Abstract
Phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) signaling pathway performs a central role in tumorigenesis and is constitutively activated in many malignancies. As a novel dual PI3K/mTOR inhibitor currently undergoing evaluation in a phase I/II clinical trial, NVP-BEZ235 indicates a significant antitumor efficacy in diverse solid tumors, including colorectal cancer (CRC). Autophagy is a catabolic process that maintains cellular homeostasis and reduces diverse stresses through lysosomal recycling of the unnecessary and damaged cell components. This process is also observed to antagonize the antitumor efficacy of PI3K/mTOR inhibitor agents such as NVP-BEZ235, via apoptosis inhibition. In the present study, we investigated anti-proliferative and apoptosis-inducing ability of NVP-BEZ235 in SW480 cells and the crosstalk between autophagy and apoptosis in SW480 cells treated with NVP-BEZ235 in combination with an autophagy inhibitor. The results revealed that, NVP-BEZ235 effectively inhibit the growth of SW480 cells by targeting the PI3K/mTOR signaling pathway and induced apoptosis. The inhibition of autophagy with 3-methyladenine or chloroquine inhibitors in combination with NVP-BEZ235 in SW480 cells enhanced the apoptotic rate as componets to NVP-BEZ235 alone. In conclusion, the findings provide a rationale for chemotherapy targeting the PI3K/mTOR signaling pathway presenting a potential therapeutic strategy to enhance the efficacy of dual PI3K/mTOR inhibitor NVP-BEZ235 in combination with an autophagy inhibitor in CRC treatment and treatment of other tumors.
Keywords: NVP-BEZ235, autophagy, apoptosis, colorectal cancer, phosphatidylinositol 3-kinase/mammalian target of rapamycin
Introduction
Colorectal cancer (CRC) is a common cancer worldwide and is the fourth leading cause of cancer-associated mortality in China (1). There are >1 million CRC incidences and 600,000 mortalities occur anually. Survival is associated with the stage at which the cancer is diagnosed (2). The TNM and Dukes' staging systems have greatly improved the rational stratification of CRC patients and the design of therapeutic strategies (3). Early diagnosis results in a highly favorable prognosis, stages 1 and 2 CRC have an 80–90% 5-year survival rate, whereas stages 3 and 4 metastatic diseases have a 5-year survival rate of 60 and 8%, respectively (4). Almost 20% of patients are diagnosed at an advanced metastatic stage, and >50% ultimately develop metastases (5).
NVP-BEZ235 as a novel dual phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitor currently in phase I/II clinical trials has demonstrated significant antitumor efficacy in diverse solid tumors, including CRC (6). NVP-BEZ235 targets PI3K/mTOR pathways by binding to the ATP-binding pocket and has a dual role in inhibiting multiple class I PI3K isoforms and mTOR kinase activity (7). The PI3K/mTOR signaling pathway is involved in various types of cancer, including CRC, and this pathway regulates tumorigenesis in many important aspects, such as cell proliferation, angiogenesis, invasion and cell motility (8–10). PI3Ks is a family of lipid kinase and each member is composed of two heterologous subunits. Active PI3Ks can convert phosphatidylinositol 4, 5-bisphosphate to phosphatidylinositol 3, 4, 5-triphosphate (PIP3) by phosphorylating at the 3-position of the inositol ring. PIP3 provides binding sites for pleckstrin homology-containing proteins such as 3-phosphoinositide-dependent protein kinase-1 (PDK1) and AKT serine/threonine kinase (11). AKT activated by PDK1 is able to phosphorylate many protein targets at the membrane such as caspase-9, tuberin, murine double minute 2, and mTOR. mTOR is a serine/threonine kinase that regulates cell proliferation and apoptosis by binding other proteins to form the mTOR complex 1 (mTORC1) or mTORC2 (12). AKT activation involves the regulation of cell proliferation, survival, and motility.
Autophagy is an intracellular catabolic process that recycles unnecessary cell components and damaged organelles in order to maintain cellular homeostasis and reduce diverse stresses, commonly through lysosomes (13). Autophagy has a dual role of tumor promoter and tumor inhibitor in the cancer cells. It leads to genetic instability by preventing inflammation and necrosis, otherwise it might provide energy via recycling mechanism that is vital to tumor progression during the unfavorable condition, such as starvation or hypoxia (14–18). Many chemotherapeutic agents, especially drugs effecting through PI3K/mTOR inhibition such as dual PI3K/mTOR inhibitor are observed to induce autophagy, and mTOR is the central checkpoint that negatively regulates autophagy (19,20).
