Abstract
Background
Dopamine β-hydroxylase (DBH) plays an indispensable role in catecholamine synthesis by converting dopamine into norepinephrine. Here, we characterized a DBH promoter polymorphism (C-2073T; rs1989787; minor allele frequency ∼16%) that influences not only gene transcription but also enzyme secretion and blood pressure (BP) in vivo.
Methods
Plasma DBH activity was measured spectrophotometrically. DBH genetic effects on BP were tested in subjects with the most extreme BP values in a large primary care population. Functional effects of promoter variants were studied by site-directed mutagenesis in DBH promoter haplotype/luciferase reporter plasmids transfected into chromaffin cells. Sequence motifs were predicted from position weight matrices, and endogenous transcription factor binding was probed by Chromatin ImmunoPrecipitation (ChIP).
Results
The T-allele of common promoter variant C-2073T was contained in a promoter haplotype that associated with plasma DBH activity, a trait also predicted by that variant itself. Promoter haplotypes including C-2073T predicted BP in the population, and the effect was also referable to C-2073T itself. Computationally, C-2073 disrupted a predicted match for transcription factor c-FOS. Site-directed mutagenesis at C-2073T altered not only basal promoter activity, but also transactivation by c-FOS, as well as the chromaffin cell secretory stimuli nicotine or pituitary adenylate cyclase-activating polypeptide (PACAP). Endogenous c-FOS bound to the motif in chromatin.
Conclusion
These results suggest that DBH promoter variant C-2073T is functional in vivo: this promoter variant seems to initiate a cascade of transcriptional and biochemical changes including augmented DBH secretion, eventuating in elevation of basal BP, and hence cardiovascular risk. The observations suggest new strategies for probing the pathophysiology, risk, and treatment of hypertension.
American Journal of Hypertension, advance online publication 2 September 2010; doi:10.1038/ajh.2010.186
Keywords: blood pressure, dopamine β-hydroxylase, hypertension, polymorphism
Excessive sympathoadrenal activity is implicated in the pathogenesis of hypertension, both primary and secondary, in both humans and experimental animals.1,2 Norepinephrine is the principal transmitter in the sympathetic nervous system, maintaining such functions as heart rate and blood pressure (BP). Dopamine β-hydroxylase (DBH) catalyzes the oxidative hydroxylation of dopamine to form norepinephrine.
In the periphery, DBH is preferentially located in the adrenal medulla and synaptic vesicles of postganglionic sympathetic neurons.3 DBH is coreleased by exocytosis together with norepinephrine.4,5 The enzymatic activity of DBH in plasma corresponds to the level of DBH protein,6,7 and family studies indicate that the effects of environmental factors such as stress and medications on DBH activity are small compared to genetic factors.8
Given its necessary role in converting dopamine to norepinephrine, as well as its hereditary determination, the potential role of DBH in hypertension attracts attention. Several lines of evidence suggest that DBH may play a role in the pathogenesis of hypertension. Genetic deficiency of DBH manifests as hypotension in both humans and knockout mice.9,10 Of note for more common varieties of BP elevation, DBH inhibition attenuates development of hypertension in the spontaneously hypertensive rat.11,12
Recently, we systematically identified common genetic variation at the human DBH locus by resequencing the gene in several human populations, and identified promoter variant C-970T that exaggerated the heritable change in BP during environmental stress in twin pairs, and also predicted higher basal BP in the population.13
In this study, we identified a second functional variant in the DBH promoter, C-2073T, which influences not only gene transcription and enzyme secretion but also BP.
Methods
Population and phenotypic characterization
San Diego population BP extremes (white). The white cohort with extreme BPs consists of 601 male and 701 female white subjects. These participants were chosen based on a diastolic BP (DBP) in the upper or lower extreme percentiles of BP distribution from 25,599 males and 27,479 females in a primary care database developed by Kaiser-Permanente of Southern California.14,15 Each subject was self-identified as of white (European) ancestry, with the same classification for the subject's four grandparents. Individuals in the highest DBP 5th percentiles were age-matched to individuals with DBP values in the lowest 5th percentiles. We ascertained 330 males with DBP >96mmHg (the upper 5th percentile of the overall male DBP distribution) and 310 age-matched males with DBP <61mmHg (corresponding to the lowest 5th percentile). Among the females, 271 were ascertained with high DBP (>92mmHg) in the upper 5th percentile of the overall DBP distribution along with 391 age-matched females with low DBP (DBP <59mmHg, representing individuals in the lower 5th percentile of the overall female distribution). Since epidemiologic data on BP may be compromised by the effects of antihypertensive medications,16,17 we accounted for the effect of such drugs in the analysis, by adding +10/+5 systolic BP/DBP mmHg to all treated values.
Molecular genetics
SNPs. Genotypes for extreme subject were scored on amplified DNA by extension based methods: matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (Sequenom), or Pyrosequencing (Uppsala, Sweden). To ensure accurate assignment, genotypes were verified by visual inspection and artifacts were excluded from further analysis. We began by typing within the 5′ block of DBH, wherein we initially identified extended promoter haplotypes spanning seven common single-nucleotide polymorphisms (SNPs) (six in the promoter region, and one in intron-A, that predicted plasma DBH enzymatic activity: GC[−2771/−2770]AT/rs1076151/rs1076152; C-2734T/rs1076150; C-2073T/rs1989787; C-1282T/rs1611114; C-970T/rs1611115; G+457C/rs2797849).13 After defining the promoter block, we “tagged” that block in subsequent studies with four common promoter SNPs: C-2734T/rs1076150; C-2073T/rs1989787; C-1282T/rs1611114; and C-970T/rs1611115.
Biochemical assays. Plasma DBH activity was measured by the spectrophotometric method in heparinized plasma.18 The principle of the method is as follows: the synthetic DBH substrate tyramine is converted by DBH (in the presence of cofactors Cu2+, N-ethylmaleimide, and fumarate) to octopamine, which is then oxidized to parahydroxybenzaldehyde by sodium periodate. The oxidation is terminated by sodium metabisulfite, and parahydroxybenzaldehyde is then quantified by its absorbance at 330nm in the UV.
