Abstract
Background
The FluoroSpot assay, an advancement of the ELISpot assay, enables simultaneous measurement of different analytes secreted at a single-cell level. This allows parallel detection of several cytokines secreted by immune cells upon antigen recognition. Easier standardization, higher sensitivity and reduced labour intensity render FluoroSpot assays an interesting alternative to flow-cytometry based assays for analysis of clinical samples. While the use of immunoassays to study immunological primary and secondary endpoints becomes increasingly attractive, assays used require pre-trial validation. Here we describe the assay validation (precision, specificity and linearity) of a FluoroSpot immunological endpoint assay detecting Interferon γ (IFNγ) and Interleukin 2 (IL2) for use in clinical trial immune monitoring.
Methods
We validated an IFNγ/IL2 FluoroSpot assay to determine Epstein-Barr virus (EBV)-specific cellular immune responses (IFNγ, IL2 and double positive IFNγ + IL2 responses), using overlapping peptide pools corresponding to EBV-proteins BZLF1 and EBNA3A. Assay validation was performed using cryopreserved PBMC of 16 EBV-seropositive and 6 EBV-seronegative donors. Precision was assessed by (i) testing 16 donors using three replicates per assay (intra-assay precision/repeatability) (ii) using two plates in parallel (intermediate precision/plate-to-plate variability) and (iii) by performing the assays on three different days (inter-assay precision/reproducibility). In addition, we determined specificity, linearity and quantification limits of the assay. Further we tested precision across the two assay systems, IFNγ/IL2 FluoroSpot and the corresponding enzymatic single cytokine ELISpot.
Results
The validation revealed: (1) a high intra-assay precision (coefficient of variation (CV) 9.96, 8.85 and 13.05 %), intermediate precision (CV 6.48, 10.20 and 12.97 %) and reproducibility (CV 20.81 %, 12,75 % and 12.07 %) depending on the analyte and antigen used; (2) a specificity of 100 %; (3) a linearity with R2 values from 0.93 to 0.99 depending on the analyte. The testing of the precision across the two assay systems, adduced a concordance correlation coefficient pc = 0.99 for IFNγ responses and pc = 0.93 for IL2 responses, indicating a large agreement between both assay methods.
Conclusions
The validated primary endpoint assay, an EBV peptide pool specific IFNγ/IL2 FluoroSpot assay was found to be suitable for the detection of EBV-specific immune responses subject to the requirement of standardized assay procedure and data analysis.
Electronic supplementary material
The online version of this article (doi:10.1186/s12967-016-0932-7) contains supplementary material, which is available to authorized users.
Keywords: Assay precision, Assay validation, Clinical trial monitoring, EBV-specific T-cell responses, FluoroSpot
Background
The enzyme-linked immuno spot (ELISpot) assay, which enumerates peripheral blood mononuclear cells releasing cytokines upon specific antigen stimulation, has become an assay of choice for evaluation of cell-mediated immune responses in many clinical trials [1–3].
The ELISpot assay is limited, however, in that only one cytokine at a time can be assessed. The FluoroSpot assay, an advancement of the ELISpot assay, enables simultaneous measurement of different analytes secreted at a single-cell level [4, 5]. This facilitates the detection of cells secreting several cytokines in parallel such as e.g. polyfunctional T cells, which have been suggested to be correlates of protection in various infectious diseases [6–8]. By detecting different cytokines with a specific fluorophore and analyzing differentially fluorescent spots by specific filter systems, cells producing single or multiple cytokines can be identified. FluoroSpot assays maintain the simplicity and sensitivity of the ELISpot assay but offer the advantage of multiplex analyses.
Investigating antigen specific immune responses as a primary endpoint in clinical trials requires highly sensitive and validated assays to determine immune cell reactivity ex vivo correlating with clinical outcome. Assays for detecting cellular immune responses in humans have already been used to determine primary endpoints in clinical trials [9, 10], but the validation of these assays has often not been approached in a manner that follows the assay validation guidelines provided for industry [11] (http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf).
Validation is a well-known process in industry, but is much less common for immune monitoring assays used in the academic and clinical settings with only few published guidelines, especially for validation of assays that are considered to be “state-of-art” [12–15]. Since 2005, two consortia have performed proficiency panel experiments to address T cell immunoassay harmonization and as a consequence the MIATA (“Minimum Information About T-cell Assays”) initiative was launched to optimize assay performance and reproducibility between different laboratories [16–18]. Implementation of cross-laboratory validation is known to support reduction of data variability, thus guaranteeing consistency of datasets generated by different clinical trials sites [19–21].
Guidelines for assay validation define eight parameters that must be investigated in order to validate a bioanalytical assay [22]: (1) specificity, (2) accuracy, (3) precision (repeatability, intermediate precision, reproducibility), (4) detection limit, (5) quantification limit, (6) linearity, (7) range and (8) robustness. Here we provide, to our knowledge, the first validation report for an IFNγ/IL2 FluoroSpot assay designed to allow qualitative and quantitative evaluation of cellular immune responses. Further we tested precision across the two related assay systems, IFNγ/IL2 FluoroSpot and the corresponding enzymatic single cytokine ELISpot.
As a result, the validated primary endpoint assay, an Epstein-Barr virus (EBV) peptide pool specific IFNγ/IL2 FluoroSpot assay was found to be suitable for the detection of EBV-specific immune responses in a clinical trial setting.
Methods
The authors acknowledge the concept of the MIATA framework which was recently published [17, 18]. Therefore, detailed information is provided as structured in the five modules proposed by MIATA (http://www.miataproject.org/) [18].
The sample
Subjects
Peripheral blood was taken by venipuncture from 30 healthy donors (20 women, 10 men) with an average age of 30 years (range 17–50 years). Donors were either EBV-seropositive or EBV-seronegative, diagnosed by pre-testing with a diagnostic assay. A pre-screening of donors for EBV-specific cell-mediated immune responses was done by ELISpot analyses prior to the FluoroSpot validation experiments, to ensure an inclusion of a broad range of EBV-specific low- and high-responders. Informed consent was obtained from all participating subjects prior to their inclusion in this validation experiments.
Cryoconservation of PBMC
Within 4 h after collection of heparinized whole blood human peripheral blood mononuclear cells (PBMC) were separated by Ficoll density gradient (human Pancoll, PAN-BIOTECH, Aidenbach, Germany) using 50 ml LeucosepTM tubes (Greiner Bio-One, Frickenhausen) and washed one time with sterile phosphate buffered saline (PBS) (Life Technologies, Darmstadt, Germany) and once with RPMI1640 medium (Life Technologies, Invitrogen, Darmstadt, Germany) following our established standard operating procedure (SOP). Trypan blue (Life Technologies, Darmstadt, Germany) staining was used to count on living cells. The median PBMC number obtained per ml whole blood was 0.7x106 PBMC. PBMC were frozen at 5 × 106 PBMC per vial in 1.8 ml cryotubes (Thermo Scientific, Roskilde, Denmark) in a concentration of 1 × 107 PBMC per 1 ml freezing medium (fetal calf serum (FCS) (Life Technologies, Darmstadt, Germany) supplemented with 10 % dimethyl sulfoxide (Sigma-Aldrich, Steinheim, Germany) using a freezing container (Mr. Frosty, Thermo Scientific, Roskilde, Denmark) and put on −80 °C. After 24 h PBMC were stored in liquid nitrogen until further use.