We hypothesized that autophagy inhibition enhance the therapeutic outcome of NVP-BEZ235 in CRC treatment based on previous studies. Thus, we examined the anticancer effect of PI3K/mTOR dual inhibitor NVP-BEZ235 on CRC and assessed whether autophagy inhibitors were able to enhance NVP-BEZ235 efficacy in the therapeutic regimen.
Materials and methods
Materials
The antibodies (p-AKT, p-S6, PARP, LC3 and P62) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). NVP-BEZ235 was purchased from LC Laboratories (Woburn, MA, USA). 3-Methyladenine (3-MA), chloroquine (CQ) and 3-(4,5-dimetrylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich China, Inc. (Shanghai, China).
Cell viability assay
SW480 cells were plated in 96-well plates at a density of 1.0×104 cells/well in 200 µl of complete medium. After NVP-BEZ235 treatment, MTT reagent (10 µl, 5 mg/ml) was added to the wells and incubated for 4 h. MTT treated cells were then dissolved in 150 µl DMSO and the absorbance was recorded at a wavelength of 490 nm using ?. Each treatment was performed in triplicate.
Western blott analysis
SW480 cells were washed with cold phosphate-buffered saline twice following treatment with NVP-BEZ235. Radioimmunoprecipitation assay buffer (200 µl) was added and the cell lysates were agitated and centrifuged for 3,000 × g for 20 min, at 4°C. Protein concentration was detected by bicinchoninic acid protein assay. Proteins (45 µg) were loaded and run through 12% (w/v) sodium dodecyl sulphate-polyacrylamide gel electrophoresis. Proteins were then transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was then blocked with 5% (w/v) skimmed milk for 120 min at room temperature (20°C) and primary antibodies were added and incubated overnight at 4°C. The following day, PVDF membranes were incubated with secondary antibodies for 60 min. Tanon Gel Imaging System (Tanon Co., Shanghai, China) was used for the semi-quantitative analysis of proteins.
Flow cytometry
Following the treatment of SW480 cells with NVP-BEZ235, SW480 cells were incubated at 37°C in the dark for 45 min with Annexin V-fluorescein isothiocyanate (Invitrogen-Life Technologies, Carlsbad, CA, USA) and propidium iodide (Invitrogen Life Technologies) to detect the apoptotic rate. The cells were analyzed using a FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Data were presented as mean ± standard deviation. Data were representative of three independent experiments performed in triplicate. P<0.05 was considered to indicate a statistically significant difference.
Results
NVP-BEZ235 inhibits the growth of SW480 cells and PI3K/AKT/mTOR signaling pathway
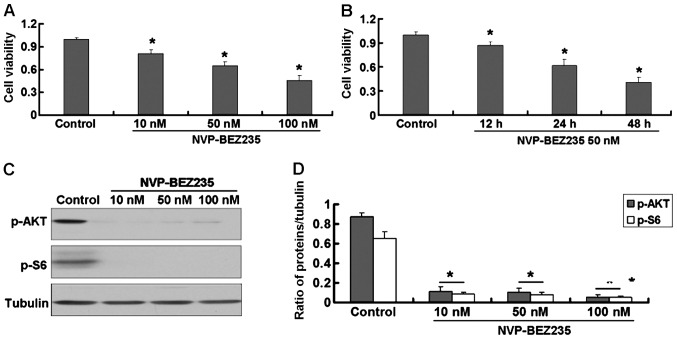
The NVP-BEZ235 treatment of SW480 cells reduced cell viability in a dose- and time-dependent manner (Fig. 1A and B). NVP-BEZ235 also caused a decrease in the expression of p-AKT and p-S6 proteins in SW480 cells (Fig. 1C and D) and this decrease was dose-dependent.
Figure 1.
(A) SW480 cells treated with NVP-BEZ235 (10, 50 and 100 nM) for 24 h. (B) SW480 cells treated with NVP-BEZ235 for 12, 24 and 48 h. MTT assay was used to detect cell viability. (C) Expression of p-AKT and p-S6 proteins in SW480 cells treated with NVP-BEZ235 (10, 50 and 100 nM) for 24 h. (D) Quantitative analysis of p-AKT and p-S6 proteins, n=3. *P<0.05 vs. control.
