Abstract
Background and Objectives:
Adhesion and colonization are prerequisites for the establishment of bacterial pathogenesis. The biofilm development of Pseudomonas aeruginosa was assessed on adhesive surfaces like dialysis membrane, stainless steel, glass and polystyrene.
Materials and Methods:
Microtiter plate biofilm assay was performed to assess the effect of nutrient medium and growth parameters of P. aeruginosa. Further, its growth on adhesive surfaces namely hydrophilic (dialysis membrane) and hydrophobic (polystyrene plate, square glass and stainless steel coupon) was assessed. The exopolysaccharide (EPS) was quantified using ruthenium red microplate assay and microscopic analysis was used to observe P. aeruginosa biofilm architecture. The anti-biofilm activity of herbal extracts on mature P. aeruginosa was performed.
Results:
The formation of large scale biofilms on dialysis membrane for 72 h was proved to be the best surface. In microscopic studies, very few exopolysaccaride fibrils, indicating a rather loose matrix was observed at 48 h. Further, thick exopolysaccaride, indicated higher adhesive properties at 72 h which is evident from ruthenium red staining. Among the plant extract used, Justicia wynaadensis leaf and Aristolochia indica (Eswari) root extract showed significant reduction of anti-biofilm activity of 0.178 OD and 0.192 OD in inhibiting mature biofilms at 0.225 OD respectively, suggesting the possible use of these extracts as efficient anti-adhesive and biofilm-disrupting agents with potential applications in controlling biofilms on surfaces.
Conclusion:
Our study facilitates better understanding in the development of P. aeruginosa biofilms on different food processing and clinical surfaces ultimately taking care of food safety and hygiene.
Keywords: Pseudomonas aeruginosa, Anti-biofilm, Exopolysaccaride, Anti-quromones
INTRODUCTION
The study of microbial development has shown that microorganisms are capable of complex differentiation and behaviors. Almost all bacterial species, under appropriate conditions, are able to adhere to various biotic (Plant and animal tissues) and abiotic (wooden log, plastic, glass, metal) surfaces (1), finally getting transformed from free-floating bacteria to sessile (non-motile) population of cells growing on a surface surrounded with an extracellular polysaccharide matrix called Biofilm (2). They are of major concern for clinicians in the treatment of infectious diseases because of their resistance to a wide range of antibiotics (3). In certain chronic bacterial diseases they are characterized by resistance to chemotherapy and resistance to clearance by humoral or cellular host defense mechanisms. Thus the above factors lead to serious hygienic problems in individuals and economic losses in food and medicinal industries (4).
One such opportunistic pathogen is Pseudomonas aeruginosa, a Gram-negative, rod shaped bacterium which is a natural habitant of soil environments and water reservoirs. This is because of their ability to colonize multiple environmental niches by using natural compounds as energy sources (5). Moreover, they have the large cascade of genes devoted to command and control systems such as transcriptional regulators which modulate biochemical abilities of this organism in changing environmental conditions and thus contribute to its resistance to antibiotics. It also encodes outer membrane proteins which are important in cell surface related properties such as adhesion and motility (6). Pseudomonas species are ubiquitous spoilage organisms found in food processing environments including drains and floors, on fruits, vegetables, meat surfaces and in low acid dairy products (4). In these industries they cause a reduction in the flow with consequent losses of capacity and product. In addition, biofilms formed on the metal surfaces offers a cross contamination, for these reasons food contact surfaces must be sanitized (7).
In order to improve the microbiological safety of food and hygiene, understanding the biofilm formation is utmost importance, in order to develop effective combative agents to be tested and discovered. In these lines of studies, we investigated the optimization of growth parameters (with different media supplements, temperature, incubation time and adhesive surfaces) for development of mature in vitro P. aeruginosa biofilms. In this study, all the parameters are designed with an intension to mimic the conditions which are widely used in food industries, so as to closely relate to the practical applications. Further, with a known fact of P. aeruginosa biofilm resistant to antimicrobial agents (8), we have also screened the anti-biofilm efficiency against P. aeruginosa biofilm of medicinal important herbal extracts (folklore literature) namely, Aristolochia indica (Eswari) root, Justicia wynaadensis (Maddhu thoppu) leaf, Gymnema sylvestre (Gurmar) leaf and Piper nigrum (White pepper) seeds of which till date as far our knowledge, the anti-biofilm activity of P. aeruginosa has not been investigated. The results of this study are known to facilitate in better understanding the development of P. aeruginosa biofilm on different food processing surfaces and associated pathogenesis, in order to improve the microbiological safety of food and hygiene, which will help in developing combating strategies and also on discovery of potent anti-biofilm drug targets (anti-quromones).
MATERIALS AND METHODS
Microorganism, media and culture conditions.