Thawing and resting of PBMC
According to our SOP, PBMC were thawed at 37 °C using Roswell Park Memorial Institute (RPMI1640) medium supplemented with 10 % FCS and 1 % penicillin–streptomycin (PenStrep, Life Technologies, Invitrogen, Darmstadt, Germany) (abbr.: RPMI-10). After two washing steps with RPMI-10, cells were counted with an automated cell counter (Vi-cell XR, Beckman Coulter, Krefeld, Germany). The median cell recovery after thawing was 5.0 × 106 PBMC per vial with a median viability of 93 %. For a standard resting procedure PBMC were incubated for 18 h at 37 °C in a humidified atmosphere at 5 % CO2 at a concentration of 2 × 106 PBMC/ml RPMI-10. After resting the median cell recovery was 4.8 × 106 PBMC per vial with a median viability of 95 %.
The assay
Stimulatory agents
The following stimulatory agents were used in this study: Overlapping peptide pools of EBV-derived proteins BZLF1 (59 peptides) and EBNA3A (234 peptides) (JPT Peptide Technologies, Berlin, Germany), consisting of 15mers overlapping 11 amino acids in a concentration of 1 µg/ml. The optimal assay concentration of both peptide pools was identified in previous titration experiments. Phytohemagglutinin (PHA-L) (Sigma-Aldrich Chemie, Schnelldorf, Germany) was used as a mitogen for stimulation in a concentration of 2 µg/ml. All experiments were performed in triplicates when cells were stimulated with antigen (BZLF1, EBNA3A) or in six replicates for the PHA-L-stimulated cells. RPMI-10 was added as a negative control in triplicates and anti-CD3 (in a dilution of 1:1000, mAb CD3-2, Mabtech AB, Nacka Strand, Sweden) was used as a positive control in a single well for each donor.
IFNγ/IL2 FluoroSpot assay
IFNγ/IL2 FluoroSpot assays (human IFNγ/IL2 FluoroSpot Kit with pre-coated plates, product code: FSP-0102-10, Mabtech AB, Nacka Strand, Sweden) were performed according to the manufacturer´s instructions, except for washing steps, which were increased to a seven-time washing. Either 2 × 105 PBMC/well for BZLF1- and EBNA3A-stimulated PBMC or 5 × 104 PBMC/well for PHA-L-stimulated PBMC were plated in a final volume of 150 µl/well.
ELISpot assays
IFNγ ELISpot assays (human IFNγ ELISpotPLUS Kit with pre-coated plates, product code: 3420-4APW-10, Mabtech AB, Nacka Strand, Sweden) and IL2 ELISpot assays (human IL2 ELISpotPLUS Kit with pre-coated plates, product code: 3445-4APW-10, Mabtech AB, Nacka Strand, Sweden) were performed according to the manufacturer´s instructions, except for washing steps, which were increased to a seven-time washing. 2 × 105 PBMC/well were plated in a final volume of 150 µl/well and stimulated with peptide pools of EBV-derived proteins BZLF1 or EBNA3A, respectively.
Data acquisition
ELISpot and FluoroSpot plates were evaluated within 3 days after assay performance using an automated reader system (CTL-ImmunoSpot® S6 Ultra-V Analyzer/CTL ImmunoSpot 5.1 Professional DC Software, CTL Europe, Bonn, Germany). ELISpot plates were scanned with automatically adjusted settings conducted by the reader. Solely the selection of the plate type and the centring of the wells were done manually. FluoroSpot plates were scanned with manual settings for both fluorophore filters (fluorescein isothiocyanate (FITC)/phycoerythrin (PE)) by adjusting the gain and exposure time of the UV-light. Counting of spot forming cells (SFC) within ELISpot and FluoroSpot plates was performed manually in compliance with the guidelines for the automated evaluation of ELISpot assays [23] and our laboratory standard counting parameters consisting of a best possible spot separation, a spot size gating from minimum to maximum and a counting mask size of 90 %. Spot counts were normalized to 100 % of the well area. The settings for sensitivity of spot counting were established and adjusted manually for each plate using antigen stimulated and negative control wells. In detail, the sensitivity for the counting of single spots was adjusted by identifying antigen-stimulated wells with spots that were well distributed and clearly distinguishable from background activity and artefacts. In a next step, the selected parameters for the sensitivity of counting spots were checked on negative control wells to prevent to count on small background spots. If necessary, the parameters for the sensitivity of counting spots were adjusted to exclude artefact or background spots. The performed adjustments were rechecked on the antigen-stimulated wells and if necessary adapted another time to make sure to count on the most distinct spots. This way of parameter checking was repeated on other sets of antigen-stimulated and negative control wells. If possible, throughout the counting procedure of one single plate, similar settings were used for replicates of one donor, and one antigen. All obtained counts were reviewed and certified by a second person during a quality control process including an exclusion of artefacts within wells or a rejection of failed wells. Figure 1 displays an image example of representative data sets obtained using the IFNγ/IL2 FluoroSpot assay in which cryopreserved PBMC of an EBV-seropositive donor were stimulated with the BZLF1 peptide pool.
Fig. 1.

Illustration of IFNγ/IL2 FluoroSpot assay images. Individual images were captured for IFNγ (left image) (FITC filter) and IL2 (central image) (PE filter) and used to generate the computerized overlay of the two filters showing double positive IFNγ + IL2 cell responses (right image). IFNγ, IL2 and IFNγ + IL2 secreting cells upon stimulation with BZLF1 are depicted as green (left image), red (central image), and yellow (right image) spots, respectively
Interpretation of results
Final results are represented as spot forming cells (SFC) per 2 × 105 PBMC for BZLF1- and EBNA3A-stimulated PBMC and 5 × 104 PBMC for PHA-L-stimulated PBMC. Unless specified differently, denoted results represent background subtracted data. The median background reactivity (spot counts in negative control wells) observed within the ELISpot assay was 0 spots per well (range 0–4 SFC/well) in IFNγ ELISpot assays and 5 spots per well (range 0–19 SFC/well) in IL2 ELISpot assays. For the IFNγ/IL2 FluoroSpot assays we observed a median background of 1 spot per well (range 0–4 SFC/well) for single positive IFNγ responses (IFNγ), 5 spots per well (range 1–10 SFC/well) for single positive IL2 responses (IL2) and 1 spots per well (range 0–2 SFC/well) for double positive IFNγ + IL2 responses (IFNγ + IL2). Positive reactivity to experimental stimulatory agents was selected as a p-value of equal or smaller than 0.05 when applying the distribution free sampling method (DFR(2x)), by using a web-based tool (http://www.scharp.org/zoe/runDFR/) [24], which compares the spot counts in antigen stimulated wells with spot counts in negative control wells. In addition, only mean spot counts of at least 11 SFC/2 × 105 PBMC (in EBV peptide pool stimulated wells) or 11 SFC/5 × 104 PBMC (in PHA-L-stimulated wells) were regarded as a positive reactivity. Outliers of the replicates, predefined as results which originate from irregular wells, were excluded during quality control. Raw data of all performed assays can be provided upon request.
