Abstract
Increasing evidence has demonstrated that malignant cells exhibit increased glucose uptake, which facilitates survival and growth in a hypoxic environment. The glucose transporter-1 (GLUT-1) is overexpressed in a variety of malignant tumors. However, the association between GLUT-1 expression and clinicopathological factors, 18F-fluorodeoxyglucose uptake and tumor proliferation in pancreatic cancer has not been investigated to date. In the present study, the expression of GLUT-1 in 53 pancreatic cancer tissues was analyzed, which revealed that GLUT-1 was overexpressed in pancreatic tissue and correlated with poor prognosis and clinicopathological characteristics, including increased tumor size, clinical stage and lymph node metastasis, maximum standardized uptake value (SUVmax) and Ki-67 expression. The receiver operating characteristic curve analysis indicated that a cut-off SUVmax value of 4.830 was associated with optimal sensitivity (88%) and specificity (71.4%) for the detection of strong positive GLUT-1 expression. In addition, as the expression of GLUT-1 was found to correlate with Ki-67 expression, GLUT-1 may exhibit a significant effect on cell proliferation in pancreatic cancer. Overall, these findings indicate that GLUT-1 may represent a prognostic indicator, and a potential therapeutic target for pancreatic cancer.
Keywords: glucose transporter-1, 18F-fluorodeoxyglucose, prognosis, Ki-67, pancreatic cancer
Introduction
Pancreatic cancer remains one of the most lethal malignancies worldwide, with a high malignant potential and a poor prognosis. The number of new cases of pancreatic cancer was almost 48,960 in 2015. It is the fourth most common cause of cancer-associated mortality in Western society, with a median survival of <6 months and a 5-year survival rate of 5% (1,2). Despite advances in cancer therapy, pancreatic cancer is unresponsive to the majority of treatments (3,4). To date, no targeted therapy to improve the clinical outcome has been identified. Consequently, development of molecular prognostic factors to improve patient selection for novel therapeutic approaches is urgently required.
Tumor hypoxia is a common phenomenon in solid tumors, and is associated with poor prognosis in several types of cancer, including laryngeal squamous cell carcinoma, ovarian cancer, breast cancer, gallbladder cancer and pancreatic cancer (5). Hypoxia leads to genetic instability and failure of DNA repair, which results in the selection of tumor cells toward a more aggressive phenotype. Under hypoxic conditions, tumor cells switch from oxygen-dependent glucose metabolism to anaerobic glycolysis (6). This cellular adaptation to hypoxia, known as the Warburg effect, is supported by an observed increase in glucose transport and consumption (7). High rates of glucose uptake and glycolysis supply the energy required for proliferation of malignant cells and tumor growth.
The glucose transporter (GLUT) family has been identified as belonging to the solute carrier 2A family (SLCZA). The members of this family differ in their affinity for glucose and their effects on physiological regulation (8,9). Glucose transporter-1 (GLUT-1) is a member of the GLUT family, which is expressed in erythrocytes, endothelial cells, placenta and blood-tissue barriers, including the blood-brain and blood-nerve barriers (10,11). Recent studies have demonstrated that GLUT-1 is often upregulated in various malignant tumors, including colorectal cancer (12), esophageal cancer (13), oral squamous cell carcinoma (14), renal cell carcinoma (15) breast cancer and lung cancer (16). It is also considered to be the predominantly elevated glucose transporter under ischemic and hypoxic conditions, whereby cells require glycolysis as an energy source. Positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG) is a non-invasive diagnostic and prognostic tool used to evaluate the hypoxic status of tumors. The expression of glucose transporter proteins, in particular GLUT-1, is hypothesized to be associated with FDG uptake (17).
In the present study, immunohistochemical analysis was used to determine the level of GLUT-1 expression in human pancreatic cancer tissues and to evaluate the association between GLUT-1 expression and clinicopathological characteristics and prognosis. In addition, the association between GLUT-1 expression, 18F-FDG accumulation and Ki-67 expression was also investigated.
Materials and methods
Clinical data
The study sample was comprised of 53 formalin-fixed and paraffin-embedded pancreatic cancer tissue specimens and adjacent healthy tissues obtained from patients with pancreatic cancer. All patients underwent surgical resection at the First Affiliated Hospital of Soochow University (Suzhou, China) between January 2010 and December 2011. Patient characteristics and tumor status are summarized in Table I. The clinical stage was classified according to the seventh edition of the TNM classification of the American Joint Committee on Cancer (18). Patients that had received preoperative chemo-, radio-or immunotherapy were excluded. The study was conducted in accordance with the Declaration of Helsinki (19) and was approved by the Ethics Committee of Soochow University.