P. aeruginosa was collected from Microbial Type Culture Collection (MTCC) with the strain No. MTTC 6642. All microbiological medium were purchased from Himedia Pvt India Ltd, Mumbai and chemicals and solvents (analytical grade) were purchased from Merck (Merck Pvt India Ltd, Bangalore). For each experiment, the frozen stocks of strain were sub cultured on tryptic soy agar (TSA) for 24 h at 37 °C. The isolated colony was inoculated into tryptic soy broth (TSB) and incubated for 24 h at 37 °C in a shaker incubator (Genei, Bangalore). The cultures were centrifuged (6000 rpm at 4 °C for 10 min) and the pellets were washed twice in phosphate-buffered saline (PBS) and centrifuged (6000 rpm at 4 °C for 10 min). The cell suspensions were then standardized to an optical density (OD) 600 nm = 0.5 using a spectrophotometer (UV Spec 1800 Shimadzu, Japan).
Effect of nutrient medium on planktonic growth of P. aeruginosa.
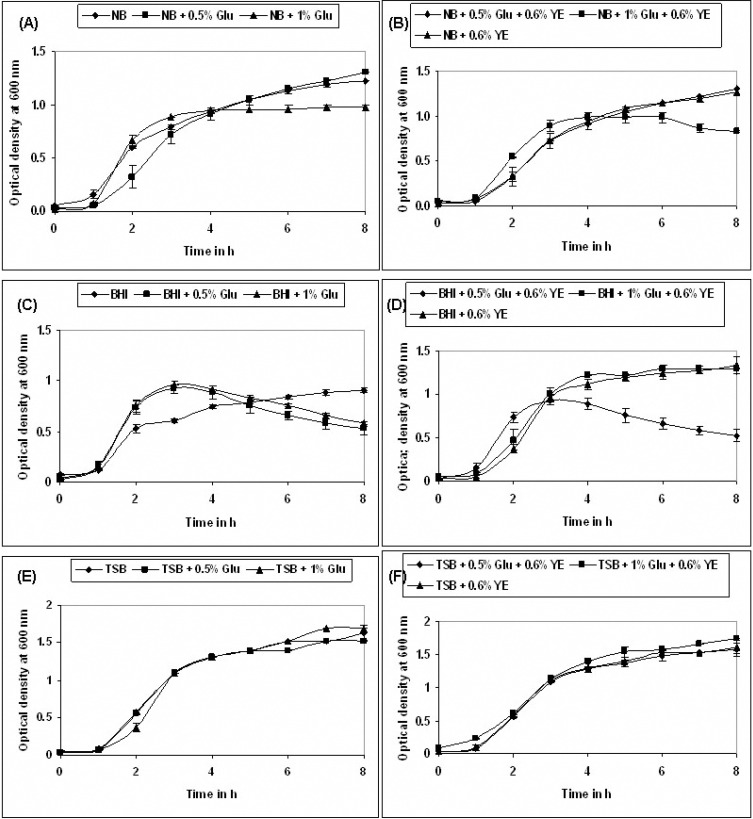
To study the effect of nutrients on the planktonic cell growth, an overnight pre-culture of the strain was prepared in 5 mL TSB incubated at 37 °C in an orbital shaker incubator at 190 rpm. The overnight pre-culture was centrifuged and the TSB media was decanted and the cells were re-suspended in 5 mL of PBS (1X) until the next transfer to the respective medium. The following medium namely Nutrient broth (NB), Brain Heart Infusion (BHI) broth and tryptic soy broth (TSB) were used individually and each were also supplemented with 0.5% glucose (Glu), 1% glucose and with 0.6% yeast extract (YE). The following is the sequence of different composition used for the study, NB, NB + 0.5% Glu, NB + 1.0% Glu, NB + 0.5% Glu + 0.6% YE, NB + 1.0% Glu + 0.6% YE, NB + 0.6% YE. BHI, BHI + 0.5% Glu, BHI + 1.0% Glu, BHI + 0.5% Glu + 0.6% YE, BHI + 1.0% Glu + 0.6% YE, BHI + 0.6% YE. TSB, TSB + 0.5% Glu, TSB + 1.0% Glu, TSB + 0.5% Glu + 0.6% YE, TSB + 1.0% Glu + 0.6% YE, TSB + 0.6% YE. The optical density (OD) was measured per every hour at 600 nm using a spectrophotometer and un-inoculated respective medium were used as the blank (1).
Cultivation of P. aeruginosa biofilms.