NVP-BEZ235 induces apoptosis in SW480 cells
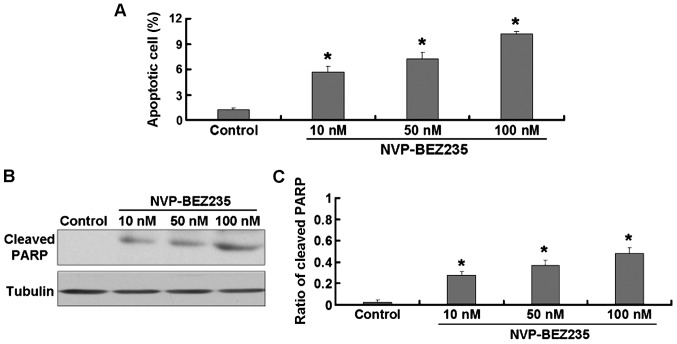
The flow cytometric analysis revealed that, the NVP-BEZ235 treatment increased the rate of apoptosis in SW480 cells (Fig. 2A). To further confirm this result, we detected the expression of cleaved PARP protein which is widely considered as an apoptosis indicator. The NVP-BEZ235 treatment significantly increased the expression of cleaved PARP protein in SW480 cells (Fig. 2B and C).
Figure 2.
SW480 cells treated with NVP-BEZ235 (10, 50 and 100 nM) for 24 h. (A) Percent age of apoptotic cells. (B) Expression of cleaved PARP. (C) Quantitative analysis of cleaved PARP. Data are presented as mean ± standard deviation, n=3. *P<0.05 vs. control group.
Autophagy occurs in SW480 cells treated with NVP-BEZ235
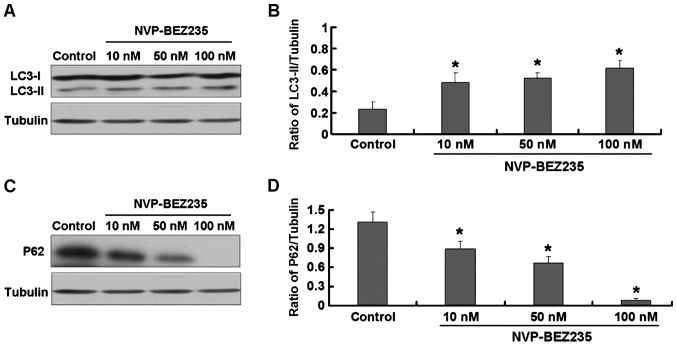
The treatment of SW480 cells with NVP-BEZ235 affected the expression of light chain 3 (LC3) (LC3-I and LC3-II) and p62 proteins involved in the process of cellular autophagy. As shown in Fig. 3A and B, NVP-BEZ235 treatment increased the expression of LC3-II, whereas it decreased the expression of p62 (Fig. 3C and D).
Figure 3.
SW480 cells treated with NVP-BEZ235 (10, 50 and 100 nM) for 24 h. (A) Expression of light chain 3 (LC3)-I and LC3-II. (B) Quantitative analysis of LC3-II. (C) Expression of p62. (D) Quantitative analysis of p62. Data are presented as mean ± standard deviation, n=3, *P<0.05 vs. control group.
Autophagy synergistically increases apoptosis induced by NVP-BEZ235
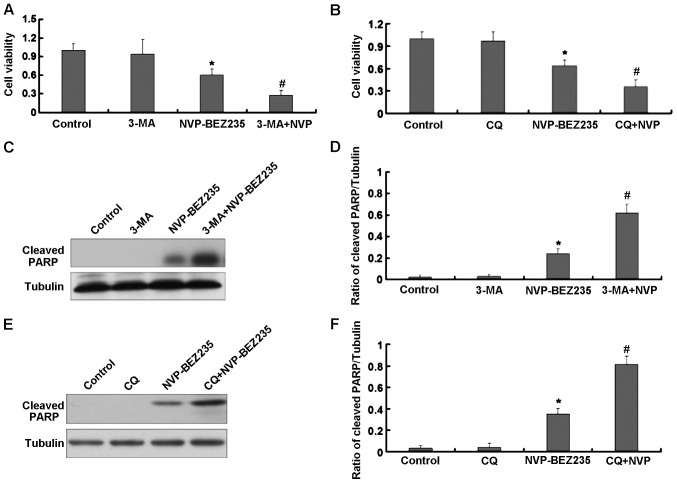
Autophagy can lead to a pro-survival or pro-death effect through apoptosis that is induced by the antitumor agent depending on different circumstances. In the present study, the autophagy inhibitors 3-MA and CQ were used to examine the role of autophagy in NVP-BEZ235-induced apoptosis in SW480 cells. The combination NVP-BEZ235+3-MA showed a higher growth inhibition in SW480 cells than NVP-BEZ235 alone (Fig. 4A). A similar result was observed in SW480 treated with the combination NVP-BEZ235+CQ (Fig. 4B). We also assessed the level of expression of cleaved PARP protein in SW480 cells treated with the combination NVP-BEZ235+3-MA and NVP-BEZ235+CQ. As shown in Fig. 4C and D, NVP-BEZ235 in combination with 3-MA enhanced the expression of cleaved PARP as combined to NVP-BEZ235 alone in SW480 cells. Similarly, the expression of cleaved PARP protein was higher in SW480 cells treated with the combination of NVP-BEZ235 and CQ as combined to NVP-BEZ235 alone (Fig. 4E and F).