Table I.
Clinicopathological characteristics of pancreatic cancer patients (n=53).
| Characteristics | Patients, n (%) |
|---|---|
| Age, years | |
| Median | 63 |
| Range | 39–72 |
| Gender | |
| Male | 29 (54.7) |
| Female | 24 (45.3) |
| Tumor size, cm | |
| Median | 3.8 |
| Range | 1.1–7.4 |
| ≤2 | 18 (34.0) |
| >2 | 35 (66.0) |
| Differentiation | |
| Well | 13 (24.5) |
| Moderate | 18 (34.0) |
| Poor | 22 (41.5) |
| Lymph node metastasis | |
| Yes | 21 (39.6) |
| No | 32 (60.4) |
| Clinical stage | |
| I | 22 (41.5) |
| II | 31 (58.5) |
Immunohistochemistry (IHC)
The samples were fixed with formalin (GE Healthcare Life Sciences, Logan, UT, USA) embedded in paraffin (GE Healthcare Life Sciences) and sectioned. Serial sections (4-µm) subjected to immunohistological staining were fixed with freshly prepared 3% H2O2 with 0.1% sodium azide to block endogenous peroxidase activity and treated with antigen retrieval solution (GE Healthcare Life Sciences) for 15 min. After placing in blocking reagent (Roche Diagnostics, Basel, Switzerland) for 15 min, the sections were incubated with primary rabbit monoclonal anti-GLUT-1 (dilution, 1:300; catalog no., ab115730; Abcam, Cambridge, MA, USA) or mouse monoclonal anti-Ki-67 (dilution, 1:500; catalog no., ab6526; Abcam) antibody overnight at 4°C, followed by incubation with horseradish peroxidase-conjugated polyclonal goat anti-rabbit IgG secondary antibody (dilution, 1:500; catalog no., ab97200; Abcam) for 2 h at 4°C. The signal was visualized by 3,3′-diaminobenzidene (Sangon Biotech Co., Ltd, Shanghai, China).
Evaluation of IHC
GLUT-1 expression was evaluated by light microscopy (Leica Microsystems, Mannheim, Germany) for immunostaining intensity and staining percentage. A total of 3 fields of view were examined at magnification, ×200. The staining intensity was classified as follows: 0, no staining; 1, weak staining; 2, moderate to strong staining. The percentage of positively stained cells was classified as follows: 0, <10%; 1, 10–50%; 2, >50%. The final intensity score was calculated by multiplying the staining intensity score by the staining percentage score. All cases were subsequently classified into the four expression groups according to the following final scores: 0, negative (−); 1, weak (+); 2, moderate (++); 3, strong (+++). Scores of ++ and +++ indicated positive GLUT-1 expression. To determine Ki-67 expression, positively stained cells were defined as those exhibiting clear nuclear staining. Tissues were considered to exhibit positive Ki-67 expression when >15% of the tumor cells were stained among ≥1,000 tumor cells.
18F-FDG PET/computed tomography (CT)
FDG-PET scans were performed on the 53 patients from mid-thigh to the head using a GE Discovery STE 16 PET/CT scanner (GE Healthcare, Piscataway, NJ, USA). Blood glucose levels were measured prior to 18F-FDG injection, and patients with a blood glucose level of >11.2 mmol/l were excluded from the study. Patients underwent FDG PET scans after ≥6 h fasting and an uptake time of 45–60 min following intravenous 18F-FDG administration (3.70–4.44 MBq/kg). An emission scan was acquired for 3 min per bed position and a whole-body scan was performed for each patient using several bed positions, which were conducted based on the height of each patient.
The whole-body PET images were independently evaluated by two nuclear medicine physicians for the presence of abnormally increased uptake in the pancreas. PET, CT and fused PET/CT images were presented on a workstation to diagnose 18F-FDG uptake in the pancreas. On the basis of regions of interest (ROIs), 18F-FDG uptake was analyzed semi-quantitatively by calculating the maximum standardized uptake value (SUVmax) according to the following equation: SUVmax = maximum pixel value within the ROI activity (MBq/kg)/(injected dose [MBq]/body weight [kg]).
