Abstract
The aim of the present study was to investigate the effect of bavachin treatment on A375 cells and the regulation of melanin synthesis. The cultured A375 cells in vitro were treated with bavachin; and the effect of bavachin on cell activity, tyrosinase (TYR) activity and melanin synthesis were respectively tested by the MTT assay, L-dopa oxidation assay and the NaOH lysis assay. The expression levels of TYR and c-Jun N-terminal kinases (JNK) proteins were tested by western blot analysis. The expression levels of TYR, tyrosinase-related protein-1 (TRP-1), TRP-2, extracellular signal-regulated kinase 1 (ERK1), ERK2 and JNK2 mRNA were tested by the reverse transcription-polymerase chain reaction assay. Simultaneously, the effect of estrogen receptor inhibitor (ICI182780) and ERK pathway inhibitor (U0126) was also tested on A375 cells following bavachin. The safe dose of bavachin significantly inhibited melanin synthesis and TYR activity. Bavachin (10 µmol/l) inhibited the expression of TYR and JNK proteins, and the expression of TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2 mRNA in A375 cells. ICI182780 and U0126 could significantly reverse the bavachin treatment on the protein expression levels and the mRNA expression of TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2. In conclusion, bavachin inhibited the synthesis of melanin on A375 cells by inhibiting the protein and mRNA expression of TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2.
Keywords: bavachin, estrogen receptor-mitogen-activated protein kinase, A375 cells, melanin
Introduction
Psoralea coryfolia is a traditional tonifying drug, which can tonify renal function and is antidiarrheal. A previous study illustrated that Psoralea coryfolia has estrogen-like effects (1). For adult ovariectomized female-mice, Psoralea coryfolia could not only increase keratinization of vaginal epithelial cells and enhance the weight of the uterus, but it also has an influence on their estrous cycles, which demonstrate the estrogen effects of Psoralea coryfolia (2). Bavachin is one of the classic flavonoid phytoestrogens extracted from Psoralea coryfolia (3–5). Phytoestrogens are one of the selective estrogen receptor (ER) modulators extracted from plants with a structure similar to estrogen, which may have an important role in the treatment of skin disease as a substitute of estrogen, due to its estrogen-like effects but mild estrogen-like side effects (6). Previously, certain studies have shown that Psoralea coryfolia and certain phytoestrogens extracted from Psoralea coryfolia that exhibit estrogen-like effects were closely connected with melanin synthesis. Psoralea coryfolia combined with psoralen combined with ultraviolet A (UVA) treatment could increase the activity of melanocytes, and reduce the degeneration of melanocytes and keratinocytes (7). Bakuchiol, bavachin and isobavachalcone can inhibit melanin synthesis in B16 mouse melanoma cells (8). Phytoestrogen can regulate melanin synthesis of melanocytes through the mitogen-activated protein kinase (MAPK) signaling pathway initiated by binding of phytoestrogen to the ER. Thus, bavachin may regulate melanin synthesis of A375 cells through the ER-MAPK signaling pathway.
Materials and methods
Cells and reagents
Human A375 cells (Cell Center of Chinese Academy of Sciences, Beijing, China), MTT (Sigma-Aldrich, St. Louis, MO, USA), Dulbecco's modified Eagle's medium (DMEM; Hyclone, Thermo Fisher Scientific, Inc., Waltham, MA, USA), cell lysis buffer for Western blotting and immunoprecipitation (Beyotime Institute of Biotechnology, Shanghai, China), goat anti-mouse immunoglobulin G conjugated with horseradish peroxidase (cat. no. BA1050; Wuhan Boster Golden Bridge Biological Technology Co., Ltd., Wuhai, China), mouse anti-β-actin monoclonal antibody (cat. no. TA-09; Beijing Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing, China), mouse anti-tyrosinase (TYR) monoclonal antibody (cat. no. sc-20035; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), mouse anti-JNK monoclonal antibody (Santa Cruz Biotechnology, Inc.), primer (Sangon Biotech Co., Ltd., Shanghai, China) and TRIzol reagent (Gibco, Thermo Fisher Scientific, Inc.) were purchased.