Function of DBH promoter variant C-2073T
Promoter/luciferase reporter activity assays. Human DBH promoter/reporter plasmids were constructed essentially as described.13,19 We obtained a human BAC genomic clone (RP11-317B10), spanning the entire DBH gene sequence, from CHORI (http://bacpac.chori.org), from which we excised 3,051 base pairs (bp) of DBH promoter (from –3,000 to +51bp, by PCR on the BAC template), containing the common polymorphic promoter sites, for insertion into the upstream/polylinker of the firefly luciferase reporter plasmid pGL3-Basic (Promega, Madison, WI). Synthetic replacements (at polymorphic sites) were made by site-directed mutagenesis (QuikChange; Stratagene) to produce the common haplotypes shown in Tables 1 and 2. All inserts were sequence-verified before use. PC12 pheochromocytoma cells were transfected (at ~50–60% confluence, 1 day after 1:4 splitting) with 1µg of supercoiled promoter haplotype/firefly luciferase reporter plasmid and 10ng of the Renilla luciferase expression plasmid pRL-TK (Promega) as an internal control per well, by the liposome method (Superfect; Qiagen, Valencia, CA). The firefly and Renilla luciferase activities in the cell lysates were measured 16h after transfection, and the results were expressed as the ratio of firefly/Renilla luciferase activity (“Stop & Glo”; Promega). Each experiment was repeated a minimum of three times.
Table 1.
DBH promoter 4-SNP haplotype distribution in the BP extreme population sample
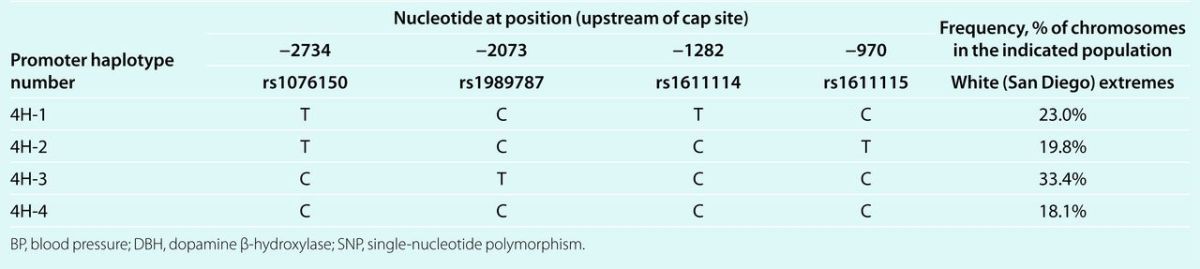
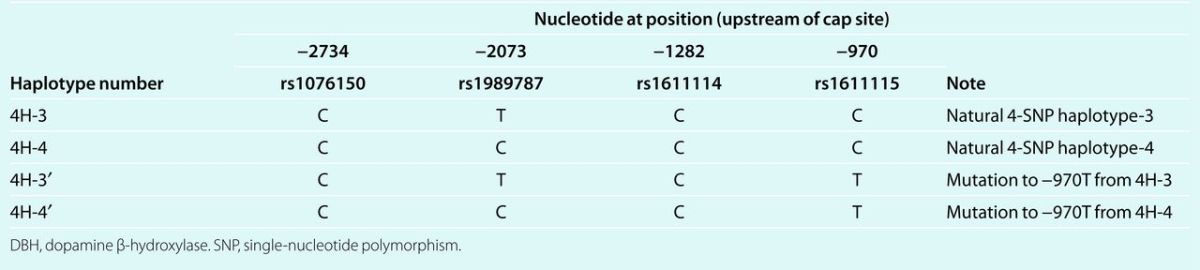
Table 2.
Description of DBH promoter natural and mutant haplotypes inserted into pGL3-basic luciferase reporter plasmids
Transactivation of the DBH promoter by cotransfected c-FOS. pCMV (pcDNA3.1 or pCMV-SPORT6) eukaryotic expression plasmids for human c-FOS were obtained from Open Biosystems (http://www.openbiosystems.com/). Fifty nanogram of each expression plasmid were cotransfected with DBH promoter/luciferase reporter plasmids (at 1µg). Firefly luciferase activities in the cell lysates were measured 16h after transfection, and the results were expressed as firefly luciferase activity/cell protein.
Secretory or transcriptional stimulation. Human DBH promoter/reporter plasmids were transfected into PC12 cells as described above, and the secretory stimuli nicotine (1mmol/l), or pituitary adenylate cyclase-activating polypeptide (PACAP) (0.2µmol/l) were added to the medium; the firefly luciferase activities in the cell lysates were measured 16h after transfection and the results were expressed as the ratio of firefly luciferase activity/cell lysate protein.
Chromatin ImmunoPrecipitation. PC12 cells were transfected with human DBH promoter haplotype/reporters to obtain the C vs. T-alleles at C-2073T. Chromatin immunoprecipitation assays were carried out using reagents and procedures from Upstate Biotechnology (Lake Placid, NY). Cells (5–10× 106) were crosslinked in 1% formaldehyde for 10min at 37°C, and washed with ice-cold phosphate-buffered saline containing a protease inhibitor mixture (Sigma P8340; 10µg/ml), then resuspended in cell lysis buffer (0.1% Triton X-100, 10mmol/l KCl, and 10mmol/l Tris, pH 8). After sonication (Branson, Danbury, CT) and centrifugation, samples were precleared with a salmon sperm DNA/protein A agarose 50% slurry to reduce nonspecific background, and incubated with factor-specific antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; or preimmune immunoglobulin G as a negative control) at 4°C overnight with rotation. The immune complex was captured with a salmon sperm DNA/protein A slurry after incubation at 4°C for 1h. The immune complex was pelleted, washed four times and eluted by 1%SDS/0.1mol/l NaHCO3, then crosslinks were reversed by heating in 0.2mol/l NaCl at 65°C for 4h, followed by proteinase K digestion at 45°C for 1h. The DNA was subsequently extracted and purified with QIAquick PCR kits (Qiagen). Immunoprecipitated nucleosomal DNA samples were analyzed by PCR using primers forming a 161-bp amplicon that encompassed the C-2073T variant in the human DBH promoter. Extracted DNA from the chromatin fractions before antibody precipitation was used as a positive control (“input DNA”). To ensure that the PCR amplification was in the linear range, reactions with different amounts of input DNA samples were carried out for various (typically 15–40) cycle numbers; a linear range of amplification occurred here at ~31 cycles. After amplification, PCR products were separated on 1.5% agarose gels, stained with ethidium bromide, and photographed. We have shown previously that transfected plasmids are incorporated into the chromatin fraction of the cell nucleus.20
Bioinformatics of variant promoter motifs: Phylogenetic footprinting. Human and other mammalian promoter reference sequences were obtained from ENSEMBL and aligned pair-wise using the Smith–Waterman Algorithm.21 Regions of the alignment with >50% human–rat conservation were scored on position weight matrices from JASPAR22 and TRANSFAC.23 databases, using the CONSITE interface <www.phylofoot.org/consite>.24 Motifs from the consensus sequences scoring >80% of maximum for the position weight matrices were considered binding candidates. Details of the scoring function are described elsewhere.25 We then ranked binding candidates in decreasing order, at the top those with maximum difference in position weight matrices score between wild type and variant human motifs (with at least one allele-score meeting the above criteria). Top hits represent transcription factors that bind to motifs containing at least one allele, are conserved between human and rat, and whose binding affinity is altered by mutation and hence likely functional.