Statistical analysis
All statistical analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Continuous variables were compared using the Mann-Whitney U test and categorical variables were compared using the χ2 test or Fisher's exact test. The overall survival time was defined as the interval between the date of tumor resection and the date of mortality or last follow-up. Overall survival was calculated using the Kaplan-Meier method and compared by the log-rank test. Multivariate analysis was performed using the Cox proportional hazards regression model. Correlation analysis was performed using Spearman's rank analysis. Receiver operating characteristic (ROC) curve analysis was used to define a cut-off SUVmax value for the optimal sensitivity and specificity in the prediction of GLUT-1 strong positive expression. P<0.05 was considered to indicate a statistically significant difference.
Results
Overexpression of GLUT-1 protein in pancreatic cancer
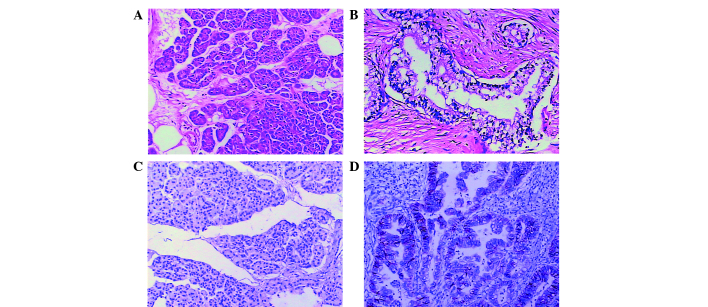
To elucidate the function of GLUT-1 in the progression of pancreatic cancer, the expression of GLUT-1 protein in clinical pancreatic cancer tissues was analyzed using IHC staining. The GLUT-1 protein was predominantly localized to the cytomembrane of cancer cells in pancreatic cancer tissues (Fig. 1). Among the 53 pancreatic cancer tissues, 39 cases (73.6%) exhibited positive GLUT-1 expression, including 25 strongly positive cases (47.2%) in tumor tissues. Among the non-tumorous tissues, 42 cases (79.2%) exhibited negative GLUT-1 expression and 11 cases exhibited positive expression (20.8%). Thus, GLUT-1 expression was significantly higher in pancreatic cancer tissues when compared with non-tumor tissues (χ2=29.681; P<0.001).
Figure 1.
Representative immunohistochemical staining of GLUT-1. Hematoxylin and eosin staining of (A) pancreatic cancer tissues and (B) paired non-tumorous tissues, showing (C) negative GLUT-1 expression in non-tumorous tissues and (D) positive GLUT-1 expression in pancreatic cancer tissues. Magnification, ×200. GLUT-1, glucose transporter-1.
Correlation between GLUT-1 protein expression and clinicopathological parameters
The associations between GLUT-1 expression and clinicopathological parameters of pancreatic cancer patients are shown in Table II. GLUT-1 expression significantly correlated with tumor size (χ2=11.908; P=0.001), clinical stage (χ2=10.764; P=0.002) and lymph node metastasis (χ2=5.105; P=0.029), however, no significant associations were identified between GLUT-1 expression and gender (χ2=0.045; P=1.000), age (χ2=1.002; P=0.365), tumor location (χ2=1.449; P=0.311), tumor differentiation (χ2=1.287, P=0.525) or vascular invasion (χ2=3.527; P=0.106). These results indicated that the overexpression of GLUT-1 may correlate with the progression of pancreatic cancer.
Table II.
Association between GLUT-1 expression and clinicopathological features of pancreatic cancer patients.
| GLUT-1 expression | |||||
|---|---|---|---|---|---|
| Parameter | n | Positive | Negative | χ2 | P-value |
| Gender | |||||
| Male | 29 | 21 | 8 | 0.045 | 1.000 |
| Female | 24 | 18 | 6 | ||
| Age, years | |||||
| ≤65 | 28 | 19 | 9 | 1.002 | 0.365 |
| >65 | 25 | 20 | 5 | ||
| Tumor locationb | |||||
| Head | 37 | 29 | 8 | 1.449 | 0.311 |
| Body and tail | 16 | 10 | 6 | ||
| Tumor size, cm | |||||
| ≤2 | 18 | 8 | 10 | 11.908 | 0.001 |
| >2 | 35 | 31 | 4 | ||
| Differentiation | |||||
| Well | 13 | 8 | 5 | 1.287 | 0.525 |
| Moderate | 18 | 14 | 4 | ||
| Poor | 22 | 17 | 5 | ||
| Clinical stage | |||||
| I | 22 | 11 | 11 | 10.764 | 0.002a |
| II | 31 | 28 | 3 | ||
| Lymph node metastasis | |||||
| Y | 21 | 19 | 2 | 5.105 | 0.029a |
| N | 32 | 20 | 12 | ||
| Vascular invasion | |||||
| Y | 10 | 5 | 5 | 3.527 | 0.106 |
| N | 43 | 34 | 9 | ||
P<0.05.