Instruments
A TCL6G-C table-top high-speed refrigerated centrifuge (Anting, Shanghai, China), MK3 Enzyme microplate reader (Redian, Shanghai, China), DYCZ-40B transfer electrophoresis cell (Liuyi, Beijing, China), Professional polymerase chain reaction (PCR) Amplification Instrument (Biometra, Gottingen, Germany), SmartChemi™ Image Analysis system (Beijing Sage Creation Science Co., Ltd., Beijing, China) and Nano-100 Spectrophotometry (Aosheng, Hangzhou, China) were the instruments used.
Drugs
Bavachin with a purity >98% (20130829; Baoji Herbest Bio-Tech Co., Ltd., Baoji, China), estradiol with a purity >98% (L750N46; Bailingwei, Beijing, China), ICI182780 (20A/129473; Tocris Bioscience, Bristol, UK) and U0126 (S110202; Selleck, Houston, TX, USA) were used for the treatments of the cells.
Drug solution preparation
Estradiol was precisely weighed (5.5 mg), dissolved with 4 ml anhydrous alcohol, and DMEM complete medium was added to a total volume of 1 liter. A stock solution with a concentration of 20 µmol/l was prepared and diluted to 10−3 µmol/l when used. Bavachin was weighed precisely (6.49 mg) and dissolved with DMEM complete medium to a total volume of 100 ml. The solution was prepared with an initial concentration of 200 µmol/l, and diluted to 10 µmol/l when used. ICI182780 was weighed precisely (5 mg), dissolved with 1% dimethyl sulphoxide (DMSO) and DMEM complete medium was added to prepare the solution with an initial concentration of 200 µmol/l. This solution was diluted to 1 µmol/l when used. U0126 was weighed precisely (8.53 mg), dissolved with 1% DMSO and DMEM complete medium was added to prepare the solution with an initial concentration of 400 µmol/l. The solution was diluted to 10 µmol/l when used. All the solutions were filtered and sterilized by 0.22-µm filter membrane, and stored at −20°C.
Cell grouping and culture
A375 cells in the logarithmic growth phase were prepared and subsequently digested, centrifuged and resuspended. The cells were transferred to 96-well plates (200 µl medium with 4,500 cells per well) or 6-well plates (2 ml medium with 24×104 cells per well), cultured in 5% CO2 at 37°C for 24 h, the medium was discarded and the cells were divided into different groups according to the drug treatments. For the control group, medium without drugs was added; for the estradiol group, estradiol solution with a concentration of 10−3 µmol/l was added; for the bavachin group, 10 µmol/l bavachin solution was added; for the bavachin + ICI182780 group, 1 µmol/l ICI182780 solution was added, and bavachin stock solution was added 1 h later, with a bavachin concentration that was identical to the bavachin group. For the bavachin + U0126 group, 10 µmol/l U0126 solution was added, and 20 min later the bavachin stock solution was added to make a concentration of bavachin that was identical to the bavachin group. All the cell groups were cultured for a further 48 h. There were 6 wells for duplicate treatments in the 96-well plates, and 4 wells for duplicate treatments in 6-well plates.
Detection index
Cell activity
MTT solution (20 µl) was added to each well of the 96-well plates, the cells were cultured for 4 h, the solution was discarded, and the purple crystal was dissolved in the wells with 150 µl DMSO solution, agitated in a 37°C incubator shaker for 10 min, and the optical density (OD) was measured at 490 nm by the microplate reader.