Laboratory environment
All experiments were performed by well-trained members of the lab in accordance with our established SOP protocols. Laboratory personnel participated regularly in external international ELISpot proficiency panels.
Statistical analyses
To define assay precision, the coefficient of variation (%CV) was calculated as the ratio of the standard deviation to the mean and expressed as a percentage value. All tests were two-sided and were conducted on exploratory 5 % significance levels. Effect measures are presented with 95 % confidence intervals. Linear regression analysis was performed to assess the coefficient of determination R2. The concordance correlation coefficient pc by Lin [25] was calculated to investigate agreement of measurements. Likewise, the Bland–Altman method was used to assess the agreement between FluoroSpot and ELISpot measurements by calculating the average difference d (FluoroSpot-ELISpot) and the 95 % limits of agreement (d ± 1.96 standard deviation (s) of the difference) [26]. The software Graph Pad Prism 5.00 (GraphPad Software, La Jolla, California, USA) and R (http://www.r-project.org/) [27] were used for statistical analyses.
Results
First we evaluated assay precision, including repeatability (intra-assay precision), intermediate precision (plate-to-plate variability) and reproducibility of the assay (day to day variability). For the complete validation process cryopreserved PBMC of one isolation batch were used to assess assay precision using the same lot of assay reagents. All samples were assayed in triplicates (BZLF1 and EBNA3A) or six replicates (PHA-L; control antigen).
Intra-assay precision
We used PBMC of 16 donors to examine intra-assay precision of the IFNγ/IL2 FluoroSpot assay. Overall we determined a high intra-assay precision for IFNγ, IL2 and IFNγ + IL2 responses for both, the EBV-derived antigens and the mitogen. Regarding all donors, a mean CV of 9.96, 8.85 and 13.05 % was obtained for IFNγ, IL2 and IFNγ + IL2 responses, respectively (Table 1A–C). These results indicate that intra-assay variability was acceptable. It also justified the analysis of samples with lower replicates when clinical material is limited.
Table 1.
Intra-assay variability of IFNγ, IL2 and IFNγ + IL2 responses in the IFNγ/IL2 FluoroSpot assay
| Donor | Antigen | Replicate 1 | Replicate 2 | Replicate 3 | Replicate 4 | Replicate 5 | Replicate 6 | Mean | SD | %CV |
|---|---|---|---|---|---|---|---|---|---|---|
| A | ||||||||||
| S09 | BZLF1 | 283 | 267 | 280 | ND | ND | ND | 277 | 8.50 | 3.07 |
| S11 | BZLF1 | 432 | 440 | 493 | ND | ND | ND | 455 | 33.15 | 7.29 |
| S14 | BZLF1 | 438 | 452 | 479 | ND | ND | ND | 456 | 20.84 | 4.57 |
| S16 | BZLF1 | 458 | 374 | 384 | ND | ND | ND | 405 | 45.88 | 11.32 |
| S18 | BZLF1 | 110 | 148 | 103 | ND | ND | ND | 120 | 24.21 | 20.12 |
| S01 | EBNA3A | 30 | 26 | 31 | ND | ND | ND | 29 | 2.65 | 9.12 |
| S10 | EBNA3A | 28 | 31 | 31 | ND | ND | ND | 30 | 1.73 | 5.77 |
| S12 | EBNA3A | 38 | 43 | 46 | ND | ND | ND | 42 | 4.04 | 9.55 |
| S20 | EBNA3A | 68 | 60 | 81 | ND | ND | ND | 70 | 10.60 | 15.21 |
| S21 | EBNA3A | 166 | 158 | 192 | ND | ND | ND | 172 | 17.78 | 10.34 |
| S12 | PHA-L | 35 | 35 | 45 | 44 | Rejected | 38 | 39 | 4.83 | 12.25 |
| S15 | PHA-L | 718 | 659 | 774 | 685 | 679 | Rejected | 703 | 45.01 | 6.40 |
| S21 | PHA-L | Rejected | 715 | 836 | 740 | 786 | 771 | 770 | 46.20 | 6.00 |
| S25 | PHA-L | 293 | 326 | 391 | 250 | 276 | Rejected | 307 | 54.37 | 17.70 |
| S26 | PHA-L | 98 | 120 | 123 | Rejected | 98 | 123 | 112 | 13.20 | 11.75 |
| S27 | PHA-L | 253 | 280 | 284 | 264 | 226 | Rejected | 261 | 23.38 | 8.95 |
| Mean | 22.27 | 9.96 | ||||||||
| B | ||||||||||
| S09 | BZLF1 | 63 | 67 | 52 | ND | ND | ND | 61 | 7.77 | 12.80 |
| S11 | BZLF1 | 28 | 27 | 30 | ND | ND | ND | 28 | 1.53 | 5.39 |
| S14 | BZLF1 | 37 | 31 | 34 | ND | ND | ND | 34 | 3.00 | 8.82 |
| S16 | BZLF1 | 39 | 39 | 43 | ND | ND | ND | 40 | 2.31 | 5.73 |
| S18 | BZLF1 | 32 | 37 | 37 | ND | ND | ND | 35 | 2.89 | 8.17 |
| S01 | EBNA3A | 33 | 29 | 21 | ND | ND | ND | 28 | 6.11 | 22.08 |
| S10 | EBNA3A | 26 | 26 | 20 | ND | ND | ND | 24 | 3.46 | 14.43 |
| S12 | EBNA3A | 47 | 44 | 51 | ND | ND | ND | 47 | 3.51 | 7.42 |
| S20 | EBNA3A | 41 | 32 | Rejected | ND | ND | ND | 37 | 6.36 | 17.44 |
| S21 | EBNA3A | 42 | 38 | 38 | ND | ND | ND | 39 | 2.31 | 5.87 |
| S12 | PHA-L | 142 | 148 | 143 | 142 | 112 | Rejected | 137 | 14.42 | 10.49 |
| S15 | PHA-L | 718 | 645 | 647 | 718 | 652 | Rejected | 676 | 38.43 | 5.68 |
| S21 | PHA-L | Rejected | 685 | 688 | 693 | 725 | 700 | 698 | 16.02 | 2.29 |
| S25 | PHA-L | 413 | 410 | 408 | 382 | Rejected | 412 | 405 | 13.00 | 3.21 |