Head, body and tail refer to the location of the tumor in the pancreas. GLUT-1, glucose transporter-1; Y, yes; N, no.
Prognostic significance of GLUT-1 overexpression
Of the 53 pancreatic cancer patients, 3 patients were lost to follow-up. As shown in Fig. 2, the median overall survival time for the GLUT-1 positive group was 12.3 months compared with 22.2 months for the GLUT-1 negative group. Kaplan-Meier curve analysis revealed that patients with positive GLUT-1 expression exhibited a significantly shorter overall survival time than those with GLUT-1 negative expression (log-rank test, P=0.001). Multivariate analysis revealed that GLUT-1 expression is an independent prognostic factor (P=0.001; Table III). These results indicated that GLUT-1 overexpression is correlated with poor prognosis of pancreatic cancer.
Figure 2.

Kaplan-Meier survival curves comparing GLUT-1 expression in pancreatic cancer patients. The survival time in the GLUT-1 positive expression group was significantly shorter than that for patients in the GLUT-1 negative expression group (P=0.001). GLUT-1, glucose transporter-1.
Table III.
Multivariate analysis of prognostic markers in pancreatic cancer patients.
| Factors | HR | 95% CI | P-value |
|---|---|---|---|
| Gender | 1.251 | 0.686–2.280 | 0.466 |
| Age | 0.638 | 0.360–1.128 | 0.122 |
| Tumor location | 1.385 | 0.690–2.778 | 0.359 |
| Tumor size | 0.425 | 0.211–0.856 | 0.017 |
| Differentiation | 1.426 | 0.697–2.915 | 0.331 |
| Clinical stage | 0.537 | 0.306–0.943 | 0.030 |
| Lymph node metastasis | 4.210 | 2.295–7.720 | <0.001 |
| Vascular invasion | 0.583 | 0.302–1.125 | 0.108 |
| GLUT-1 expression | 0.294 | 0.153–0.568 | <0.001 |
GLUT-1, glucose transporter-1; HR, hazard ratio; CI, confidence interval.
Association between GLUT-1 expression and SUVmax
All patients were examined by 18F-FDG PET/CT. The median SUVmax was 4.90 (range, 1.93–13.22; 25–75% percentile, 2.96–7.04). As shown in Fig. 3A, the patients with positive GLUT-1 expression exhibited a significantly higher SUVmax than those exhibiting negative GLUT-1 expression (median SUVmax, 6.07 vs. 2.84; P<0.001). In addition, Spearman's rank analysis indicated that SUVmax is positively correlated with GLUT-1 expression in pancreatic cancer tissues (r=0.6885; P<0.001; Fig. 3B).
Figure 3.
The association between GLUT-1 expression and SUVmax. (A) SUVmax was measured by 18F-fluorodeoxyglucose positron emission tomography/computed tomography. (B) The correlation between SUVmax and GLUT-1 expression. (C) The sensitivity and specificity in the detection of GLUT-1 strong expression according to the ROC curve. The arrow indicates the optimal sensitivity (88%) and specificity (71.4%) at a cut-off SUVmax value of 4.830. (D) Distribution of GLUT-1 expression according to the value of SUVmax. GLUT-1, glucose transporter-1; SUVmax, maximum standardized uptake value; ROC, receiver operating characteristic.