Statistical analyses. Haplotypes were estimated from unphased diploid genotypes by HAP.26 Haplotype homozygous individuals were confirmed by visual inspection. One way post hoc analysis of variance using Bonferroni or Dunnett multiple comparison tests was performed in SPSS to test the significance of in vivo associations between SNPs or haplotypes with plasma DBH levels, and in vitro reporter activity assays. Univariate analyses (i.e., analysis of variance with one dependent phenotypic variable) within general linear models in SPSS evaluated the effects of genotype or haplotype on plasma DBH activity, BP (mmHg), or dichotomous disease status, adjusted by age, sex, and body mass index (as covariates). In analyses of C-2073 effects, C-970T was entered as a covariate, to test whether C-2073 effects were specific, given its statistical correlation with C-970T. Within a haplotype block, SNPSpD27 was used to estimate the experiment-wide significance threshold required to maintain the type-I error rate (α) at 5% or less.
Results
DBH promoter haplotypes and common promoter variant C-2073T: Effect on plasma DBH enzymatic activity
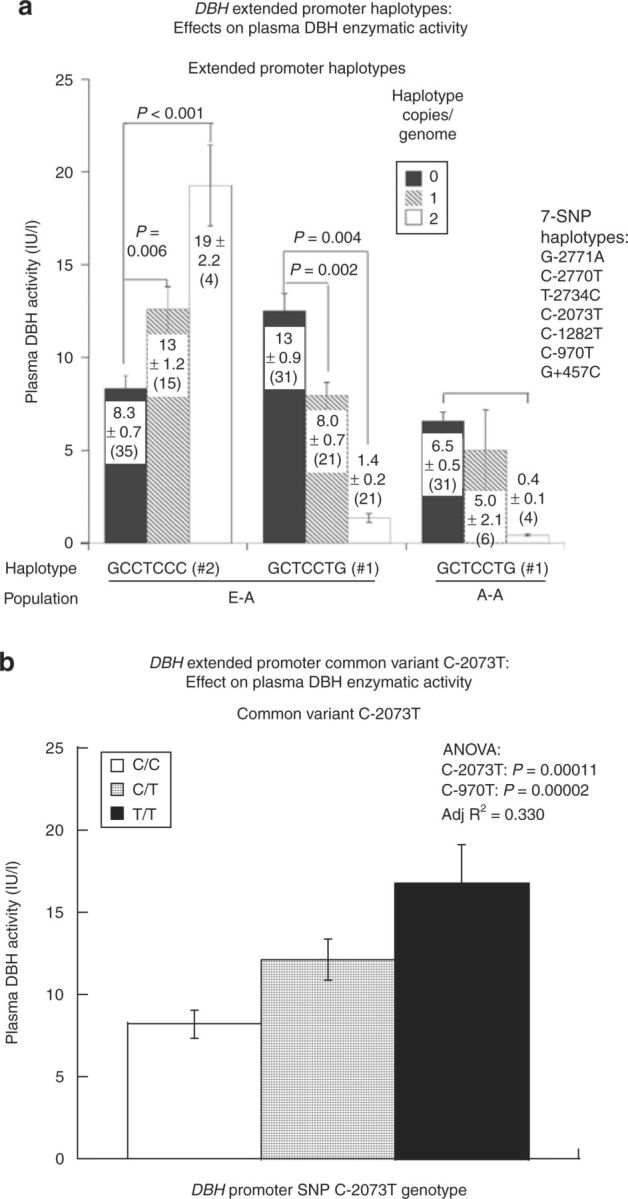
DBH resequencing identified common variants in the promoter region that formed a tightly linked 5′ haplotype block in white individuals.13 We phenotyped 54 healthy subjects of European ancestry, whom we also genotyped at 18 common polymorphic sites across the DBH locus by dideoxy/capillary sequencing. Within the 5′ block, we identified extended promoter haplotypes spanning seven common SNPs (six in the promoter region, and one in intron-A: GC[–2771/–2770]AT/rs1076151/rs1076152; C-2734T/rs1076150; C-2073T/rs1989787; C-1282T/rs1611114; C-970T/rs1611115; G+457C/rs2797849).13 These DBH promoter haplotypes predicted plasma DBH activity in vivo: haplotype-1 reduced DBH activity, whereas haplotype-2, increased DBH activity (Figure 1a). C-2073T itself also showed significant association with DBH activity in an additive fashion (T>C) (Figure 1b). For both haplotypes and C-2073T itself, the –2073T allele gave rise to higher DBH activity.
Figure 1. DBH extended promoter haplotypes and common variant C-2073T: effects on plasma DBH enzymatic activity. Error bars indicate mean ± s.e.m., and P values by ANOVA are shown. Subjects are of European ancestry (E-A), by self-identification. Plasma DBH activity data displayed skewness (0.825) and kurtosis (0.922), and therefore a square root (√) transformation was applied before ANOVA, resulting in diminished skewness (−0.189) and kurtosis (0.032). (a) Extended promoter haplotypes. Seven SNPs span the 5′ haplotype block in subjects of E-A (European ancestry; six in the promoter, one in intron-A). Two promoter haplotypes are significantly associated with DBH activity, shown as the effect of haplotype copy number on activity. (b) Common promoter variant C-2073T. Plasma DBH activity shows significant association to common promoter variant C-2073T. For specificity, C-970T was included as a covariate in the analysis. ANOVA, analysis of variance; DBH, dopamine β-hydroxylase; SNP, single-nucleotide polymorphism.