Figure 4.
SW480 cells treated with NVP-BEZ235 in combination with autophagy inhibitors. (A) SW480 cells treated with NVP-BEZ235 (50 nM)+3-methyladenine (3-MA) (5 mM) for 24 h. (B) SW480 cells treated with NVP-BEZ235 (50 nM)+chloroquine (CQ) (10 mM) for 24 h. (C) Expression of cleaved PARP in SW480 cells treated with NVP-BEZ235 (50 nM)+3-MA (5 mM) for 24 h. (D) Quantitative analysis of cleaved PARP. (E) Expression of cleaved PARP in SW480 cells treated with NVP-BEZ235 (50 nM)+CQ (10 mM) for 24 h. (F) Quantitative analysis of cleaved PARP. Data are presented as mean ± standard deviation, n=3, *P<0.05 vs. control group, #P<0.05 vs. NVP-BEZ235.
Discussion
CRC is a heterogeneous disease and its development commonly spans approximately 10–15 years as a result of the accumulation of diverse genetic and epigenetic alterations. Early detected CRC is potentially curable via surgical resection followed with adjuvant chemotherapy or radiotherapy. However, the treatment is of limited efficacy for advanced CRC, which increases the mortality to a high level (21,22).
The PI3K/mTOR signaling pathway is involved in tumorigenesis in many important aspects, such as cell proliferation, angiogenesis, invasion, as well as cell survival and motility (10). Previous studies identified PI3K/mTOR signaling downregulation in various types of cancer, including breast cancer (23), hepatocellular cancer (24), lung cancer (25), pancreatic adenocarcinoma (26), and CRC (27). Thus, targeting the PI3K/mTOR pathway has become a promising and intense research field over the last 10 years aiming to develop effective novel anticancer agents. The PI3K/mTOR inhibitor has experienced two generations of the drugs LY294002, wortmannin and rapamycin, and its derivatives. These drugs belong to the first-generation inhibitor with low efficacy and has severe side effects (28–30). NVP-BEZ235 is one of the second generation inhibitors with improved pharmacological properties currently under evaluation in a phase I/II clinical trial (6,31,32). In the present study, we firstly examined the impact of NVP-BEZ235 on SW480 CRC cells and our results supported those of previous studies, in which NVP-BEZ235 effectively inhibited the survival of CRC cells in a time- and dose-dependent manner.
As mentioned earlier, AKT is the key node of the PI3K/mTOR signaling pathway and its activation by PDK1 is able to phosphorylate many protein targets, including mTOR, at the membrane thereby regulating cell proliferation, apoptosis, survival, and motility (8–12). AKT phosphorylation involves the activity of PI3K and AKT kinases, whereas S6K phosphorylation directly reflects mTOR kinase activity (33). Thus, we further investigated the abundance of phosphorylated AKT and S6K in SW480 cells after NVP-BEZ235 treatment. The results were in accordance with studies of other tumors in which NVP-BEZ235 inhibited the PI3K/mTOR signaling pathway (6,31,34–36).
Our study also showed that, NVP-BEZ235 induced apoptosis and autophagy in a dose-dependent manner in SW480 CRC cells. Autophagy is known as the basic catabolic mechanism that degrades cellular components, which are unnecessary or dysfunctional, through the lysosomal pathway (13). In recent studies, autophagy is well demonstrated as a tumor promoter via a recycling mechanism that is vital to tumor progression during chemotherapy with antitumor agents (14–18). Previous findings have revealed that, the PI3K/mTOR pathway inhibition induced autophagy as a mechanism of cell death or drug resistance (37,38). Autophagy is reported to have a crosstalk with apoptosis that under certain circumstances may suppress apoptosis serving as a cell survival pathway (39). On the other hand, autophagy blockage may enhance the pro-apoptotic effects of PI3K/mTOR inhibitors in preclinical studies (26,40) and it is evident that our results support the latter.