Melanin content
The cultured solution in the 6-well plates was discarded, the cells were washed with phosphate-buffered saline (PBS) twice and were digested with 1 ml 0.25% pancreatin for 1 min. DMEM total medium was added (1 ml) to stop the digestion, the cell suspension was collected in a 15-ml centrifuge tube, the sample underwent centrifugation at a speed of 2,000 × g for 5 min and the supernatant was discarded. Following this, 100 µl of 1 mol/l NaOH solution was added, mixed and placed in a centrifuge tube in water at 37°C for 1 h. Subsequently, 400 µl double-distilled water was added, the sample was mixed, and a 100 µl solution was removed from each centrifuge tube to 96-well plates, and the OD was measured at 490 nm by the microplate reader.
TYR activity
The culture solution in the 96-well plates was discarded, the cells were washed with PBS twice, 100 µl 1% Triton-X-100 was added in each well, and the cell samples were rapidly frozen at −80°C for 30 min. Following this, 50 µl PBS [with 0.2% L-dopa (pH 6.8)] was added to each well, the cells were cultured at 37°C for 3 h, and the OD at a wavelength of 490 nm was detected by the microplate reader.
Western blot analysis of TYR and c-Jun N-terminal kinases (JNK) protein expression levels
The total protein of each group in the 6-well plates was collected and detected. Polyacrylamide gel electrophoresis was used to separate the protein, which was transferred to a polyvinylidene difluoride membrane, blocked with non-specific binding using 5% non-fat milk for 2 h, following which the samples were incubated with primary antibodies (1:300) overnight at 4°C. Following this, the samples were incubated with secondary antibodies in a separate process, and colored with electrochemiluminescence. Images of each sample were captured and the gray value of each band was analyzed. The amount of target protein is shown by the ratio of its gray value to the internal reference.
Analysis of TYR, tyrosinase-related protein-1 (TRP-1), TRP-2, extracellular signal-regulated kinase 1 (ERK1), ERK2 and JNK2 mRNA expression levels by reverse transcription (RT)-PCR
The total cells in the 6-well plates of each group were collected. The total RNA was extracted according to the manufacturer's protocol for TRIzol. For RT, 3 µg RNA was collected from each group. The reaction conditions were as follows: Sample was incubated at 25°C for 10 min, at 42°C for 60 min and at 70°C for 10 min, before terminating the reaction and obtaining the cDNA. Primer sequences were designed and composed by Sangon Biotech Co., Ltd. The primer sequences, with the reference gene β-actin, are shown in Table I. Amplification conditions were as follows: Initial denaturation at 94°C for 2 min, denaturation for 30 sec at 94°C, annealing for 40 sec (TYR at 60°C, TRP-1 at 58°C, TYP-2 at 58°C, ERK1 at 58°C, ERK2 at 55°C, JNK2 at 55°C and β-actin at 55°C, respectively), and the extension was for 40 sec at 70°C. A total of 35 PCR cycles were used for amplification of the samples. The PCR amplification products (5 µl) were mixed with 1 µl of 6X DNA loading buffer. The PCR products were fractionated by 1.5% agarose gel electrophoresis at a constant voltage of 120 V for 30 min. The results were detected using a gel imaging system, and were analyzed with gel-pro analysis software (Media Cybernetics, Inc., Rockville, MD, USA). The mRNA levels of each group were calculated as the relative expression ratio to that of β-actin (TYR/β-actin, TRP-1/β-actin, TRP-2/β-actin, ERK1/β-actin, ERK2/β-actin and JNK2/β-actin).
Table I.