DBH 4-SNP haplotype and common promoter variant C-2073T: Prediction of BP in the population
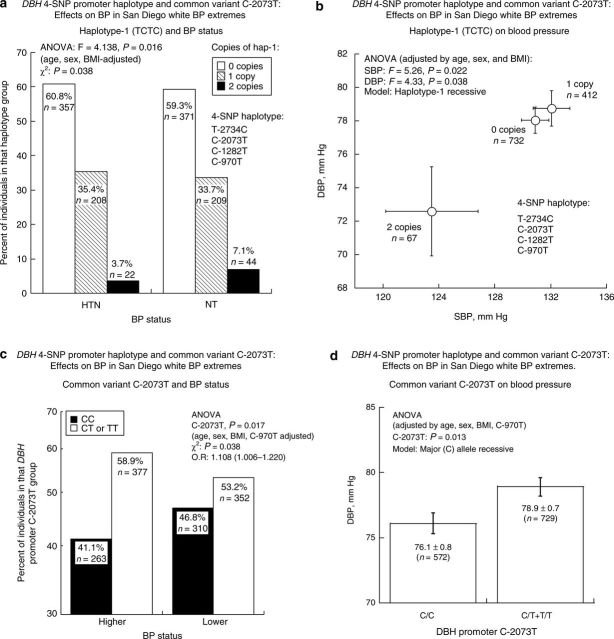
Since the common variants in the promoter region formed a tightly linked block in white individuals, we began DBH marker-on-BP studies by typing a 4-SNP haplotype (Table 1; all in the promoter (C-2734T/rs1076150; C-2073T/rs1989787; C-1282T/rs1611114; and C-970T/rs1611115), in n = 1,302 subjects with the most extreme BP values in a large primary care population.14,23 Haplotype-1 (TCTC), the second most common haplotype in the population, predicted lower BP, both for dichotomous BP status (hypertension vs. normotension), and as the continuous traits (systolic BP/DBP, in mmHg) (Figure 2a,b).
Figure 2. DBH 4-SNP promoter haplotype and common variant C-2073T: effects on BP in San Diego white BP extremes. Mean values ± s.e.m. are shown, and P values by ANOVA were adjusted by age, sex, and BMI. N = 1,211 had genetic information at all four SNP positions, whereas n = 1,302 had information at C-2073T. (a) Haplotype-1 (TCTC) and BP status (lower/higher). (b) Haplotype-1 (TCTC) on blood pressure (mmHg). (c) Common variant C-2073T and BP status (lower/higher). For specificity, C-970T was included as a covariate in the analysis. (d) Common variant C-2073T on blood pressure (mmHg). For specificity, C-970T was included as a covariate. ANOVA, analysis of variance; BMI, body mass index; BP, blood pressure; DBH, dopamine β-hydroxylase; DBP, diastolic BP; HTN, hypertension; NT, normotension; SBP, systolic BP; SNP, single-nucleotide polymorphism.
Among the individual variants within the 5′ block, C-2073T was significantly associated with BP: homozygotes for the major allele (C) displayed less frequent hypertension status as well as lower DBP (Figure 2c,d), even in the face of covariate adjustment for functional variant C-970T. By SNPSpD27 within the promoter block, the experiment-wide significance threshold required to maintain the type-I error rate at <5% remained α < 0.05 (reflecting the high degree of LD within this block); this threshold was exceeded by C-2073T.
Function of DBH promoter variant C-2073T: Transcriptional activity
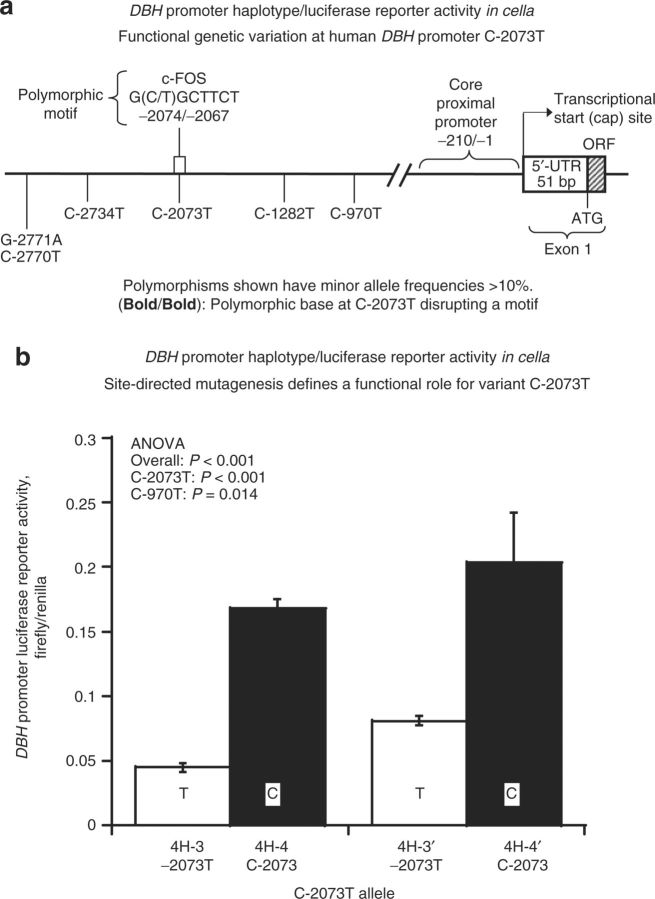
Basal activity of transfected promoters in chromaffin cells. We constructed luciferase reporter plasmids (Table 2) for the four common naturally occurring DBH promoter haplotypes (Table 1). Table 2 describes the strategy for mutagenesis to create DBH promoter/luciferase reporter pairs differing only at C-2073T.
Naturally occurring 4-SNP haplotype-3 (4H-3) and haplotype-4 (4H-4) differ only at position –2,073. We then created two mutant haplotypes by altering position –970: on 4H-3, from C-970→–970T, forming 4H-3′; or on 4H-4, from T-970→–970C, forming 4H-4′.
Since variant C-2073T appeared to be crucial in influencing BP (Figure 2) and plasma DBH activity (Figure 1), we took advantage of these two pairs of haplotypes (4H-3&4H-4; 4H-3′&4H-4′) to isolate the function of individual variants in cella. Higher gene expression resulted from the C allele at C-2073T (Figure 3), when tested upon balanced/matched haplotype backgrounds.