| S26 | PHA-L | 488 | 505 | 511 | 554 | 501 | 524 | 514 | 22.96 | 4.47 |
| S27 | PHA-L | 374 | 423 | 430 | 445 | 385 | 383 | 407 | 29.59 | 7.28 |
| Mean | 10.85 | 8.85 | ||||||||
| C | ||||||||||
| S09 | BZLF1 | 50 | 51 | 57 | ND | ND | ND | 53 | 3.79 | 7.19 |
| S11 | BZLF1 | 19 | 18 | 21 | ND | ND | ND | 19 | 1.53 | 7.90 |
| S14 | BZLF1 | 24 | 18 | 18 | ND | ND | ND | 20 | 3.46 | 17.32 |
| S16 | BZLF1 | 26 | 23 | 26 | ND | ND | ND | 25 | 1.73 | 6.93 |
| S18 | BZLF1 | 23 | 29 | 29 | ND | ND | ND | 27 | 3.46 | 12.83 |
| S01 | EBNA3A | 18 | 11 | 11 | ND | ND | ND | 13 | 4.04 | 30.31 |
| S10 | EBNA3A | 13 | 18 | 11 | ND | ND | ND | 14 | 3.61 | 25.75 |
| S12 | EBNA3A | 23 | 25 | 27 | ND | ND | ND | 25 | 2.00 | 8.00 |
| S20 | EBNA3A | 10 | 14 | 16 | ND | ND | ND | 13 | 3.06 | 22.91 |
| S21 | EBNA3A | 22 | 17 | 21 | ND | ND | ND | 20 | 2.65 | 13.23 |
| S12 | PHA-L | 0 | 0 | 0 | 0 | 0 | 0 | 0 | – | – |
| S15 | PHA-L | Rejected | 317 | 372 | 345 | 363 | Rejected | 349 | 24.25 | 6.94 |
| S21 | PHA-L | Rejected | 460 | 454 | 437 | Rejected | 489 | 460 | 21.65 | 4.71 |
| S25 | PHA-L | 113 | 118 | 124 | Rejected | 125 | Rejected | 120 | 5.60 | 4.66 |
| S26 | PHA-L | 66 | 79 | 89 | 68 | 70 | 93 | 78 | 11.43 | 14.75 |
| S27 | PHA-L | 175 | 181 | 200 | 189 | 142 | Rejected | 177 | 21.89 | 12.34 |
| Mean | 7.61 | 13.05 | ||||||||
Values represent the number of detected antigen-specific IFNγ (A), IL2 (B) and IFNγ + IL2 (C) SFC/2 × 105 PBMC (stimulated with 1 µg/ml BZLF1 or EBNA3A peptide pools) or mitogen-specific IFNγ (A), IL2 (B) and IFNγ + IL2 (C) SFC/5 × 104 PBMC (stimulated with 2 µg/ml PHA-L) in the IFNγ/IL2 FluoroSpot assay (data is not background subtracted); “rejected” wells were not accepted because they did not pass the quality control
ND not done, SD standard deviation, CV coefficient of variation
Inter-assay precision
To evaluate inter-assay variability we used PBMC of eight donors plated into two different assay plates in parallel. Inter-assay precision of the two assays performed on the same day showed low inter-plate variability. The mean CV for all tested donors was 6.48, 10.20 and 12.97 % for IFNγ, IL2 and IFNγ + IL2 responses, respectively (Table 2A–C).
Table 2.
Inter-assay variability of IFNγ, IL2 and IFNγ + IL2 responses in the IFNγ/IL2 FluoroSpot assay
| Donor | Antigen | Plate 1 | Plate 2 | Plate 1 and plate 2 | ||
|---|---|---|---|---|---|---|
| Mean | Mean | Mean | SD | % CV | ||
| A | ||||||
| S09 | BZLF1 | 274 | 233 | 254 | 28.99 | 11.44 |
| S11 | BZLF1 | 317 | 326 | 322 | 6.36 | 1.98 |
| S12 | EBNA3A | 92 | 94 | 93 | 1.41 | 1.52 |
| S13 | EBNA3A | 65 | 63 | 64 | 1.41 | 2.21 |
| S15 | PHA-L | 645 | 680 | 663 | 24.75 | 3.74 |
| S21 | PHA-L | 668 | 753 | 711 | 60.10 | 8.46 |
| S25 | PHA-L | 234 | 287 | 261 | 37.48 | 14.39 |
| S26 | PHA-L | 214 | 240 | 227 | 18.38 | 8.10 |
| Mean | 22.36 | 6.48 | ||||
| B | ||||||
| S09 | BZLF1 | 56 | 47 | 52 | 6.36 | 12.36 |
| S11 | BZLF1 | 52 | 86 | 69 | 24.04 | 34.84 |
| S12 | EBNA3A | 33 | 35 | 34 | 1.41 | 4.16 |
| S13 | EBNA3A | 0 | 0 | 0 | – | – |
| S15 | PHA-L | 575 | 657 | 616 | 57.98 | 9.41 |
| S21 | PHA-L | 698 | 691 | 695 | 4.95 | 0.71 |
| S25 | PHA-L | 411 | 403 | 407 | 5.66 | 1.39 |
| S26 | PHA-L | 547 | 617 | 582 | 49.50 | 8.50 |
| Mean | 21.42 | 10.20 | ||||
| C | ||||||
| S09 | BZLF1 | 52 | 49 | 51 | 2.12 | 4.20 |
| S11 | BZLF1 | 61 | 85 | 73 | 16.97 | 23.25 |
| S12 | EBNA3A | 19 | 28 | 24 | 6.36 | 27.08 |
| S13 | EBNA3A | 0 | 0 | 0 | – | – |
| S15 | PHA-L | 329 | 349 | 339 | 14.14 | 4.17 |
| S21 | PHA-L | 453 | 459 | 456 | 4.24 | 0.93 |
| S25 | PHA-L | 86 | 105 | 96 | 13.44 | 14.07 |
| S26 | PHA-L | 102 | 130 | 116 | 19.80 | 17.07 |
| Mean | 11.01 | 12.97 | ||||
Values represent the mean number of antigen-specific IFNγ (A), IL2 (B) and IFNγ + IL2 (C) SFC/2 × 105 PBMC (stimulated with 1 µg/ml BZLF1 or EBNA3A peptide pools) or mitogen-specific IFNγ (A), IL2 (B) and IFNγ + IL2 (C) SFC/2 × 105 PBMC/5 × 104 PBMC (stimulated with 2 µg/ml PHA-L) detected in two different assay plates performed on the same day in parallel
SD standard deviation, CV coefficient of variation
We assessed inter-assay variability also in assays performed on three consecutive days (i.e. reproducibility) using PBMC of ten donors. The mean CV was 20.81, 12.75 and 12.07 % for IFNγ, IL2 and IFNγ + IL2 responses, respectively (Table 3A–C). The inter-assay testing revealed that inter-day variability was only slightly higher than the inter-plate variability for IL2 and IFNγ + IL2 responses. The CV for IFNγ responses, however, was clearly higher when we performed the assay at different days, but still acceptable.
Table 3.