Polymerase chain reaction primer sequences.
| Primer name | Upstream primer | Downstream primer | Amplified fragment, base pairs |
|---|---|---|---|
| TYR | 5′TCACGGCTCTGTTGAATGTCT3′ | 5′CTGAAGTTGGGCGAGATGAT3′ | 300 |
| TRY | 5′ACATCATTCCCTCACCAAAGAC3′ | 5′AGAAGTCCGAAAGCCAAGTAAA3′ | 303 |
| TRP-2 | 5′GTTCCTTTCTTCCCTCCAGTG3′ | 5′TTCCTTTATTGTCAGCGTCAGA3′ | 300 |
| ERK1 | 5′GGGAGGTGGAGATGGTGAAG3′ | 5′AGCAGGTTGGAGGGCTTTAGAT3′ | 441 |
| ERK2 | 5′ACCCACACAAGAGGATTGAAGT3′ | 5′AAAAGCCACAACTACCAGAAAC3′ | 353 |
| JNK2 | 5′CCTTCTTTACCAGATGCTTTGTG3′ | 5′ATACGGTCAGTGCCTTGGAATA3′ | 303 |
| β-actin | 5′CGTGGACATCCGCAAAGAC3′ | 5′AAGAAAGGGTGTAACGCAACTA3′ | 302 |
TYR, tyrosinase; TRP, tyrosinase-related protein; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinases.
Statistical analysis
The obtained data are expressed as mean ± standard deviation, statistically analyzed by one-way analysis of variance using the SPSS software (version 18.0; SPSS, Inc., Chicago, IL, USA). A 2:2 comparison was conducted by least significant difference. P<0.05 was considered to indicate a statistically significant difference.
Results
Effect on cell activity
The effects of bavachin on A375 cells activity are shown in Table II. Treatment with 10 µmol/l bavachin had no significant effect on A375 cells activity when compared to the control group. Therefore, it could be used in the follow-up experiments.
Table II.
Effects of bavachin on A375 cells activity.
| Group (µmol/l) | OD | Cell proliferation rate, % |
|---|---|---|
| Control (0) | 0.466±0.029 | 100.00 |
| Estradiol (10−3) | 0.564±0.029a | 121.03a |
| Bavachin (10) | 0.436±0.020 | 93.56 |
Compared with the control group
P<0.01. Data are mean ± standard deviation and n=6. OD, optical density at 490 nm.
Effects of bavachin on melanin synthesis of the A375 cells
The results for the effect of bavachin treatment on melanin synthesis in A375 cells are shown in Table III. Bavachin significantly inhibited melanin synthesis, which was 55.07% of the control group (P<0.01). ICI182780 and U0126 could reverse the melanin decline induced by bavachin, and the differences were significant when compared with the bavachin group (P<0.01).
Table III.
Effects of bavachin on melanin synthesis in A375 cells.
| Group (µmol/l) | OD | ODtreatment group/ODcontrol group, % |
|---|---|---|
| Control (0) | 0.138±0.014 | 100.00 |
| Estradiol (10−3) | 0.168±0.009a | 121.74a |
| Bavachin (10) | 0.076±0.016a | 55.07a |
| Bavachin (10) + | 0.114±0.004a,b | 82.61a,b |
| ICI182780 (1) | ||
| Bavachin (10) + | 0.109±0.010a,b | 78.99a,b |
| U0126 (10) |
Compared with the control group
P<0.01; compared with the bavachin group
P<0.01. Data are mean ± standard deviation and n=4. OD, optical density at 490 nm.
Effects of bavachin on TYR activity of A375 cells
The results for the effects of bavachin on TYR activity are shown in Table IV. Bavachin significantly inhibited TYR activity, which was 72.41% of the control group (P<0.01). ICI182780 and U0126 reversed the decline of TYR activity induced by bavachin, and the differences were significant compared with the bavachin group (P<0.01).
Table IV.
Effects of bavachin on TYR activity of A375 cells.
| Group (µmol/l) | OD | ODtreatment group/ODcontrol group, % |
|---|---|---|
| Control (0) | 0.087±0.006 | 100.00 |
| Estradiol (10−3) | 0.107±0.004a | 122.99a |
| Bavachin (10) | 0.063±0.004a | 72.41a |
| Bavachin (10) + | 0.080±0.004b,c | 91.95b,c |
| ICI182780 (1) | ||
| Bavachin (10) + | 0.077±0.009a,c | 88.51a,c |
| U0126 (10) |
Compared with the control group
P<0.05
P<0.01; compared with the bavachin group
P<0.01. Data are mean ± standard deviation and n=6. TYR, tyrosinase; OD, optical density at 490 nm.