Figure 3. DBH promoter haplotype/luciferase reporter activity in transfected chromaffin cells. (a) Functional domains in the proximal human DBH promoter: role of C-2073T. (b) Site-directed mutagenesis defines a functional role for variant C-2073T. Two natural and two mutant haplotypes, 4H-3/4H-4, and 4H-3′/4H-4′, form pairs which have different alleles at C-2073T, but otherwise identical haplotype backgrounds. Results are compared with ANOVA, with C-970T as a covariate in the analysis, for specificity. Each experiment was performed in triplicate, and such experiments were repeated at least three times. Fifty micro liter from the 500-µl cell lysate was used for luciferase enzymatic activity measurement. ANOVA, analysis of variance; DBH, dopamine β-hydroxylase.
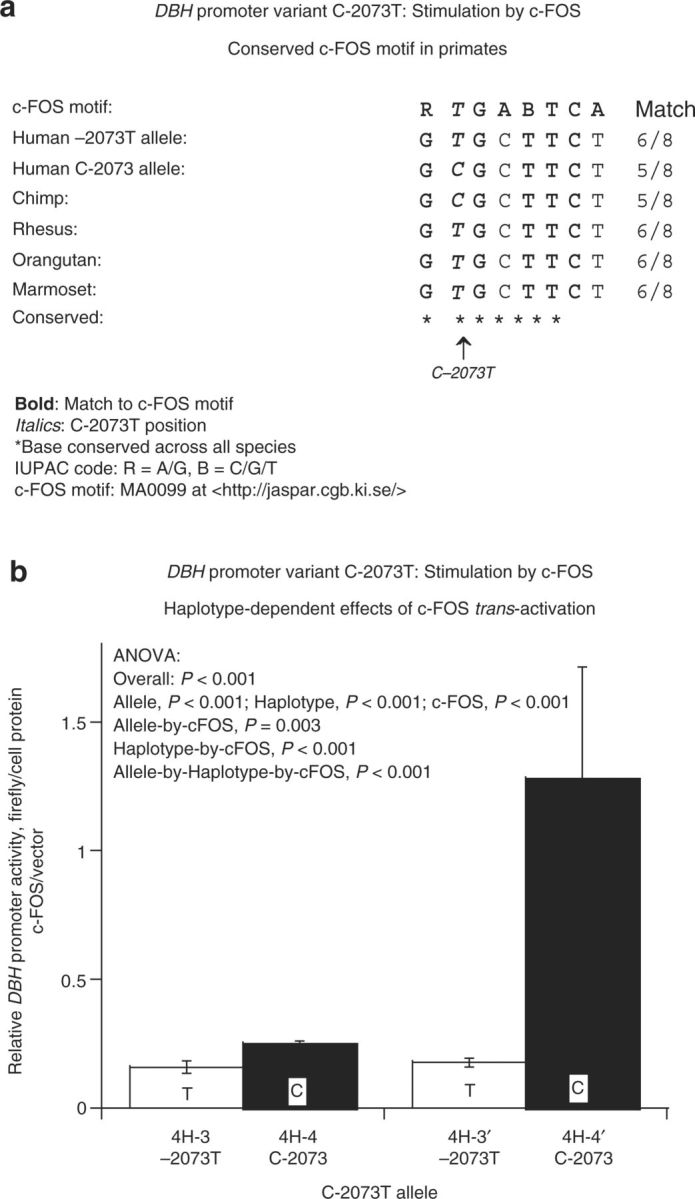
Differential transactivation by c-FOS. c-FOS was identified as a partial (6/8 base) match spanning C-2073T (Figure 4a); contrary to expectation from the superior match of the –2073T allele for the c-FOS motif, the T-allele exhibited less transactivation of expression than the C-allele, in response to cotransfected/expressed c-FOS (Figure 4b).
Figure 4. DBH promoter variant C-2073T: Stimulation by c-FOS. (a) DBH promoter variant C-2073T: Conserved c-FOS motif. The c-FOS motif match in the sequence is shown, as well as primate interspecies homology in the region. (b) DBH promoter variant C-2073T: haplotype-dependent effects of c-FOS transactivation. Results are shown as stimulated activity over control activity (i.e., without c-FOS stimulation). Each experiment was performed in triplicate, and such experiments were repeated at least three times. ANOVA, analysis of variance; DBH, dopamine β-hydroxylase.
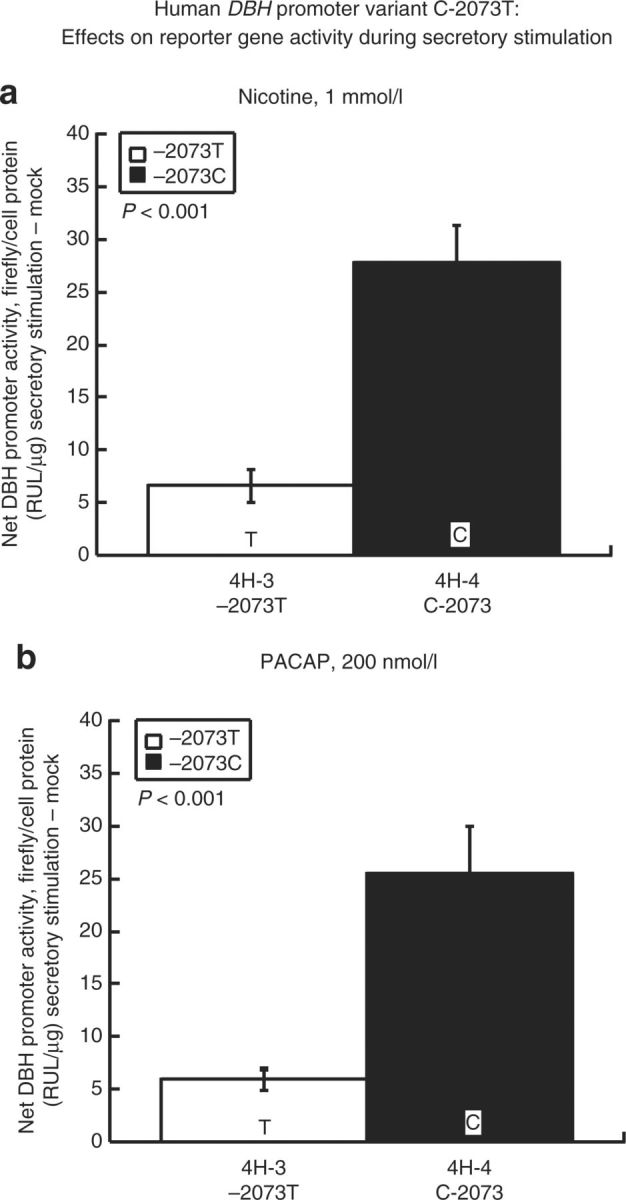
Differential effects of secretory stimulation. We tested the responses of C-2073T variation on balanced haplotype backgrounds to preganglionic secretory stimuli (Figure 5): a nicotinic cholinergic agonist (nicotine), or the neuropeptide PACAP. The C-allele demonstrated consistently increased responses to both nicotine and PACAP, once again in contrast to the expectation generated by the superior c-FOS match to the T-allele at that position (Figure 4a).