Inter-day variability of IFNγ, IL2, IFNγ + IL2 responses in the IFNγ/IL2 FluoroSpot assay
| Donor | Antigen | Day 1 | Day 2 | Day 3 | Mean | SD | %CV |
|---|---|---|---|---|---|---|---|
| A | |||||||
| S09 | BZLF1 | 191 | 145 | 149 | 162 | 25.48 | 15.76 |
| S11 | BZLF1 | 326 | 344 | 351 | 340 | 12.90 | 3.79 |
| S14 | BZLF1 | 223 | 161 | 175 | 186 | 32.52 | 17.45 |
| S18 | BZLF1 | 257 | 194 | 189 | 213 | 37.90 | 17.77 |
| S10 | EBNA3A | 27 | 27 | 19 | 24 | 4.62 | 18.98 |
| S12 | EBNA3A | 92 | 44 | 35 | 57 | 30.64 | 53.76 |
| S19 | EBNA3A | 144 | 127 | 67 | 113 | 40.45 | 35.90 |
| S15 | PHA-L | 304 | 358 | 311 | 324 | 29.37 | 9.05 |
| S25 | PHA-L | 77 | 88 | 60 | 75 | 14.11 | 18.81 |
| S26 | PHA-L | 214 | 242 | 297 | 251 | 42.23 | 16.82 |
| Mean | 27.02 | 20.81 | |||||
| B | |||||||
| S09 | BZLF1 | 69 | 62 | 67 | 66 | 3.61 | 5.46 |
| S11 | BZLF1 | 18 | 20 | 17 | 18 | 1.53 | 8.33 |
| S14 | BZLF1 | 48 | 57 | 62 | 56 | 7.09 | 12.74 |
| S18 | BZLF1 | 27 | 29 | 48 | 35 | 11.59 | 33.43 |
| S10 | EBNA3A | 17 | 18 | 15 | 17 | 1.53 | 9.17 |
| S12 | EBNA3A | 33 | 34 | 29 | 32 | 2.65 | 8.27 |
| S19 | EBNA3A | 17 | 12 | 20 | 16 | 4.04 | 24.74 |
| S15 | PHA-L | 492 | 615 | 572 | 560 | 62.42 | 11.15 |
| S25 | PHA-L | 344 | 359 | 348 | 350 | 7.77 | 2.22 |
| S26 | PHA-L | 521 | 617 | 662 | 600 | 72.02 | 12.00 |
| Mean | 17.42 | 12.75 | |||||
| C | |||||||
| S09 | BZLF1 | 69 | 56 | 63 | 63 | 6.51 | 10.38 |
| S11 | BZLF1 | 20 | 19 | 18 | 19 | 1.00 | 5.26 |
| S14 | BZLF1 | 54 | 60 | 54 | 56 | 3.46 | 6.19 |
| S18 | BZLF1 | 32 | 32 | 42 | 35 | 5.77 | 16.34 |
| S10 | EBNA3A | 14 | 13 | 13 | 13 | 0.58 | 4.33 |
| S12 | EBNA3A | 19 | 26 | 20 | 22 | 3.79 | 17.47 |
| S19 | EBNA3A | 12 | 11 | 14 | 12 | 1.53 | 12.39 |
| S15 | PHA-L | 215 | 276 | 258 | 250 | 31.34 | 12.55 |
| S25 | PHA-L | 50 | 59 | 47 | 52 | 6.24 | 12.01 |
| S26 | PHA-L | 95 | 130 | 155 | 127 | 30.14 | 23.79 |
| Mean | 9.04 | 12.07 | |||||
Values represent the mean number of antigen-specific IFNγ (A), IL2 (B) and IFNγ + IL2 (C) SFC/2 × 105 PBMC (stimulated with 1 µg/ml BZLF1 or EBNA3A peptide pools) or mitogen-specific IFNγ (A), IL2 (B) and IFNγ + IL2 (C) SFC/2 × 105 PBMC/5 × 104 PBMC (stimulated with 2 µg/ml PHA-L) detected on three different assay plates performed on three consecutive days
SD standard deviation, CV coefficient of variation
Concerning the obtained CVs for low counts of cytokine secreting cells one should keep in mind, that for mathematical reasons, high CV values tend to be determined when spot numbers are of low frequency.
Next, we analyzed the sensitivity of the assay and determined its quantitative range and linearity.
Limit of detection
The limit of detection (LOD) of the ELISpot/FluoroSpot assay is defined as the lowest number of spots that is precisely distinguishable from an unstimulated control well (O) with LOD = O + 2SD of O [11, 28]. For the IFNγ/IL2 FluoroSpot assay we determined mean background levels of 2 SFC/well (range 0–4, SD 1.37), 5 SFC/well (range 1–10, SD 2.53) and 1 SFC/well (range 0–2, SD 0.54) for IFNγ, IL2, and IFNγ + IL-2 responses, respectively (Additional file 1: Table S1). Based on these results and in line with the determined LLOQ (lower limit of quantification) we set a consistent LOD for the IFNγ/IL2 FluoroSpot assay at 10 SFC/2x105 PBMC.
Lower limit of quantification
To specify the lower limit of quantification (LLOQ), which is defined as the lowest value that can be quantitatively determined with acceptable precision and accuracy [13], of the IFNγ/IL2 FluoroSpot assay we analyzed the intra-assay CV of antigen-specific IFNγ, IL2 and IFNγ + IL2 responses of donors with numbers of SFC ranging between 1–10 SFC/well. We calculated an intra-assay CV of 46.28, 43.48 and 69.74 % for IFNγ, IL2 and IFNγ + IL2 responses, respectively (Additional file 2: Table S2a–c). These results indicate that SFC counts below 11 SFC/well are of low precision, but it should be taken into account that the high variability in low counts expressed as CV, could be a result of the mathematical equation of the CV, which presents the ratio of the standard deviation to the mean. In contrast, SFC counts of “low responders” ranging between 11–50 SFC/well revealed an acceptable intra-assay CV (<25 %) of 10.70, 9.29 and 16.13 % for IFNγ, IL2, and IFNγ + IL2 responses, respectively (Additional file 3: Table S3a–c). In line with these observations, we set the LLOQ for the IFNγ/IL2 FluoroSpot assay to 11 SFC/well.
Upper limit of quantification
The automated reader system we used is able to count SFC numbers up to approximately 1000 SFC per well. Intra-assay variability of those high SFC numbers is still acceptable (%CV < 25; data not shown), the setting of counting parameters, however, is difficult, because fusion of several adjacent single spots results in spot aggregates. We used cryopreserved PBMC of six donors to determine the upper limit of quantification (ULOQ), which is defined as the highest value that can be quantitatively determined with acceptable precision and accuracy [11]. All samples were stimulated with PHA-L and assayed in six replicates. Based on the morphology and the ability to count on clearly separated single spots we defined the ULOQ of an IFNγ/IL2 FluoroSpot assay as 700 SFC/well (data not shown). Magnitude of EBV-specific responses was always below this ULOQ.