Effects of bavachin on the expression levels of the TYR and JNK proteins
The effects of bavachin treatment on TYR and JNK proteins are shown in Table V and Fig. 1. Bavachin significantly inhibited the expression levels of the TYR and JNK proteins when compared with the control group (P<0.01). ICI182780 reversed the decline of the TYR protein and the JNK protein as induced by bavachin, and the differences were significant compared to the bavachin group (P<0.01).
Table V.
Expression levels of TYR and JNK proteins in the different groups.
| Group (µmol/l) | TYR/β-actin | JNK/β-actin |
|---|---|---|
| Control (0) | 0.558±0.018 | 0.477±0.018 |
| Estradiol (10−3) | 0.693±0.030a | 0.583±0.011a |
| Bavachin (10) | 0.358±0.032a | 0.221±0.003a |
| Bavachin (10) + | 0.477±0.005a,b | 0.332±0.029a,b |
| ICI182780 (1) |
Compared with the control group
P<0.01; compared with the bavachin group
P<0.01. Data are mean ± standard deviation and n=4. TYR, tyrosinase; JNK, c-Jun N-terminal kinases.
Figure 1.
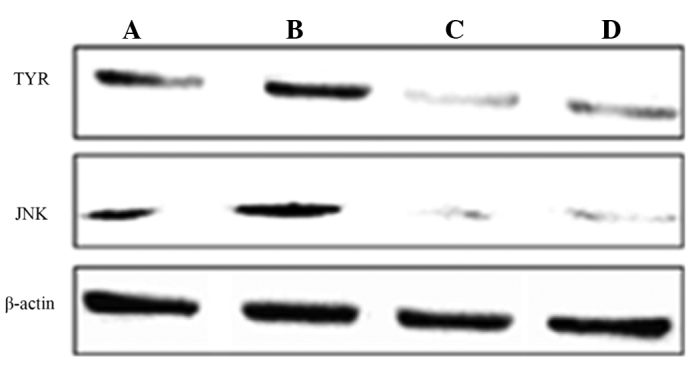
Expression levels of TYR and JNK proteins in the different groups. (A) Control; (B) estradiol; (C) bavachin; and (D) bavachin + ICI182780 groups.
RT-PCR results
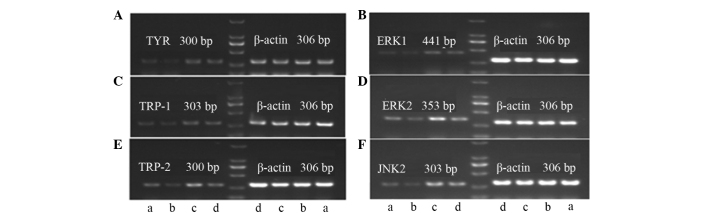
Effects of bavachin and ICI182780 on the expression levels of the TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2 mRNA in the A375 cells
The effects of bavachin and ICI182780 are shown in Table VI and Fig. 2. Bavachin significantly inhibited the expression levels of TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2 mRNA when compared with the control group (P<0.01). ICI182780 reversed the decline of the TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2 mRNA as induced by bavachin, and the differences were significant when comparing with the bavachin group (P<0.05 or P<0.01).
Table VI.