Figure 5. Human DBH promoter variant C-2073T: Effects on reporter gene activity during secretory stimulation. Results are shown as stimulated activity minus control activity (i.e., without secretory stimulation). Each experiment was performed in triplicate, and such experiments were repeated at least three times. DBH, dopamine β-hydroxylase. PACAP, pituitary adenylate cyclase-activating polypeptide.
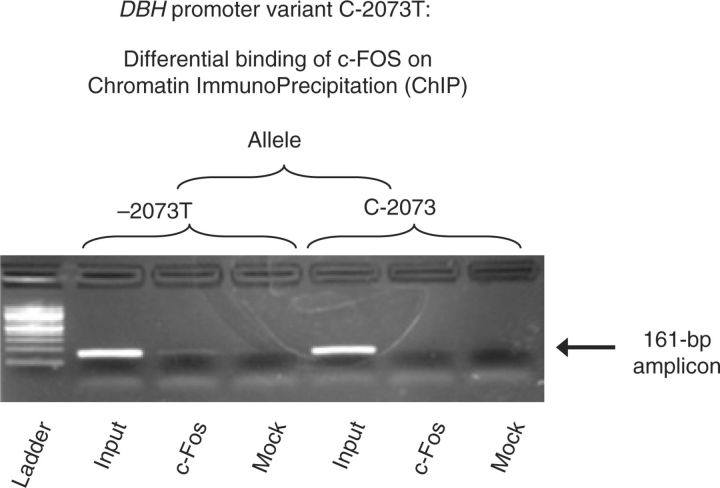
Endogenous transcription factor binding to C-2073T. c-FOS (by chromatin immunoprecipitation). During chromatin immunoprecipitation (Figure 6), transcription factor c-FOS on the target promoter could be captured by specific antibody immunoprecipitation, with a greater signal than immunoglobulin G control serum (“mock”); the T-allele exhibited a greater signal than the C allele, as expected for the c-FOS homology match (Figure 4a).
Figure 6. Interaction of endogenous c-FOS with DBH promoter variant C-2073T in cella: ChIP (Chromatin ImmunoPrecipitation). Results of nucleosomal immunoprecipitates from PC12 cells. “Input” DNA derives from the nucleosomal preparation before immunoprecipitation. Antibodies directed against c-FOS (or preimmune serum, “mock”) were used to precipitate nucleosomes derived from PC12 cells transfected with either the C-2073 or −2073T alleles, on promoter/reporter plasmids. The nucleosomal immunoprecipitates were interrogated with PCR primers producing a 161-bp amplicon spanning C-2073T. DBH, dopamine β-hydroxylase.
Discussion
Overview
In this report, we approach the effect of common DBH promoter variant C-2073T for expression and activity of the enzyme, and finally for cardiovascular disease (hypertension) risk. We found that a promoter haplotype (TCTC, encompassing the C-2073 allele) was associated with higher systolic BP/DBP; within that haplotype, C-2073T had a significant effect on BP, with elevation by the –2073T allele. C-2073T also associated with DBH secretion, as estimated by plasma DBH enzymatic activity; the –2073T allele elevated DBH activity. Studies with isolated/transfected DBH promoters showed that the T-allele at C-2073T altered expression of a reporter gene, when compared to C allele. C-2073T disrupted a consensus transcriptional motif for c-FOS, affecting not only basal expression of a luciferase reporter, but also the response to c-FOS transactivation. Under stimulation by triggers to chromaffin cell exocytosis, such as nicotine or PACAP, both alleles were activated, but the increment differed between the T and C alleles. The motif also bound endogenous c-FOS in the chromatin fraction of the cell. These results suggest a mechanistic chain of events whereby common DBH promoter variant C-2073T plays a role in the determination of human BP.
Of note for cardiovascular disease risk in the population, the minor (T) allele at C-2073T has an overall frequency of ~16%, ranging from 0% to ~44% in different biogeographic ancestry subgroups;13 thus, the T-allele is extraordinarily common as a risk factor in the population.
Mechanisms in cella vs. those in vivo
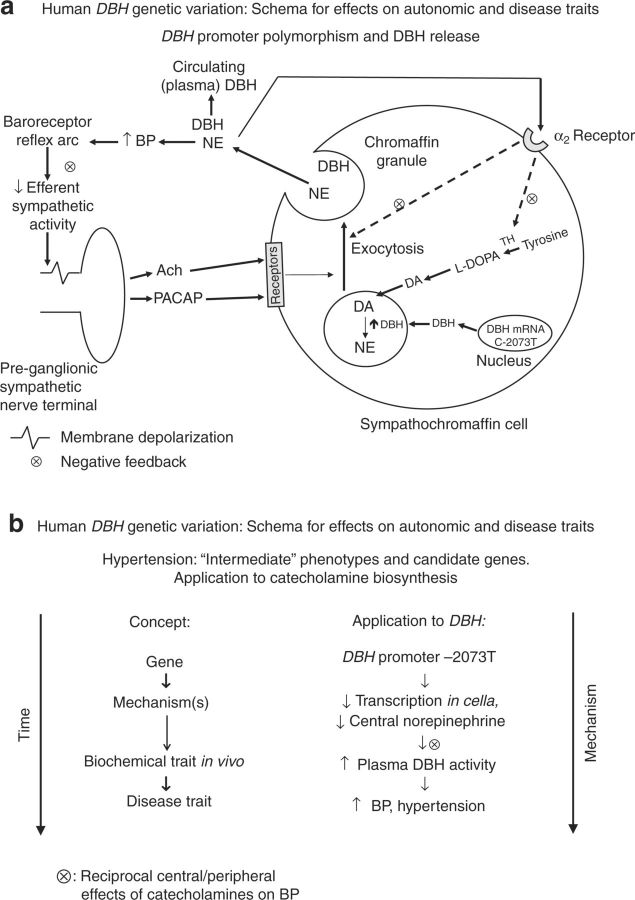
Much as we found for DBH promoter variant C-970T,13 we note that C-2073T also displayed directionally opposite effects on transcription in cella (reporter gene activity in transfected cells) and plasma DBH activity in vivo. We previously hypothesized that the reciprocal actions of central vs. peripheral noradrenergic transmission on BP, perhaps mediated by negative feedback mechanisms (Figure 7a), can be proposed to reconcile the contrasting results in vivo and in cella.13 After exocytosis, norepinephrine acts via presynaptic α-2-adrenergic receptors on sympathetic termini, thus decreasing both catecholamine biosynthesis and vesicle exocytosis. BP elevation by catecholamines also activates the baroreceptor mechanism, thereby diminishing efferent sympathetic activity and consequently decreasing vesicle exocytosis (Figure 7a). We have previously reported a similar effect for the catecholamine release-inhibitory catestatin region of CHGA, for which we proposed reciprocal central and peripheral actions on BP.19
Figure 7. Human DBH genetic variation: schema for effects on autonomic and disease traits. (a) DBH promoter polymorphism and DBH release. Hypothesis for a negative feedback mechanism(s) whereby DBH promoter variants may influence catecholamine release, with indirect/systemic/feedback effects upon subsequent DBH release. (b) “Intermediate” phenotype hypothesis for DBH. Framework for integration of the experimental results for DBH genetic variation, early/proximate autonomic traits (such as DBH secretion into plasma), and later disease traits (such as hypertension). BP, blood pressure; DBH, dopamine β-hydroxylase; PACAP, pituitary adenylate cyclase-activating polypeptide.