The sensitivity and specificity for the detection of GLUT-1 strong positive expression at different cutoff values of SUVmax in pancreatic cancer patients were determined according to the ROC curve (Fig. 3C). A cutoff SUVmax value of 4.830 exhibited the highest Youden's index (20) of 0.594, which was associated with optimal sensitivity (88%) and specificity (71.4%). The area under the ROC curve was 0.844 (95% confidence interval, 0.7405–0.9480; P<0.001). According to the cutoff value, the 53 pancreatic cancer patients were divided into two groups: high and low SUVmax groups. Among the 33 patients of the high SUVmax group, 69.7% (23/33) exhibited strong positive GLUT-1 expression, while the remaining 30.3% (10/33) of patients exhibited weak or moderate GLUT-1 expression. Of the 20 patients in the low SUVmax group, 10% (2/20) exhibited strong positive GLUT-1 expression, while 90% (18/20) exhibited weak or moderate GLUT-1 expression. (Fig. 3D).
Association between GLUT-1 expression and Ki-67
To clarify the association between GLUT-1 and cell proliferation, the correlation between GLUT-1 and Ki-67 expression was examined in pancreatic cancer tissues (Fig. 4). Positive Ki-67 expression was observed in 79.2% (42/53) of pancreatic cancer tissues and 22.7% (12/53) of adjacent non-tumorous tissues. Among the 53 tumor specimens, GLUT-1 expression was positively correlated with the Ki-67 expression (r=0.327; P=0.017; Table IV).
Figure 4.
Expression of Ki-67 in two groups of pancreatic cancer tissues with (A) GLUT-1 negative expression and (B) GLUT-1 positive expression (magnification, ×200). GLUT-1, glucose transporter-1.
Table IV.
Correlation between GLUT-1 and Ki-67 expression in pancreatic cancer patients.
| GLUT-1 expression | ||||
|---|---|---|---|---|
| Ki-67 expression | Positive, n | Negative, n | r | P-value |
| Positive, n | 34 | 8 | ||
| Negative, n | 5 | 6 | 0.327 | 0.017 |
GLUT-1, glucose transporter-1.
Discussion
In the present study, the expression of GLUT-1 was examined in 53 pairs of paraffin-embedded pancreatic cancer tissues. The results revealed that GLUT-1 was overexpressed in pancreatic cancer tissues and its expression positively correlated with increased tumor size, higher clinical stage and lymph node metastasis. Additionally, GLUT-1 was identified as an independent prognostic factor for pancreatic cancer.
GLUT-1, a member of GLUT family, facilitates the entry of glucose across the plasma membrane. A number of studies have demonstrated a close association between GLUT-1 expression and malignant mesothelium, which is relevant for the clinical behavior of the tumor (14,21,22). The results of the present study indicated that GLUT-1 was overexpressed in pancreatic cancer and was associated with clinicopathological characteristics, including tumor size, clinical stage and lymph node metastasis. In particular, the expression of GLUT-1 exhibited a significant effect on patient survival. Elevated GLUT-1 expression in tumor tissues reflects the requirement for a corresponding increase in glucose. Two possible mechanisms have been postulated to explain the overexpression of GLUT-1 in tumors. Firstly, local ischemia and hypoxia in the tumor may result in adaptive glycolytic metabolism and GLUT-1 expression (23). Secondly, GLUT-1 activity is widely upregulated via hypoxia-inducible factor-1 in hypoxic conditions (24,25).
Certain factors affect FDG uptake, including hypoxia, cell density and expression of glycolysis-associated proteins (26,27). In the present study, SUVmax was significantly associated with the intensity of GLUT-1 expression and low GLUT-1 expression also corresponded to a low SUVmax. This may indicate that the sensitivity of SUVmax for the pancreatic cancer patients with positive GLUT-1 expression is higher than those with negative expression. In ROC analysis, the positive and negative predictive values of SUVmax for identifying GLUT-1 strong expression were 69.7% (22/33) and 90% (18/20), respectively. We hypothesize that glucose consumption, as calculated by SUVmax using 18F-FDG/PET, predicted the level of GLUT-1 expression in pancreatic cancer patients. In addition, the cutoff value of SUVmax may aid in the selection of patients for more aggressive gene therapy, particularly for advanced pancreatic cancer that is not suitable for resection.
In general, hypoxia leads to reduced proliferation and increased apoptosis. However, certain cancer cells in the hypoxic environment undergo adaptive changes and produce energy via anaerobic glycolysis, enabling their survival and proliferation (28,29). Ki-67, a proliferation-related nuclear protein, is expressed in proliferating cells during all active phase of the cell cycle (30,31). The results of the present study revealed a positive correlation between GLUT-1 expression and Ki-67 expression. This indicates that proliferation and hypoxia are not exclusive, and that GLUT-1 may present a potential therapeutic target to limit glucose uptake, thereby limiting the proliferation of pancreatic cancer cells.