Taking the above findings into account, it was found that inhibiting autophagy enhanced the efficacy of NVP-BEZ235 in CRC. The synergistic effect of the autophagy inhibitor observed in the present study was also observed in glioma (41) and malignant peripheral nerve sheath tumors in previous studies (42).
Collectively, our findings suggest autophagy inhibition as a potential strategy to enhance the therapeutic efficacy of dual PI3K/mTOR inhibitor in CRC treatment.
References
- 1.Li Q, Wang D, Li J, Chen P. Clinicopathological and prognostic significance of HER-2/neu and VEGF expression in colon carcinomas. BMC Cancer. 2011;11:277. doi: 10.1186/1471-2407-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J. UK Flexible Sigmoidoscopy Trial Investigators: Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: A multicentre randomised controlled trial. Lancet. 2010;375:1624–1633. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 3.Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int J Cancer. 2014;134:2403–2413. doi: 10.1002/ijc.28536. [DOI] [PubMed] [Google Scholar]
- 4.Mathur A, Ware C, Davis L, Gazdar A, Pan BS, Lutterbach B. FGFR2 is amplified in the NCI-H716 colorectal cancer cell line and is required for growth and survival. PLoS One. 2014;9:e98515. doi: 10.1371/journal.pone.0098515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zimonin FS. Reliable protection of chicks against pullorum disease. Veterinariia. 1973;8:69–70. (In Russian) [PubMed] [Google Scholar]
- 6.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 7.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448:439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 10.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 11.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 12.Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: Apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–975. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 15.Denton D, Nicolson S, Kumar S. Cell death by autophagy: Facts and apparent artefacts. Cell Death Differ. 2012;19:87–95. doi: 10.1038/cdd.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scarlatti F, Granata R, Meijer AJ, Codogno P. Does autophagy have a license to kill mammalian cells? Cell Death Differ. 2009;16:12–20. doi: 10.1038/cdd.2008.101. [DOI] [PubMed] [Google Scholar]
- 17.Chen HY, White E. Role of autophagy in cancer prevention. Cancer Prev Res (Phila) 2011;4:973–983. doi: 10.1158/1940-6207.CAPR-10-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: Implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janku F, McConkey DJ, Hong DS, Kurzrock R. Autophagy as a target for anticancer therapy. Nat Rev Clin Oncol. 2011;8:528–539. doi: 10.1038/nrclinonc.2011.71. [DOI] [PubMed] [Google Scholar]
- 20.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–730. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogino S, Goel A. Molecular classification and correlates in colorectal cancer. J Mol Diagn. 2008;10:13–27. doi: 10.2353/jmoldx.2008.070082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol. 2011;8:686–700. doi: 10.1038/nrgastro.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Generali D, Fox SB, Brizzi MP, Allevi G, Bonardi S, Aguggini S, Milani M, Bersiga A, Campo L, Dionisio R, et al. Down-regulation of phosphatidylinositol 3′-kinase/AKT/molecular target of rapamycin metabolic pathway by primary letrozole-based therapy in human breast cancer. Clin Cancer Res. 2008;14:2673–2680. doi: 10.1158/1078-0432.CCR-07-1046. [DOI] [PubMed] [Google Scholar]
- 24.Masuda M, Shimomura M, Kobayashi K, Kojima S, Nakatsura T. Growth inhibition by NVP-BEZ235, a dual PI3K/mTOR inhibitor, in hepatocellular carcinoma cell lines. Oncol Rep. 2011;26:1273–1279. doi: 10.3892/or.2011.1370. [DOI] [PubMed] [Google Scholar]
- 25.Herrera VA, Zeindl-Eberhart E, Jung A, Huber RM, Bergner A. The dual PI3K/mTOR inhibitor BEZ235 is effective in lung cancer cell lines. Anticancer Res. 2011;31:849–854. [PubMed] [Google Scholar]
- 26.Mirzoeva OK, Hann B, Hom YK, Debnath J, Aftab D, Shokat K, Korn WM. Autophagy suppression promotes apoptotic cell death in response to inhibition of the PI3K-mTOR pathway in pancreatic adenocarcinoma. J Mol Med Berl. 2011;89:877–889. doi: 10.1007/s00109-011-0774-y. [DOI] [PubMed] [Google Scholar]