Polymerase chain reaction analysis of the TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2 mRNA expression levels in the different groups.
| mRNA | Control group (0 µmol/l) | Estradiol group (10−3 µmol/l) | Bavachin group (10 µmol/l) | Bavachin (10 µmol/l) + ICI182780 (1 µmol/l) |
|---|---|---|---|---|
| TYR/β-actin | 0.583±0.006 | 0.691±0.017a | 0.332±0.038a | 0.470±0.047a,b |
| TRP-1/β-actin | 0.424±0.022 | 0.561±0.012a | 0.251±0.029a | 0.357±0.013a,b |
| TRP-2/β-actin | 0.427±0.010 | 0.532±0.014a | 0.289±0.022a | 0.346±0.036a,c |
| ERK1/β-actin | 0.461±0.038 | 0.561±0.021a | 0.299±0.009a | 0.357±0.047a,b |
| ERK2/β-actin | 0.507±0.019 | 0.620±0.031a | 0.320±0.020a | 0.417±0.027a,c |
| JNK2/β-actin | 0.491±0.013 | 0.591±0.012a | 0.276±0.040a | 0.357±0.033a,c |
Compared with the control group
P<0.01; compared with the bavachin group
P<0.05
P<0.01. Data are mean ± standard deviation and n=4. TYR, tyrosinase; TRP, tyrosinase-related protein; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinases.
Figure 2.
Polymerase chain reaction analysis of (A) TYR, (B) ERK1, (C) TRP-1, (D) ERK2, (E) TRP-2 and (F) JNK2 mRNA expression levels in the A375 cells in the different groups. (a) Bavachin + ICI182780; (b) bavachin; (c) estradiol; and (d) control groups. TYR, tyrosinase; TRP, tyrosinase-related protein; ERK, extracellular signal-regulated kinase; JNK, c-Jun N-terminal kinases; bp, base pairs.
Effects of bavachin and U0126 on expression of TYR, TRP-1 and TRP-2 mRNA in A375 cells
The results of the effects of bavachin and U0126 are shown in Table VII and Fig. 3. Bavachin significantly inhibited the expression of TYR, TRP-1 and TRP-2 mRNA, as compared with the control group (P<0.01). U0126 reversed the decline of the TYR, TRP-1 and TRP-2 mRNA induced by bavachin, and the differences were significant when compared with the bavachin group (P<0.01 or P<0.05).
Table VII.
Polymerase chain reaction analysis of TYR, TRP-1 and TRP-2 mRNA expression levels in the different groups.
| mRNA | Control group (0 µmol/l) | Estradiol group (10−3 µmol/l) | Bavachin group (10 µmol/l) | Bavachin (10 µmol/l) + U0126 (1 µmol/l) |
|---|---|---|---|---|
| TYR/β-actin | 0.564±0.016 | 0.676±0.011a | 0.368±0.034a | 0.413±0.037a,b |
| TRP-1/β-actin | 0.458±0.010 | 0.547±0.022a | 0.291±0.009a | 0.392±0.015a,c |
| TRP-2/β-actin | 0.411±0.007 | 0.549±0.016a | 0.265±0.033a | 0.348±0.008a,c |
Compared with the control group
P<0.01; compared with the bavachin group
P<0.05
P<0.01. Data are mean ± standard deviation and n=4. TYR, tyrosinase; TRP, tyrosinase-related protein.
Figure 3.

Polymerase chain reaction analysis of (A) TYR, (B) TRP-1 and (C) TRP-2 mRNA expression in the A375 cells in different groups. (a) Wogonin + U0126; (b) wogonin; (c) estradiol; (d) control groups. TYR, tyrosinase; TRP, tyrosinase-related protein; bp, base pairs.