In conclusion, the present study demonstrated that the overexpression of GLUT-1 in pancreatic cancer tissues is significantly associated with the clinicopathological characteristics and prognosis of pancreatic cancer patients. In addition, the expression of GLUT-1 was positively associated with 18F-FDG uptake and cell proliferation in pancreatic cancer. These findings suggest that GLUT-1 may present an underlying prognostic indicator and a potential therapeutic target for pancreatic cancer.
Acknowledgements
The present study was supported by the Project of Nature Science Foundation of China (grant no. 81201905), the Nature Science Research Grants at the University of Jiangsu Province of P.R. China (grant no. 14KJB320019) and the Project of Medical Research of Jiangsu Province (grant no. Q201402).
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 3.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diener MK, Combs SE, Buchler MW. Chemoradiotherapy for locally advanced pancreatic cancer. Lancet Oncol. 2013;14:269–270. doi: 10.1016/S1470-2045(13)70091-3. [DOI] [PubMed] [Google Scholar]
- 5.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto S, Yasui H, Mitchell JB, Krishna MC. Imaging cycling tumor hypoxia. Cancer Res. 2010;70:10019–10023. doi: 10.1158/0008-5472.CAN-10-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milane L, Ganesh S, Shah S, Duan ZF, Amiji M. Multi-modal strategies for overcoming tumor drug resistance: Hypoxia, the Warburg effect, stem cells, and multifunctional nanotechnology. J Control Release. 2011;155:237–247. doi: 10.1016/j.jconrel.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010;298:E141–E145. doi: 10.1152/ajpendo.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adekola K, Rosen ST, Shanmugam M. Glucose transporters in cancer metabolism. Curr Opin Oncol. 2012;24:650–654. doi: 10.1097/CCO.0b013e328356da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szablewski L. Expression of glucose transporters in cancers. Biochim Biophys Acta. 2013;1835:164–169. doi: 10.1016/j.bbcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Krzeslak A, Wojcik-Krowiranda K, Forma E, Jozwiak P, Romanowicz H, Bienkiewicz A, Brys M. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol Oncol Res. 2012;18:721–728. doi: 10.1007/s12253-012-9500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wincewicz A, Sulkowska M, Koda M, Kanczuga-Koda L, Witkowska E, Sulkowski S. Significant coexpression of GLUT-1, Bcl-xL and Bax in colorectal cancer. Ann N Y Acad Sci. 2007;1095:53–61. doi: 10.1196/annals.1397.007. [DOI] [PubMed] [Google Scholar]
- 13.Chiba I, Ogawa K, Morioka T, Shimoji H, Sunagawa N, Iraha S, Nishimaki T, Yoshimi N, Murayama S. Clinical significance of GLUT-1 expression in patients with esophageal cancer treated with concurrent chemoradiotherapy. Oncol Lett. 2011;2:21–28. doi: 10.3892/ol.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohba S, Fujii H, Ito S, Fujimaki M, Matsumoto F, Furukawa M, Yokoyama J, Kusunoki T, Ikeda K, Hino O. Overexpression of GLUT-1 in the invasion front is associated with depth of oral squamous cell carcinoma and prognosis. J Oral Pathol Med. 2010;39:74–78. doi: 10.1111/j.1600-0714.2009.00814.x. [DOI] [PubMed] [Google Scholar]
- 15.Brophy S, Sheehan KM, McNamara DA, Deasy J, Bouchier-Hayes DJ, Kay EW. GLUT-1 expression and response to chemoradiotherapy in rectal cancer. Int J Cancer. 2009;125:2778–2782. doi: 10.1002/ijc.24693. [DOI] [PubMed] [Google Scholar]
