibrillation | 59 (8.6) | 21 (13.3) | .072 |
| Prior stroke | 97 (14.0) | 29 (18.2) | .174 |
| NIHSS, median (interquartile range) | 1 (0-3) | 3 (1-4) | <.001 |
| NIHSS items, n (%) | |||
| Level of consciousness (LOC) | 7 (1.0) | 7 (4.3) | .008 |
| Best gaze | 19 (2.7) | 6 (3.7) | .446 |
| Visual | 33 (4.7) | 11 (6.8) | .320 |
| Facial | 234 (33.5) | 66 (40.7) | .083 |
| Motor | 224 (32.0) | 89 (54.9) | <.001 |
| Ataxia | 79 (11.3) | 40 (24.7) | <.001 |
| Sensory | 137 (19.6) | 35 (21.6) | .739 |
| Language | 64 (9.2) | 12 (7.4) | .542 |
| Dysarthria | 95 (13.6) | 30 (18.5) | .008 |
| Neglect | 29 (4.1) | 11 (6.8) | .275 |
Abbreviations: SD, standard deviation; NIHSS, National Institutes of Health Stroke Scale; LOC, level of consciousness.
Figure 2.
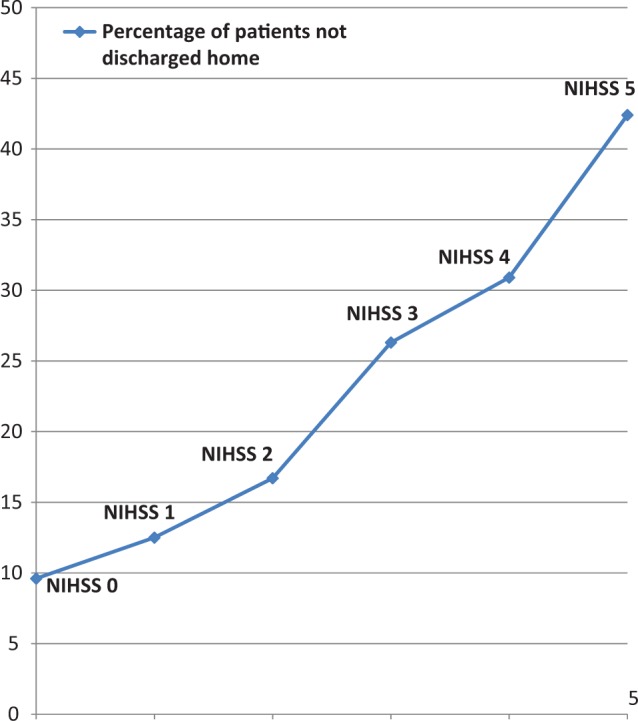
Graph showing a graded relationship between National Institutes of Health Stroke Scale (NIHSS) score and discharge disposition.
In multivariable logistic regression adjusted for demographics, stroke risk factors, and NIHSS, age (OR = 1.02, P < .001) and NIHSS score (OR = 1.51 per 1 unit increase, P < .001) were associated with poor outcome (Table 2).
Table 2.
Predictors of Not Being Discharged Home Among Participants With a National Institutes of Health Stroke Scale Score of 0 to 5.a
| Adjusted Odds Ratio | P Value | ||
|---|---|---|---|
| Model 1 | Age | 1.02 | .001 |
| National Institutes of Health Stroke Scale (NIHSS)—per unit increase | 1.51 | <.001 | |
| Model 2 | Age | 1.02 | .001 |
| Best gaze | 1.35 | .5 | |
| Motor | 2.32 | <.001 | |
| Facial | 1.18 | .4 | |
| Level of consciousness | 6.61 | .004 | |
| Ataxia | 3.01 | <.001 | |
| Neglect | 1.45 | .3 | |
| Dysarthria | 1.54 | .07 | |
| Sensory | 0.96 | .9 | |
| Language | 0.67 | .3 |
aModel 1: Adjusted for age, gender, race–ethnicity, National Institutes of Health Stroke Scale (NIHSS), hypertension, diabetes, atrial fibrillation, and prior stroke. Model 2: Adjusted for age, gender, race–ethnicity, NIHSS components, hypertension, diabetes, atrial fibrillation, and prior stroke.