Biological role of C-2073T
Our results suggest that a discrete variant in the DBH promoter (C-2073T) may play a role in control of BP, with sequential effects beginning at transcriptional control, proceeding through an intermediate/risk phenotype, and eventuating in basal BP changes in the population.
Previous DBH promoter/reporter gene studies using isolated polymorphic sites have not completely resolved the source of functional variation in the gene.28,29 In the present study, we used a longer ~3-kbp DBH promoter fragment, which may more accurately reflect the regulatory architecture of the endogenous DBH promoter by allowing interaction among a larger number of cis-regulatory sites.
With the longer ~3-kbp DBH promoter haplotypes, we attempted to understand how the two alleles at C-2073T influence gene expression, under not only the basal state, but also secretion-stimulatory conditions, as well as transactivation by c-FOS.
Expression of DBH promoter variants on a variety of haplotypic backgrounds, both natural and artificial, indicated that C-2073T exerted functional changes upon reporter activity. Follow-up experiments indicated that this variant harbored a partial consensus match for a transcriptional control motif: c-FOS. Here, we found that c-FOS exerted differential effects on the C and T alleles, with the C response exaggerated, initially unexpected in that the T-allele provides a better c-FOS consensus match. c-FOS, a cellular protooncogene belonging to the immediate/early b-Zip family of transcription factors, is widely expressed in neurons and adrenal gland, and involved in the regulation of catecholamine-synthesizing enzymes, including DBH, TH, and PNMT.30 The chromatin immunoprecipitation experiment (Figure 6) confirmed that endogenous c-FOS bound to the region, with T>C signal intensity, in accordance with the homology prediction (Figure 4b).
The chromaffin cell secretory stimulus (nicotinic cholinergic agonist) nicotine-activated transcription from both C-2073T alleles, though stimulation of the C allele was greater than the T-allele, consistent with the basal transcription results. Nicotine elevates DBH mRNA levels in vivo,30 and nicotine consumption is a risk factor for cardiovascular diseases such as hypertension.31 The secretory stimulus PACAP also augmented DBH transcription, with a magnified response by the C allele. PACAP also upregulates DBH synthesis in chromaffin cells.32 Taken together, these complementary results indicate that several specific stimuli function differentially at C-2073T, with a consistent C>T expression pattern.
Conclusions and perspectives
We began with an analysis of common haplotypes in the DBH promoter, and first established an effect DBH secretion, and then moved to basal/resting BP in the population. Within the haplotype, in each case (plasma DBH or BP) the effect was found for promoter variant C-2073T. The C-2073T site altered a consensus c-FOS transcriptional motif, and site-directed mutagenesis of the site altered not only basal transcription, but also the response of the DBH promoter to exogenous (cotransfected) c-FOS, as well as the chromaffin secretory stimuli nicotine and PACAP. The findings are consistent with a cascade of events (Figure 7b), beginning with the C-2073T variant and eventuating in alterations of BP. Further analysis of genetic interactions among variants at DBH and components of the catecholamine biosynthetic pathway may assist in refining the contribution of that pathway to susceptibility for hypertension.
Acknowledgments
This work was supported by National Institutes of Health, Department of Veterans Affairs. Y.C. was supported by an International Society of Nephrology fellowship.
Disclosure:
The authors declared no conflict of interest.