- 27.Potter DS, Kelly P, Denneny O, Juvin V, Stephens LR, Dive C, Morrow CJ. BMX acts downstream of PI3K to promote colorectal cancer cell survival and pathway inhibition sensitizes to the BH3 mimetic ABT-737. Neoplasia. 2014;16:147–157. doi: 10.1593/neo.131376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway - beyond rapalogs. Oncotarget. 2010;1:530–543. doi: 10.18632/oncotarget.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prevo R, Deutsch E, Sampson O, Diplexcito J, Cengel K, Harper J, O'Neill P, McKenna WG, Patel S, Bernhard EJ. Class I PI3 kinase inhibition by the pyridinylfuranopyrimidine inhibitor PI-103 enhances tumor radiosensitivity. Cancer Res. 2008;68:5915–5923. doi: 10.1158/0008-5472.CAN-08-0757. [DOI] [PubMed] [Google Scholar]
- 30.Wan X, Harkavy B, Shen N, Grohar P, Helman LJ. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 31.Fan QW, Knight ZA, Goldenberg DD, Yu W, Mostov KE, Stokoe D, Shokat KM, Weiss WA. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurtz JE, Ray-Coquard I. PI3 kinase inhibitors in the clinic: an update. Anticancer Res. 2012;32:2463–2470. [PubMed] [Google Scholar]
- 33.Yang F, Qian XJ, Qin W, Deng R, Wu XQ, Qin J, Feng GK, Zhu XF. Dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235 has a therapeutic potential and sensitizes cisplatin in nasopharyngeal carcinoma. PLoS One. 2013;8:e59879. doi: 10.1371/journal.pone.0059879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu TJ, Koul D, LaFortune T, Tiao N, Shen RJ, Maira SM, Garcia-Echevrria C, Yung WK. NVP-BEZ235, a novel dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor, elicits multifaceted antitumor activities in human gliomas. Mol Cancer Ther. 2009;8:2204–2210. doi: 10.1158/1535-7163.MCT-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, Wang S, Garcia-Echeverria C, Maira SM. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci USA. 2009;106:22299–22304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMillin DW, Ooi M, Delmore J, Negri J, Hayden P, Mitsiades N, Jakubikova J, Maira SM, Garcia-Echeverria C, Schlossman R, et al. Antimyeloma activity of the orally bioavailable dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Cancer Res. 2009;69:5835–5842. doi: 10.1158/0008-5472.CAN-08-4285. [DOI] [PubMed] [Google Scholar]
- 37.Fujiwara K, Iwado E, Mills GB, Sawaya R, Kondo S, Kondo Y. Akt inhibitor shows anticancer and radiosensitizing effects in malignant glioma cells by inducing autophagy. Int J Oncol. 2007;31:753–760. [PubMed] [Google Scholar]
- 38.Yang S, Xiao X, Meng X, Leslie KK. A mechanism for synergy with combined mTOR and PI3 kinase inhibitors. PLoS One. 2011;6:e26343. doi: 10.1371/journal.pone.0026343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang Y, Xing D, Zhou F, Chen Q. Mitochondrial autophagy protects against heat shock-induced apoptosis through reducing cytosolic cytochrome c release and downstream caspase-3 activation. Biochem Biophys Res Commun. 2010;395:190–195. doi: 10.1016/j.bbrc.2010.03.155. [DOI] [PubMed] [Google Scholar]
- 40.Xu CX, Zhao L, Yue P, Fang G, Tao H, Owonikoko TK, Ramalingam SS, Khuri FR, Sun SY. Augmentation of NVP-BEZ235′s anticancer activity against human lung cancer cells by blockage of autophagy. Cancer Biol Ther. 2011;12:549–555. doi: 10.4161/cbt.12.6.16397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerniglia GJ, Karar J, Tyagi S, Christofidou-Solomidou M, Rengan R, Koumenis C, Maity A. Inhibition of autophagy as a strategy to augment radiosensitization by the dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-BEZ235. Mol Pharmacol. 2012;82:1230–1240. doi: 10.1124/mol.112.080408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghadimi MP, Lopez G, Torres KE, Belousov R, Young ED, Liu J, Brewer KJ, Hoffman A, Lusby K, Lazar AJ, et al. Targeting the PI3K/mTOR axis, alone and in combination with autophagy blockade, for the treatment of malignant peripheral nerve sheath tumors. Mol Cancer Ther. 2012;11:1758–1769. doi: 10.1158/1535-7163.MCT-12-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]