Discussion
Melanin is produced in the melanosome of melanocytes regulated by TYR, which is the main rate-limiting enzyme of this process. The TYR genes mainly includes TYR, TRP-1 and TRP-2 (9–11). Estrogen, as an extracellular stimuli, can bind to ER, which also exists in melanocytes, and subsequently activate the extracellular signal transduction pathway directly or indirectly, which can induce a nongenomic effect. The ER-related regulation is well-connected with the MAPK signaling pathway, which is a common pathway of physiological reactions responding to extracellular stimuli. MAPK belongs to the serine-threonine kinases. A previous study also demonstrated that the MAPK pathway is involved in the regulation of melanin synthesis (12). The MAPK family mainly consists of ERK1/2, JNK1/2 and p38 MAPK (13). The studies have shown that activation of MAPK signaling may be an important pathway involved in melanoma transformation. Inhibition of MAPK signaling may be useful in the prevention and treatment of melanoma (14). The study by Yanase et al (15) demonstrated that the expression of TYR mRNA and protein increase phosphorylation, and activity of ERK1/2 elevated following UVA irradiation. However, treatment with a tyrosine kinase receptor inhibitor reduced ERK1/2 activation following UVA-irradiation, which suggested that UVA irradiation-induced melanogenesis is associated with the activation of ERK1/2 by upstream signals that originate from reactive oxygen species or from activated tyrosine kinase receptors (15). Hata et al (16) reported that activation of the p38 MAPK signaling pathway induced differentiation of B16 melanoma cells. In a previous study, Ali et al (17) indicated that phytoestrogen extracted from Psoralea coryfolia is connected with melanin synthesis, the lyophilized extracts of Psoralea corylifolia seeds and pure psoralen induced melanin dispersal effects; however, the underlying regulation mechanism remains to be elucidated.
In the present study, the safe dose of bavachin could significantly inhibit melanin synthesis and TYR activity in A375 cells, which could be reversed by ICI182780 and U0126. This suggested that bavachin inhibits melanin synthesis by decreasing TYR activity, which was achieved by the ER-ERK pathway. Western blot analysis shows that bavachin could significantly inhibit the expression of TYR and JNK protein in the A375 cells, which could be reversed by ICI182780. This indicates that lessening the expression of TYR and JNK protein is connected with regulation of melanin synthesis. RT-PCR showed that bavachin could significantly inhibit the expression of TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2 mRNA, which may be involved in the regulation of melanin synthesis. Notably, bavachin inhibited the synthesis of melanin on A375 cells by inhibiting expression of TYR, TRP-1, TRP-2, ERK1, ERK2 and JNK2 protein and mRNA.
Acknowledgements
The study was sponsorted by grants from the National Natural Science Foundation of China (General Program; no. 81274035); Postdoctoral Research Starting Capital of Heilongjiang Province (no. LBH-Q13162); and the Excellent Innovation Talents of Heilongjiang University of Chinese Medicine.
References
- 1.Xu Y, Zhang ZJ, Geng F, Su SB, White KN, Bligh SW, Branford-White CJ, Wang ZT. Treatment with Qing'E, a kidney-invigorating Chinese herbal formula, antagonizes the estrogen decline in ovariectomized mice. Rejuvenation Res. 2010;13:479–488. doi: 10.1089/rej.2009.1000. [DOI] [PubMed] [Google Scholar]
- 2.Research Office of family planning, stitute of Chinese Traditional Medicine: Academy of Traditional Chinese Medicine. Research Data Traditional Chinese Medicine of Meijing. 1979;1:16–18. [Google Scholar]