Model 2 included further adjustment for all itemized components of the NIHSS, and motor (OR = 2.32, P < .001), LOC (OR = 6.62, P = .004), and ataxia (OR = 3.10, P < .001) were associated with not being discharged home (Table 2). The results remained unchanged after adjusting for the variables “marital status” and “lives alone”.
Using ROC curves, motor deficits (AUC 0.623) appeared to be the most predictive of discharge outcome, followed by ataxia (AUC 0.569) and LOC deficits (AUC 0.517) which were weakly predictive. Other examination features did not perform better than 0.5. In addition, although the admission NIHSS was most predictive of discharge outcome, it only had a fair correlation with discharge outcome (AUC 0.683; Figure 3).
Figure 3.
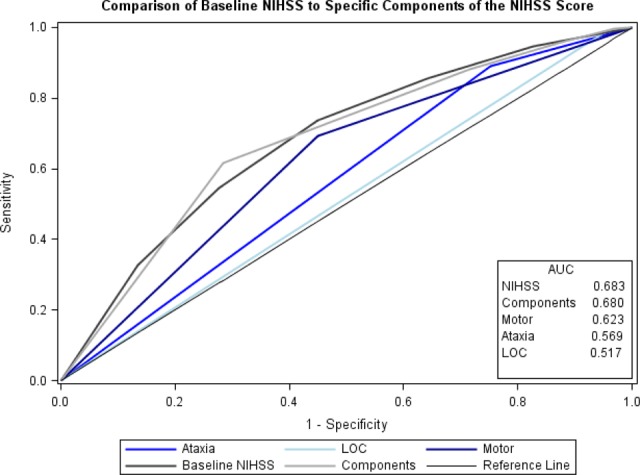
Area under curve for National Institutes of Health Stroke Scale (NIHSS) score components and NIHSS score in predicting discharge home.
Discussion
The results of our study indicate that patients with any abnormal scores on the itemized items for LOC, motor, or ataxia were less likely to be discharged home, with the motor subset having the strongest effect. The total NIHSS score as well as the itemized NIHSS subsets had only a fair correlation with discharge outcome. Our results suggest that factors not captured by the total NIHSS score which influence discharge disposition in patients with minor stroke include the nature of the deficit.
Clinical Implications
The results of our study are in line with another study showing that in addition to age and NIHSS score, motor scores strongly influence short- and long-term outcomes among patients who were not treated with thrombolysis.17 This same study also concluded that an NIHSS ≤3 had the highest sensitivity in predicting good outcome after ischemic stroke.17 Another study limited by a smaller sample size that included patients with NIHSS score ≤6 treated or untreated with intravenous thrombolysis showed that aphasia predicted poor 3-month outcome.18
Our study advocates that certain items in the NIHSS score are more likely to cause short-term disability than others in patients with minor deficits, though the overall predictive ability is modest. The nature of the neurological deficit, particularly those not captured by the NIHSS, may help risk stratify patients most suitable for randomized clinical trials in minor stroke. For example, ambulation, fine finger movements, cognitive measures, and subtle nondominant hemisphere symptoms are not captured by the NIHSS. These factors could influence discharge and long-term outcomes in patients with minor stroke. The NIHSS does not capture gait or mobility measures, such as gait speed, which can strongly predict outcome in stroke. Distal hand weakness may be an isolated disabling symptom in a number of patients with stroke which is not well captured in the arm drift item. In addition, certain measures of cognition, which may be affected by stroke including visuospatial and executive function19 are not captured by the stroke scale. Capturing a more sensitive impairment measure in research studies is necessary. These assessments could include gait performance measures or simplified versions of neuropsychological tests. Some of these measures may be obtained quickly and have previously been correlated with functional outcomes in stroke. Perhaps adding those items to the NIHSS score may potentially aid in making treatment decisions in patients with mild deficits otherwise. In addition to the type of deficit, subjective measures related to the patient such as occupation, hobbies, and baseline functional status may play a role in determining the degree to which their deficit impacts their functional outcome.