Linearity
To determine linearity of the IFNγ/IL2 FluoroSpot assay we tested PBMC of two different donors in six replicates using cell numbers of 1.25 × 104, 2.5 × 104, 5 × 104 and 1 × 105/well. PBMC were stimulated with the mitogen PHA-L, to ensure adequate numbers of IL2-secreting cells. Stimulating PBMC with peptide pools of EBV-derived proteins BZLF1 and EBNA3A did not reveal high enough frequencies of IL2-secreting cells to assess linearity of the IFNγ/IL2 FluoroSpot assay.
For both donors we observed a linear relationship for IFNγ (R2 = 0.99, respectively), IL2 (R2 = 0.98 and 0.93, respectively), and IFNγ + IL2 responses (R2 = 0.99, respectively) (Fig. 2). These results suggest that the analysis of less cells per well is feasible, when clinical material is limited. The authors recommend, however, re-assessing linearity using the IFNγ/IL2 FluoroSpot assay for clinical trial monitoring. A lower cell concentration can only be recommended if an appropriate cell-to-cell contact (e.g. in a smaller well format (384 well plate)) and an effective way of antigen presentation is ensured, and a true linear correlation between the plated cell number and the spot number exists.
Fig. 2.
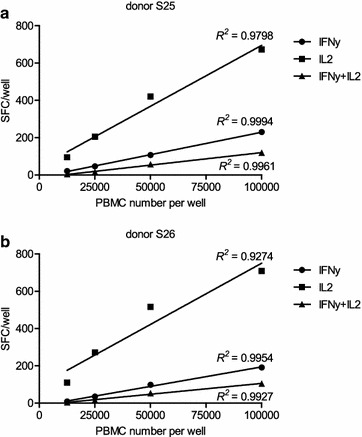
Linearity of the IFNγ/IL2 FluoroSpot assay. Magnitude of IFNγ, IL2, and IFNγ + IL2 responses of donor S25 (a) and S26 (b) within the IFNγ/IL2 FluoroSpot assay as a function of cell density. Depicted are the number of mitogen-specific IFNγ, IL2 and IFNγ + IL2 SFC/well after stimulation with 2 µg/ml PHA-L; SFC spot forming cells. R 2 = coefficient of determination
Diagnostic specificity and sensitivity
Among donors with confirmed positive EBV-serostatus the EBV-specific IFNγ/IL2 FluoroSpot was positive in all 16/16 donors (diagnostic sensitivity: 100 %) with mean frequencies of BZLF1- and EBNA3A-specific T cells ranging from 11–411 SFC/2 × 105 PBMC (median 90 SFC/2 × 105 PBMC) and 12–167 SFC/2 × 105 PBMC (median 54 SFC/2 × 105 PBMC), respectively (Additional file 4: Table S4).
An EBV-specific IFNγ/IL2 FluoroSpot assay with PBMC of the control group (n = 6 EBV-seronegative donors) was negative (Additional file 4: Table S4). This result proved a specificity of the EBV-specific IFNγ/IL2 FluoroSpot assay of 100 %, thus allowing the analysis of EBV-specific immune responses in a clinical trial setting.
Precision across assays: FluoroSpot vs. ELISpot assay
Finally we tested the concordance between the two assay systems by determining numbers of IFNγ- and IL2-secreting cells in the FluoroSpot- and the corresponding single cytokine ELISpot assay. We used cryopreserved PBMC of ten and eight donors to examine number of IFNγ and IL2 secreting cells, respectively. PBMC stimulated with BZLF1 or EBNA3A were assayed in triplicates.
Calculating the concordance of the two assay systems we obtained a mean difference d between the two assay systems of d = 1.95 SFC/2 × 105 PBMC (95 % limits of agreement: −7.80 to 11.70) for IFNγ responses and d = −0.13 SFC/2 × 105 PBMC (95 % limits of agreement: −9.10 to 8.85) for IL2 responses with a concordance correlation coefficient pc = 0.99 and pc = 0.93, respectively (Fig. 3), indicating that both assay methods give congruent results.
Fig. 3.
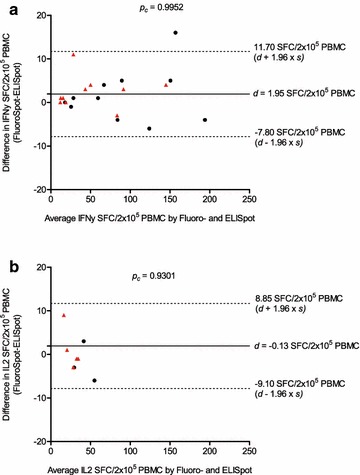
Precision across assays: FluoroSpot vs. ELISpot assay. Concordance between numbers of antigen-specific (BZLF1 and EBNA3A peptide pools) IFNγ- (a) and IL2 (b) SFC/2x105 PBMC detected within the IFNγ/IL2 FluoroSpot assay and an enzymatic IFNγ and IL2 ELISpot assay. Plotted is the difference in IFNγ (a) and IL2 (b) SFC/2x105 PBMC detected after ex vivo restimulation with BZLF1 (black circle) or EBNA3A (red triangle) peptide pools within the FluoroSpot- or ELISpot assay plotted against the average of IFNγ or IL2 SFC detected in either of the two assays. Concordance between FluoroSpot and ELISpot results was assessed using the concordance correlation coefficient p c by Lin. Descriptive statistics are the average difference d (horizontal solid line) and the limits of agreement (d ± 1.96 × s) (dashed line) of the detected T cell responses of both assay systems. d bias of measurements; s standard deviation; p c concordance correlation coefficient by Lin
Discussion
Although assay validation is a time consuming process it is a prerequisite for valid clinical trial monitoring using immunoassays (i.e. ELISpot, FluoroSpot or flow-cytometry-based assays). Following the guidelines for assay validation [22], we determined specificity, precision, detection limit, quantification limit and linearity of an IFNγ/IL2 FluoroSpot assay.
ELISpot assays are very well-suited for high-throughput analyses and have become a standard technique to assess T cell responses within a clinical trial setting [29, 30]. The applicability of the assay system in clinical practice has been confirmed with the approval of a diagnostic ELISpot for the detection of latent tuberculosis infection and disease [31]. Assay performance and data analysis is less time-consuming compared to flow cytometry-based assays (e.g. intracellular cytokine staining). In addition, assay performance can readily be standardized, validated according to international guidelines [11], and quality-controlled in internationally conducted proficiency panels [32, 33] (http://www.proficiencypanel.com/).
A major limitation of the ELISpot assay is its restriction to single parameter analyses. Several studies showed that immune monitoring using single cytokine detection is insufficient to provide an overall assessment of the T cell response and that the identification of protective functional immune signatures requires polyfunctional analysis [34]. Multiparametric FluoroSpot, an advancement of the ELISpot assay, allows the simultaneous assessment of multiple parameters in one well. Currently commercially available FluoroSpot assays are restricted to a maximum of three parameters, but technically up to six parameters are possible.