- 16.Rastogi S, Banerjee S, Chellappan S, Simon GR. Glut-1 antibodies induce growth arrest and apoptosis in human cancer cell lines. Cancer Lett. 2007;257:244–251. doi: 10.1016/j.canlet.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 17.Alakus H, Batur M, Schmidt M, Drebber U, Baldus SE, Vallböhmer D, Prenzel KL, Metzger R, Bollschweiler E, Hölscher AH, Mönig SP. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun. 2010;31:532–538. doi: 10.1097/MNM.0b013e32833823ac. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds T. Declaration of Helsinki revised. J Natl Cancer Inst. 2000;92:1801–1803. doi: 10.1093/jnci/92.22.1801. [DOI] [PubMed] [Google Scholar]
- 20.Böhning D, Böhning W, Holling H. Revisiting Youden's index as a useful measure of the misclassification error in meta-analysis of diagnostic studies. Stat Methods Med Res. 2008;17:543–554. doi: 10.1177/0962280207081867. [DOI] [PubMed] [Google Scholar]
- 21.Cho H, Lee YS, Kim J, Chung JY, Kim JH. Overexpression of glucose transporter-1 (GLUT-1) predicts poor prognosis in epithelial ovarian cancer. Cancer Invest. 2013;31:607–615. doi: 10.3109/07357907.2013.849722. [DOI] [PubMed] [Google Scholar]
- 22.Sulkowska M, Wincewicz A, Sulkowski S, Koda M, Kanczuga-Koda L. Relations of TGF-beta1 with HIF-1 alpha, GLUT-1 and longer survival of colorectal cancer patients. Pathology. 2009;41:254–260. doi: 10.1080/00313020802579318. [DOI] [PubMed] [Google Scholar]
- 23.Mayer A, Schmidt M, Seeger A, Serras AF, Vaupel P, Schmidberger H. GLUT-1 expression is largely unrelated to both hypoxia and the Warburg phenotype in squamous cell carcinomas of the vulva. BMC Cancer. 2014;14:760. doi: 10.1186/1471-2407-14-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melstrom LG, Salabat MR, Ding XZ, Strouch MJ, Grippo PJ, Mirzoeva S, Pelling JC, Bentrem DJ. Apigenin down-regulates the hypoxia response genes: HIF-1α, GLUT-1 and VEGF in human pancreatic cancer cells. J Surg Res. 2011;167:173–181. doi: 10.1016/j.jss.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Fraga A, Ribeiro R, Medeiros R. Tumor hypoxia: The role of HIF. Actas Urol Esp. 2009;33:941–951. doi: 10.1016/S0210-4806(09)72891-8. [DOI] [PubMed] [Google Scholar]
- 26.Pugachev A, Ruan S, Carlin S, Larson SM, Campa J, Ling CC, Humm JL. Dependence of FDG uptake on tumor microenvironment. Int J Radiat Oncol Biol Phys. 2005;62:545–553. doi: 10.1016/j.ijrobp.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Huang T, Civelek AC, Li J, Jiang H, Ng CK, Postel GC, Shen B, Li XF. Tumor microenvironment-dependent 18F-FDG, 18F-fluorothymidine, and 18F-misonidazole uptake: A pilot study in mouse models of human non-small cell lung cancer. J Nucl Med. 2012;53:1262–1268. doi: 10.2967/jnumed.111.098087. [DOI] [PubMed] [Google Scholar]
- 28.Jiang J, Tang YL, Liang XH. EMT: A new vision of hypoxia promoting cancer progression. Cancer Biol Ther. 2011;11:714–723. doi: 10.4161/cbt.11.8.15274. [DOI] [PubMed] [Google Scholar]
- 29.Osinsky S, Zavelevich M, Vaupel P. Tumor hypoxia and malignant progression. Exp Oncol. 2009;31:80–86. [PubMed] [Google Scholar]
- 30.Lee HE, Kim MA, Lee BL, Kim WH. Low Ki-67 proliferation index is an indicator of poor prognosis in gastric cancer. J Surg Oncol. 2010;102:201–206. doi: 10.1002/jso.21583. [DOI] [PubMed] [Google Scholar]
- 31.Viale G, Giobbie-Hurder A, Regan MM, Coates AS, Mastropasqua MG, Dell'Orto P, Maiorano E, MacGrogan G, Braye SG, Ohlschlegel C, et al. Prognostic and predictive value of centrally reviewed Ki-67 labeling index in postmenopausal women with endocrine-responsive breast cancer: Results from breast international group trial 1–98 comparing adjuvant tamoxifen with letrozole. J Clin Oncol. 2008;26:5569–5575. doi: 10.1200/JCO.2008.17.0829. [DOI] [PMC free article] [PubMed] [Google Scholar]