Identifying predictors of outcome in patients with minor stroke is of paramount importance. It can aid physicians and patients make acute treatment decisions including thrombolytic therapy and help in their triaging and disposition. In addition, it leads to identifying which patients are best suited for new clinical trials investigating early aggressive treatments aimed to improve functional outcome.
Strengths and Limitations
Our study has several limitations including its retrospective nature and using the discharge disposition outcome which is not a measure of functional outcome and does not take into account disabling cognitive deficits and nondisabling neurological deficits which could have resulted in home discharge. We did not have complete 3-month mRS that has been commonly used in clinical trials and studies in stroke. For patients and providers, however, being discharged home with a minor deficit is considered a clinically meaningful outcome. In addition, discharge outcome and discharge disposition have been shown to strongly correlate with the 90-day mRS.20,21 One-half of the patients in our sample presented within 12 hours such that many patients would not have been eligible for thrombolysis for other reasons. This makes it difficult in our study to answer who would benefit from thrombolysis, though it also allows for having a broader picture of the distribution and predictors of outcomes in untreated patients with minor stroke. Moreover, by excluding patients who received thrombolysis, we removed the potential confounding of thrombolysis on the outcome. In addition, due to the small sample of patients discharged to subacute or long-term nursing facilities (2%) in our cohort, we could not include them in separate analyses. Finally, despite adjusting for demographics and risk factors, our study lacked data on the presence of a large-vessel occlusion, which is one of the predictors of outcome in the population with minor stroke.
Conclusion
In our study, the itemized scale provided only a fair predictive ability. There is a need for ongoing research to better delineate factors that better predict poor outcome in patients with mild deficits beyond the NIHSS. Such prediction tools may allow for further tailoring of decisions on thrombolysis and using appropriately designed clinical trials.
Footnotes
Authors’ Note: Shadi Yaghi contributed to preparing the manuscript. Joshua Willey contributed to preparing and revising the manuscript. Howard Andrews and Amelia Boehme contributed to analysis of data. Randolph Marshall contributed to revising the manuscript. Bernadette Boden-Albala contributed to revising the manuscript and PI of SWIFT.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Shadi Yaghi received funds from NINDS StrokeNet. Joshua Willey received funds from the NIH. Randolph Marshall received funds from NIH. Bernadette Boden-Albala received funds from NIH and is the PI of SWIFT.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Dhamoon MS, Moon YP, Paik MC, et al. Long-term functional recovery after first ischemic stroke: The northern manhattan study. Stroke. 2009;40(8):2805–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reeves M, Khoury J, Alwell K, et al. Distribution of national institutes of health stroke scale in the Cincinnati/Northern Kentucky stroke study. Stroke. 2013;44(11):3211–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. George MG, Tong X, McGruder H, et al. Paul coverdell national acute stroke registry surveillance - four states, 2005-2007. MMWR Surveill Summ. 2009;58:(7)1–23. [PubMed] [Google Scholar]
- 4. Smith EE, Fonarow GC, Reeves MJ, et al. Outcomes in mild or rapidly improving stroke not treated with intravenous recombinant tissue-type plasminogen activator: findings from get with the guidelines-stroke. Stroke. 2011;42(11):3110–3115. [DOI] [PubMed] [Google Scholar]