- 3.Lim SH, Ha TY, Ahn J, Kim S. Estrogenic activities of Psoralea corylifolia L. seed extracts and main constituents. Phytomedicine. 2011;18:425–430. doi: 10.1016/j.phymed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Lim SH, Ha TY, Kim SR, Ahn J, Park HJ, Kim S. Ethanol extract of Psoralea corylifolia L. and its main constituent, bakuchiol, reduce bone loss in ovariectomised Sprague-Dawley rats. Br J Nutr. 2009;101:1031–1039. doi: 10.1017/S0007114508066750. [DOI] [PubMed] [Google Scholar]
- 5.Xin D, Wang H, Yang J, Su YF, Fan GW, Wang YF, Zhu Y, Gao XM. Phytoestrogens from Psoralea corylifolia reveal estrogen receptor-subtype selectivity. Phytomedicine. 2010;17:126–131. doi: 10.1016/j.phymed.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 6.Shelly W, Draper MW, Krishnan V, Wong M, Jaffe RB. Selective estrogen receptor modulators: An update on recent clinical findings. Obstet Gynecol Surv. 2008;63:163–181. doi: 10.1097/OGX.0b013e31816400d7. [DOI] [PubMed] [Google Scholar]
- 7.Anbar TS, El-Sawy AE, Attia SK, Barakat MT, Moftah NH, El-Ammawy TS, Abdel-Rahman AT, El-Tonsy MH. Effect of PUVA therapy on melanocytes and keratinocytes in non-segmental vitiligo: Histopathological, immuno-histochemical and ultrastructural study. Photodermatol Photoimmunol Photomed. 2012;28:17–25. doi: 10.1111/j.1600-0781.2011.00631.x. [DOI] [PubMed] [Google Scholar]
- 8.Ohno O, Watabe T, Nakamura K, Kawagoshi M, Uotsu N, Chiba T, Yamada M, Yamaguchi K, Yamada K, Miyamoto K, Uemura D. Inhibitory effects of bakuchiol, bavachin, and isobavachalcone isolated from Piper longum on melanin production in B16 mouse melanoma cells. Biosci Biotechnol Biochem. 2010;74:1504–1506. doi: 10.1271/bbb.100221. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto Y, Ito Y, Kato T, Motokawa T, Katagiri T, Itoh M. Expression profiles of melanogenesis-related genes and proteins in acquired melanocytic nevus. J Cutan Pathol. 2006;33:207–215. doi: 10.1111/j.0303-6987.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 10.Lu F, Yan D, Zhou X, Hu DN, Qu J. Expression of melanin-related genes in cultured adult human retinal pigment epithelium and uveal melanoma cells. Mol Vis. 2007;13:2066–2072. [PubMed] [Google Scholar]
- 11.Fang D, Kute T, Setaluri V. Regulation of tyrosinase-related protein-2 (TYRP2) in human melanocytes: Relationship to growth and morphology. Pigment Cell Res. 2001;14:132–139. doi: 10.1034/j.1600-0749.2001.140209.x. [DOI] [PubMed] [Google Scholar]
- 12.Jiang ZQ. The Mechanism of Active Componets of Two Chinese Traditional Medicines on Melanogenesis (unpublished PhD thesis) Huazhong University of Science and Technology. 2009:1114360302971160. [Google Scholar]
- 13.Cohen P. The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 1997;7:353–361. doi: 10.1016/S0962-8924(97)01105-7. [DOI] [PubMed] [Google Scholar]
- 14.Govindarajan B, Bai X, Cohen C, Zhong H, Kilroy S, Louis G, Moses M, Arbiser JL. Malignant transformation of melanocytes to melanoma by constitutive activation of mitogen-activated protein kinase kinase (MAPKK) signaling. J Biol Chem. 2003;278:9790–9795. doi: 10.1074/jbc.M212929200. [DOI] [PubMed] [Google Scholar]
- 15.Yanase H, Ando H, Horikawa M, Watanabe M, Mori T, Matsuda N. Possible involvement of ERK 1/2 in UVA-induced melanogenesis in cultured normal human epidermal melanocytes. Pigment Cell Res. 2001;14:103–109. doi: 10.1034/j.1600-0749.2001.140205.x. [DOI] [PubMed] [Google Scholar]
- 16.Hata K, Hori K, Takahashi S. Role of p38 MAPK in lupeol-induced B16 2F2 mouse melanoma cell differentiation. J Biochem. 2003;134:441–445. doi: 10.1093/jb/mvg162. [DOI] [PubMed] [Google Scholar]
- 17.Ali SA, Sultan T, Galgut JM, Sharma R, Meitei KV, Ali AS. In vitro responses of fish melanophores to lyophilized extracts of Psoralea corylifolia seeds and pure psoralen. Pharm Biol. 2011;49:422–427. doi: 10.3109/13880209.2010.521164. [DOI] [PubMed] [Google Scholar]