References
- 1.Esler M, Ferrier C, Lambert G, Eisenhofer G, Cox H, Jennings G. Biochemical evidence of sympathetic hyperactivity in human hypertension. Hypertension 1991;17:III29–III35. [DOI] [PubMed] [Google Scholar]
- 2.Ferrier C, Cox H, Esler M. Elevated total body noradrenaline spillover in normotensive members of hypertensive families. Clin Sci 1993;84:225–230. [DOI] [PubMed] [Google Scholar]
- 3.Kim CH, Zabetian CP, Cubells JF, Cho S, Biaggioni I, Cohen BM, Robertson D, Kim KS. Mutations in the dopamine β-hydroxylase gene are associated with human norepinephrine deficiency. Am J Med Genet 2002;108:140–147. [PubMed] [Google Scholar]
- 4.De Potter WP, De Schaepdryver AF, Smith AD. Release of chromogranin A and dopamine-β-hydroxylase from adrenergic nerves during nerve stimulation. Acta Physiol Scand Suppl 1970;357:8. [PubMed] [Google Scholar]
- 5.Weinshilboum RM, Thoa NB, Johnson DG, Kopin IJ, Axelrod J. Proportional release of norepinephrine and dopamine- -hydroxylase from sympathetic nerves. Science 1971;174:1349–1351. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor DT, Cervenka JH, Stone RA, Levine GL, Parmer RJ, Franco-Bourland RE, Madrazo I, Langlais PJ, Robertson D, Biaggioni I. Dopamine β-hydroxylase immunoreactivity in human cerebrospinal fluid: properties, relationship to central noradrenergic neuronal activity and variation in Parkinson's disease and congenital dopamine β-hydroxylase deficiency. Clin Sci 1994;86:149–158. [DOI] [PubMed] [Google Scholar]
- 7.Weinshilboum RM, Raymond FA, Elveback LR, Weidman WH. Serum dopamine-β-hydroxylase activity: sibling-sibling correlation. Science 1973;181:943–945. [DOI] [PubMed] [Google Scholar]
- 8.Weinshilboum RM, Schorott HG, Raymond FA, Weidman WH, Elveback LR. Inheritance of very low serum dopamine-β-hydroxylase activity. Am J Hum Genet 1975;27:573–585. [PMC free article] [PubMed] [Google Scholar]
- 9.Robertson D, Haile V, Perry SE, Robertson RM, Phillips JA, 3rd, Biaggioni I. Dopamine β-hydroxylase deficiency. A genetic disorder of cardiovascular regulation. Hypertension 1991;18:1–8. [DOI] [PubMed] [Google Scholar]
- 10.Swoap SJ, Weinshenker D, Palmiter RD, Garber G. Dbh(-/-) mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. Am J Physiol Regul Integr Comp Physiol 2004;286:R108–R113. [DOI] [PubMed] [Google Scholar]
- 11.Ohlstein EH, Kruse LI, Ezekiel M, Sherman SS, Erickson R, DeWolf WE, Jr, Berkowitz BA. Cardiovascular effects of a new potent dopamine β-hydroxylase inhibitor in spontaneously hypertensive rats. J Pharmacol Exp Ther 1987;241:554–559. [PubMed] [Google Scholar]
- 12.Ross ST, Kruse LI, Ohlstein EH, Erickson RW, Ezekiel M, Flaim KE, Sawyer JL, Berkowitz BA. Inhibitors of dopamine β-hydroxylase. 3. Some 1-(pyridylmethyl)imidazole-2-thiones. J Med Chem 1987;30:1309–1313. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Wen G, Rao F, Zhang K, Wang L, Rodriguez-Flores JL, Sanchez AP, Mahata M, Taupenot L, Sun P, Mahata SK, Tayo B, Schork NJ, Ziegler MG, Hamilton BA, O'Connor DT. Human dopamine β-hydroxylase (DBH) regulatory polymorphism that influences enzymatic activity, autonomic function, and blood pressure. J Hypertens 2010;28:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rana BK, Insel PA, Payne SH, Abel K, Beutler E, Ziegler MG, Schork NJ, O'Connor DT. Population-based sample reveals gene-gender interactions in blood pressure in White Americans. Hypertension 2007;49:96–106. [DOI] [PubMed] [Google Scholar]
- 15.Waalen J, Felitti V, Gelbart T, Ho NJ, Beutler E. Prevalence of coronary heart disease associated with HFE mutations in adults attending a health appraisal center. Am J Med 2002;113:472–479. [DOI] [PubMed] [Google Scholar]
- 16.Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension 2003;41:207–210. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Kraja AT, Oberman A, Lewis CE, Ellison RC, Arnett DK, Heiss G, Lalouel JM, Turner ST, Hunt SC, Province MA, Rao DC. A summary of the effects of antihypertensive medications on measured blood pressure. Am J Hypertens 2005;18:935–942. [DOI] [PubMed] [Google Scholar]
- 18.O'Connor DT, Levine GL, Frigon RP. Homologous radio-immunoassay of human plasma dopamine-β-hydroxylase: analysis of homospecific activity, circulating plasma pool and intergroup differences based on race, blood pressure and cardiac function. J Hypertens 1983;1:227–233. [DOI] [PubMed] [Google Scholar]
- 19.Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, Rao F, Stridsberg M, Smith DW, Mahboubi P, Schork NJ, O'Connor DT, Hamilton BA. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet 2004;74:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang K, Zhang L, Rao F, Brar B, Rodriguez-Flores JL, Taupenot L, O'Connor DT. Human tyrosine hydroxylase natural genetic variation: delineation of functional transcriptional control motifs disrupted in the proximal promoter. Circ Cardiovasc Genet 2010;3:187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith TF, Waterman MS. Identification of common molecular subsequences. J Mol Biol 1981;147:195–197. [DOI] [PubMed] [Google Scholar]
- 22.Vlieghe D, Sandelin A, De Bleser PJ, Vleminckx K, Wasserman WW, van Roy F, Lenhard B. A new generation of JASPAR, the open-access repository for transcription factor binding site profiles. Nucleic Acids Res 2006;34:D95–D97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingender E, Dietze P, Karas H, Knüppel R. TRANSFAC: a database on transcription factors and their DNA binding sites. Nucleic Acids Res 1996;24:238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandelin A, Wasserman WW, Lenhard B. ConSite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acids Res 2004;32:W249–W252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wasserman WW, Sandelin A. Applied bioinformatics for the identification of regulatory elements. Nat Rev Genet 2004;5:276–287. [DOI] [PubMed] [Google Scholar]
- 26.Eskin E, Sharan R, Halperin E. A note on phasing long genomic regions using local haplotype predictions. J Bioinform Comput Biol 2006;4:639–647. [DOI] [PubMed] [Google Scholar]
- 27.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet 2004;74:765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cubells JF, Zabetian CP. Human genetics of plasma dopamine β-hydroxylase activity: applications to research in psychiatry and neurology. Psychopharmacology (Berl) 2004;174:463–476. [DOI] [PubMed] [Google Scholar]
- 29.Zabetian CP, Anderson GM, Buxbaum SG, Elston RC, Ichinose H, Nagatsu T, Kim KS, Kim CH, Malison RT, Gelernter J, Cubells JF. A quantitative-trait analysis of human plasma-dopamine β-hydroxylase activity: evidence for a major functional polymorphism at the DBH locus. Am J Hum Genet 2001;68:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hochstenbach SL, Ciriello J. Plasma hypernatremia induces c-fos activity in medullary catecholaminergic neurons. Brain Res 1995;674:46–54. [DOI] [PubMed] [Google Scholar]
- 31.Pardell H, Rodicio JL. High blood pressure, smoking and cardiovascular risk. J Hypertens 2005;23:219–221. [DOI] [PubMed] [Google Scholar]
- 32.Choi HJ, Park SY, Hwang O. Differential involvement of PKA and PKC in regulation of catecholamine enzyme genes by PACAP. Peptides 1999;20:817–822. [DOI] [PubMed] [Google Scholar]