- 5. Fischer U, Baumgartner A, Arnold M, et al. What is a minor stroke? Stroke. 2010;41(4):661–666. [DOI] [PubMed] [Google Scholar]
- 6. Khatri P, Conaway MR, Johnston KC. Ninety-day outcome rates of a prospective cohort of consecutive patients with mild ischemic stroke. Stroke. 2012;43(2):560–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Willey JZ, Stillman J, Rivolta JA, et al. Too good to treat? Outcomes in patients not receiving thrombolysis due to mild deficits or rapidly improving symptoms. Int J Stroke. 2012;7(3):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nedeltchev K, Schwegler B, Haefeli T, et al. Outcome of stroke with mild or rapidly improving symptoms. Stroke. 2007;38(9):2531–2535. [DOI] [PubMed] [Google Scholar]
- 9. Rajajee V, Kidwell C, Starkman S, et al. Early mri and outcomes of untreated patients with mild or improving ischemic stroke. Neurology. 2006;67(6):980–984. [DOI] [PubMed] [Google Scholar]
- 10. Leira EC, Ludwig BR, Gurol ME, Torner JC, Adams HP., Jr The types of neurological deficits might not justify withholding treatment in patients with low total national institutes of health stroke scale scores. Stroke. 2012;43(3):782–786. [DOI] [PubMed] [Google Scholar]
- 11. Wendt M, Tutuncu S, Fiebach JB, Scheitz JF, Audebert HJ, Nolte CH. Preclusion of ischemic stroke patients from intravenous tissue plasminogen activator treatment for mild symptoms should not be based on low national institutes of health stroke scale scores. J Stroke Cerebrovasc Dis. 2013;22(4):550–553. [DOI] [PubMed] [Google Scholar]
- 12. Sato S, Uehara T, Ohara T, Suzuki R, Toyoda K, Minematsu K. Factors associated with unfavorable outcome in minor ischemic stroke. Neurology. 2014;83(2):174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604–607. [DOI] [PubMed] [Google Scholar]
- 14. Boden-Albala B, Stillman J, Perez T, et al. A stroke preparedness rct in a multi-ethnic cohort: design and methods. Contemp Clin Trials. 2010;31(3):235–241. [DOI] [PubMed] [Google Scholar]
- 15. Easton JD, Saver JL, Albers GW, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the american heart association/american stroke association stroke council; council on cardiovascular surgery and anesthesia; council on cardiovascular radiology and intervention; council on cardiovascular nursing; and the interdisciplinary council on peripheral vascular disease. The american academy of neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009;40(6):2276–2293. [DOI] [PubMed] [Google Scholar]
- 16. Johnston SC, Easton JD, Farrant M, et al. Platelet-oriented inhibition in new tia and minor ischemic stroke (point) trial: Rationale and design. Int J Stroke. 2013;8(6):479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Strambo D, Zambon AA, Roveri L, et al. Defining minor symptoms in acute ischemic stroke. Cerebrovascular diseases (Basel, Switzerland). 2015;39(3-4):209–215. [DOI] [PubMed] [Google Scholar]
- 18. Nesi M, Lucente G, Nencini P, Fancellu L, Inzitari D. Aphasia predicts unfavorable outcome in mild ischemic stroke patients and prompts thrombolytic treatment. J Stroke Cerebrovasc Dis. 2014;23(2):204–208. [DOI] [PubMed] [Google Scholar]
- 19. Cumming TB, Marshall RS, Lazar RM. Stroke, cognitive deficits, and rehabilitation: still an incomplete picture. Int J Stroke. 2013;8(1):38–45. [DOI] [PubMed] [Google Scholar]
- 20. Ovbiagele B, Saver JL. Day-90 acute ischemic stroke outcomes can be derived from early functional activity level. Cerebrovasc Dis (Basel, Switzerland). 2010;29(1):50–56. [DOI] [PubMed] [Google Scholar]
- 21. Qureshi AI, Chaudhry SA, Sapkota BL, Rodriguez GJ, Suri MF. Discharge destination as a surrogate for modified Rankin scale defined outcomes at 3- and 12-months poststroke among stroke survivors. Arch Physical Med Rehabil. 2012;93(8):1408–1413.e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]