Some studies have demonstrated that ELISpot assays have a higher CV for intra- and inter-assay precision compared with flow cytometry-based assays (e.g. intracellular cytokine staining, ICS) [35]. In our hands, ELISpot and ICS show a relatively high level of concordance with comparable CV values [36], but we did not test whether this applies also to the IFNγ/IL2 FluoroSpot assay. International proficiency panels have shown that ELISpot assays give reproducible results among different laboratories and the inter-laboratory CV was found to be less than 20 % [37, 38]. For the IFNγ/IL2 FluoroSpot assay we showed intra- and inter-assay precision with CV values clearly within this acceptable level.
Linearity is another important aspect of cell-based immunoassay validation [35]. The IFNγ/IL2 FluoroSpot assay showed a high linearity upon mitogen stimulation of PBMC, offering the possibility of using reduced cell numbers per well. But the minimal applicable number of cells per well must be adjusted for each antigen to the expected frequency of responding cells which may be present at or near the assay detection limit. In addition a check of assay linearity with the respective stimulating-antigen is always required, due to the need for optimal cell-to-cell contact (with e.g. antigen-presenting cells) for sufficient stimulation.
Evaluating the limits of detection and quantification is important when establishing the parameters of an acceptable positive immune response [39]. Theoretically the detection limit of an ELISpot/FluoroSpot assay is extremely low, but due to a high variability at lower concentrations, the detection limit may not be accurate. In contrast, the lowest limit of quantification (LLOQ) is the lowest concentration that can be defined with highest accuracy and precision. It is often postulated that the detection limit of the ELISpot//FluoroSpot assay can be as low as 1/100,000 cells, thus at least ten times lower than ICS [40]. Our results show, however, that CV values are unacceptably high when dealing with these low frequencies of antigen-specific cells. Nonetheless, one should be aware that high variability in low counts expressed by the CV, could be a result of the mathematical equation of the CV, which presents the standard deviation to the mean. For the IFNγ/IL2 FluoroSpot assay we determined a detection limit of 0.005 % which is similar to the LLOQ we determined in previous validation studies for the ELISpot and ICS and what has been reported by others [24].
To address the diagnostic specificity and diagnostic sensitivity of the IFNγ/IL2 FluoroSpot assay for use in an envisaged clinical trial setting we determined these parameters in an existing cohort of individuals with confirmed positive or negative EBV-serostatus. Both specificity and sensitivity was very high, proving the assay very suitable for monitoring in a clinical trial setting as it fulfills the acceptance criteria for biomarker [11] assays.
Finally, we also compared precision across two assay systems, the IFNγ/IL2 FluoroSpot assay and the corresponding enzymatic single cytokine ELISpot assay. We obtained very high concordance between the results of the two assays with an equivalent sensitivity (concordance correlation coefficient pc = 0.99 and pc = 0.93 for IFNγ and IL2 responses, respectively) as already reported by others [41]. This allows for comparability of already existing ELISpot-based data (e.g. from previous trial monitoring) with data obtained with the more advanced IFNγ/IL2 FluoroSpot assay.
In summary, the IFNγ/IL2 FluoroSpot assay showed high precision in combination with very high sensitivity and specificity. The broad linear range allows for more flexible specimen volume and permits analysis of fewer cells per assay when clinical material is limited. The IFNγ/IL2 FluoroSpot assay passed all validation checks and is suitable for the detection of EBV-specific immune responses in a clinical trial setting.
Conclusions
Investigating antigen specific immune responses as a primary endpoint in clinical trials requires highly sensitive and validated assays to determine immune cell reactivity ex vivo correlating with clinical outcome. The FluoroSpot assay enables simultaneous analysis of single cells secreting multiple cytokines thus overcoming an important current limitation of single color enzymatic ELISpot assays. The FluoroSpot assay allows monitoring of polyfunctional T cells, which have been suggested to be correlates of protection in various infectious diseases. Our data show that the validated primary endpoint assay, an IFNγ/IL2 FluoroSpot assay is suitable for the detection of EBV-specific immune responses in a clinical trial setting, subject to the requirement of standardized assay procedure and data analysis.
Authors’ contributions
NK, TB and UP conceived and designed the experiments. UB provided blood samples. NK performed the experiments and acquired the data. NK and TB analysed the data. NK, TB, UB and UP interpreted the data. AH did the evaluation of the statistical analysis. NK and TB drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Kim Dietrich and Mirjana Lekic (Institute of Virology, Technische Universität München/Helmholtz Zentrum München, Munich, Germany) provided technical help during the experimental work. We are grateful to all volunteer blood donors.
Competing interests
The authors declare that they have no competing interests.
Compliance with ethical guidelines
Studies on material of human origin were approved by the ethics committee of the Technische Universität München and informed consent was obtained from all donors.
Abbreviations
- CV
coefficient of variation
- EBV
Epstein–Barr virus
- ELISpot
enzyme-linked immuno spot
- FCS
fetal calf serum
- FITC
fluorescein isothiocyanate
- ICS
intracellular cytokine staining
- IFN
interferon
- IL
interleukin
- LOD
limit of detection
- LLOQ
lower limit of quantification
- MIATA
minimum information about T-cell assays
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate buffered saline
- PE
phycoerythrin
- PHA-L
phytohemagglutinin
- RPMI
Roswell Park Memorial Institute
- SD
standard deviation
- SFC
spot forming cells
- SOP
standard operating procedure
- ULOQ
upper limit of quantification
Additional files
10.1186/s12967-016-0932-7 Magnitude of background activity measured in unstimulated control wells.
10.1186/s12967-016-0932-7 Intra-assay variability of antigen-specific IFNγ, IL2, and IFNγ + IL2 responses ranging between 1-10 SFC/well.
10.1186/s12967-016-0932-7 Intra-assay variability of antigen-specific IFNγ, IL2, and IFNγ + IL2 responses ranging between 11-50 SFC/well.
10.1186/s12967-016-0932-7 Diagnostic specificity and sensitivity of the EBV-specific IFNγ/IL2 FluoroSpot.
Contributor Information
Nina Körber, Email: nina.koerber@tum.de.
Uta Behrends, Email: uta.behrends@mri.tum.de.
Alexander Hapfelmeier, Email: alexander.hapfelmeier@tum.de.
Ulrike Protzer, Email: protzer@tum.de.
Tanja Bauer, Phone: 089/4140-7360, Email: tanja.bauer@helmholtz-muenchen.de.
References
- 1.Cox JH, Ferrari G, Janetzki S. Measurement of cytokine release at the single cell level using the ELISPOT assay. Methods. 2006;38(4):274–282. doi: 10.1016/j.ymeth.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Slota M, et al. ELISpot for measuring human immune responses to vaccines. Expert Rev Vaccines. 2011;10(3):299–306. doi: 10.1586/erv.10.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lehmann PV, Zhang W. Unique strengths of ELISPOT for T cell diagnostics. Methods Mol Biol. 2012;792:3–23. doi: 10.1007/978-1-61779-325-7_1. [DOI] [PubMed] [Google Scholar]
- 4.Janetzki S, Rueger M, Dillenbeck T. Stepping up ELISpot: multi-level analysis in FluoroSpot Assays. Cells. 2014;3(4):1102–1115. doi: 10.3390/cells3041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gazagne A, et al. A Fluorospot assay to detect single T lymphocytes simultaneously producing multiple cytokines. J Immunol Methods. 2003;283(1–2):91–98. doi: 10.1016/j.jim.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Caccamo N, et al. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40(8):2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 7.Duvall MG, et al. Polyfunctional T cell responses are a hallmark of HIV-2 infection. Eur J Immunol. 2008;38(2):350–363. doi: 10.1002/eji.200737768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts MR, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoos A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez AM, et al. The external quality assurance oversight laboratory (EQAPOL) proficiency program for IFN-gamma enzyme-linked immunospot (IFN-gamma ELISpot) assay. J Immunol Methods. 2014;409:31–43. doi: 10.1016/j.jim.2014.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FDA. Guidance for industry, bioanalytical method validation. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm368107.pdf. Accessed 14 Jul 2014.
- 12.Janetzki S, Britten CM. The impact of harmonization on ELISPOT assay performance. Methods Mol Biol. 2012;792:25–36. doi: 10.1007/978-1-61779-325-7_2. [DOI] [PubMed] [Google Scholar]
- 13.Wood B, et al. Validation of cell-based fluorescence assays: practice guidelines from the ICSH and ICCS—part V—assay performance criteria. Cytometry B Clin Cytom. 2013;84(5):315–323. doi: 10.1002/cyto.b.21108. [DOI] [PubMed] [Google Scholar]
- 14.O’Hara DM, et al. Recommendations for the validation of flow cytometric testing during drug development: II assays. J Immunol Methods. 2011;363(2):120–134. doi: 10.1016/j.jim.2010.09.036. [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, et al. The simultaneous ex vivo detection of low-frequency antigen-specific CD4+ and CD8+ T-cell responses using overlapping peptide pools. Cancer Immunol Immunother. 2012;61(11):1953–1963. doi: 10.1007/s00262-012-1251-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britten CM, et al. T cell assays and MIATA: the essential minimum for maximum impact. Immunity. 2012;37(1):1–2. doi: 10.1016/j.immuni.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Britten CM, et al. Minimal information about T cell assays: the process of reaching the community of T cell immunologists in cancer and beyond. Cancer Immunol Immunother. 2011;60(1):15–22. doi: 10.1007/s00262-010-0940-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janetzki S, et al. “MIATA”-minimal information about T cell assays. Immunity. 2009;31(4):527–528. doi: 10.1016/j.immuni.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Burg SH, et al. Harmonization of immune biomarker assays for clinical studies. Sci Transl Med. 2011;3:108ps44. doi: 10.1126/scitranslmed.3002785. [DOI] [PubMed] [Google Scholar]
- 20.Ashoor I, et al. Standardization and cross validation of alloreactive IFNγ ELISPOT assays within the clinical trials in organ transplantation consortium. Am J Transplant. 2013;13(7):1871–1879. doi: 10.1111/ajt.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bestard O, et al. Cross-validation of IFN-γ Elispot assay for measuring alloreactive memory/effector T cell responses in renal transplant recipients. Am J Transplant. 2013;13(7):1880–1890. doi: 10.1111/ajt.12285. [DOI] [PubMed] [Google Scholar]
- 22.Butterfield LH, et al. Recommendations from the iSBTc-SITC/FDA/NCI workshop on immunotherapy biomarkers. Clin Cancer Res. 2011;17(10):3064–3076. doi: 10.1158/1078-0432.CCR-10-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janetzki S, et al. Guidelines for the automated evaluation of Elispot assays. Nat Protoc. 2015;10(7):1098–1115. doi: 10.1038/nprot.2015.068. [DOI] [PubMed] [Google Scholar]
- 24.Moodie Z, et al. Response definition criteria for ELISPOT assays revisited. Cancer Immunol Immunother. 2010;59(10):1489–1501. doi: 10.1007/s00262-010-0875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45(1):255–268. doi: 10.2307/2532051. [DOI] [PubMed] [Google Scholar]
- 26.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 27.Hornik K. “The R FAQ”; The R project for statistical computing. https://www.r-project.org/. Accessed 18 May 2015.
- 28.Tary-Lehmann M, Hamm CD, Lehmann PV. Validating reference samples for comparison in a regulated ELISPOT assay, in validation of cell-based assays in the GLP setting: a practical guide. In: Prabhakar U, Kelley M, editors. London: Wiley; 2008. p. 127–146.
- 29.Diez-Domingo J, et al. Comparison of intramuscular and subcutaneous administration of a herpes zoster live-attenuated vaccine in adults aged >/=50 years: a randomised non-inferiority clinical trial. Vaccine. 2015;33(6):789–795. doi: 10.1016/j.vaccine.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 30.Walker KM, et al. Antibody and T-cell responses associated with experimental human malaria infection or vaccination show limited relationships. Immunology. 2015;145(1):71–81. doi: 10.1111/imm.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meier T, et al. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis. 2005;24(8):529–536. doi: 10.1007/s10096-005-1377-8. [DOI] [PubMed] [Google Scholar]
- 32.IMMUDEX. http://www.immudex.com/proficiency-panels.aspx. Accessed 10 Jul 2015.
- 33.Janetzki S, et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI) Cancer Immunol Immunother. 2008;57(3):303–315. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harari A, et al. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 35.Maecker HT, et al. Precision and linearity targets for validation of an IFNgamma ELISPOT, cytokine flow cytometry, and tetramer assay using CMV peptides. BMC Immunol. 2008;9:9. doi: 10.1186/1471-2172-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kutscher S, et al. The intracellular detection of MIP-1beta enhances the capacity to detect IFN-gamma mediated HIV-1-specific CD8 T-cell responses in a flow cytometric setting providing a sensitive alternative to the ELISPOT. AIDS Res Ther. 2008;5:22. doi: 10.1186/1742-6405-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boaz MJ, et al. Concordant proficiency in measurement of T-cell immunity in human immunodeficiency virus vaccine clinical trials by peripheral blood mononuclear cell and enzyme-linked immunospot assays in laboratories from three continents. Clin Vaccine Immunol. 2009;16(2):147–155. doi: 10.1128/CVI.00326-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samri A, et al. Evaluation of the interlaboratory concordance in quantification of human immunodeficiency virus-specific T cells with a gamma interferon enzyme-linked immunospot assay. Clin Vaccine Immunol. 2006;13(6):684–697. doi: 10.1128/CVI.00387-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lathey J. Preliminary steps toward validating a clinical bioassay. BioPharm Int. 2003;16(Issue 3):42–50. [Google Scholar]
- 40.Asai T, Storkus WJ, Whiteside TL. Evaluation of the modified ELISPOT assay for gamma interferon production in cancer patients receiving antitumor vaccines. Clin Diagn Lab Immunol. 2000;7(2):145–154. doi: 10.1128/cdli.7.2.145-154.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hallengard D, et al. Comparison of plasmid vaccine immunization schedules using intradermal in vivo electroporation. Clin Vaccine Immunol. 2011;18(9):1577–1581. doi: 10.1128/CVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