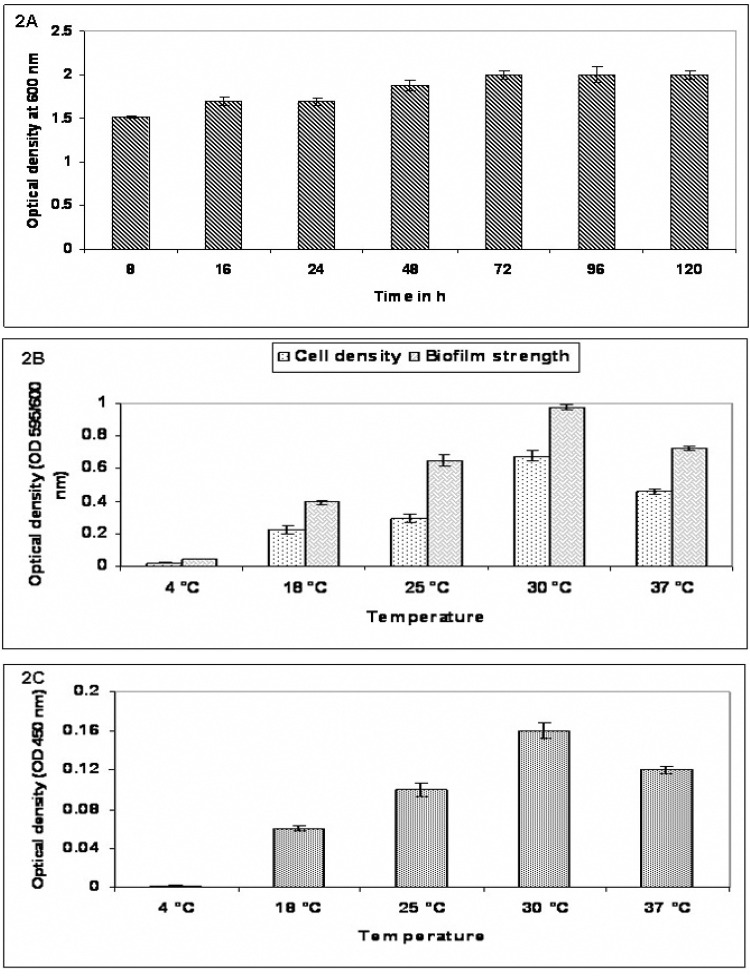
An overnight resuspended pre-culture was diluted 5000 folds (approximately 5 X 106 cfu/mL) with TSB + 1% Glu + 0.6% YE, which proved to be the best medium in planktonic conditions. Two hundred microlitres each of the suspension were added to eight wells of 96-well sterile polystyrene tissue culture (flat bottom) microtiter plates (Tarson, Bangalore) four wells with medium only served as blank. To determine the incubation duration, 2 set of plates were incubated for seven different time intervals (8 h, 16 h, 24 h, 48 h, 72 h, 96 h and 120 h) out of which 72 h proved to establish a mature biofilm. Further, this incubation time of 72 h was considered and another 2 set of plates were incubated at 5 different temperatures (4 °C, 18 °C, 25 °C, 30 °C and 37 °C) to determine the impact of temperature over biofilm formation. The optical density was read at 600 nm to determine growth cell density (CD) and biofilm strength (BS) at 595 nm using crystal violet staining, the exopolysaccharide (EPS) formed at different temperatures were quantified using ruthenium red staining at OD 450 nm using microplate reader (Systronics, Bangalore). Un-inoculated respective media were included as a control for both the experiments. The experiment was performed in duplicates in three independent sets. The mean + standard deviation of the three sets were considered (1).
Effect of adhesive surface on P. aeruginosa biofilms.
Two major kinds of adhesive surfaces were mainly used namely hydrophilic (dialysis membrane) and hydrophobic (polystyrene plate, square glass and stainless steel coupon) which would mimic industrial food processing and medical surfaces (1).
Preparation of hydrophilic test surface.
Dialysis membranes NadirR of 38 mm (Himedia Pvt India Ltd, Mumbai) were cut with the help of the fabricated tool into 7–8 cm diameter (the membrane should fit into 1 cm less than Petri plate diameter used). The dialysis membranes were activated using 2% Sodium bicarbonate and 1mM EDTA for 3 h at 60 °C. Upon activation the membranes were repeatedly washed using distilled water for at least 8–10 times (until the activation solution is drained off). The membranes were stored in Petri plates containing sterile distilled water at 4 °C until the next use. The membranes were sterilized by immersing them in 70% ethanol for about 1 h and dried in a laminar air flow hood before it was placed on the solid nutrient medium (TSA + 1.0% Glu + 0.6% YE) (1).
Preparation of hydrophobic test surface.
Polystyrene 96 well sterile tissue culture microtiter plates, square glass of 3 cm × 3 cm × 0.15 mm (Length × Breadth × Thickness) and circular stainless steel coupons of 5 cm diameter with 1.5 mm thickness were used to test the biofilms on the hydrophobic surfaces. All the surfaces were sterilized by incubating with 70% ethanol for 30 min. The ethanol was aseptically removed by pipetting and was air dried for 30 min at 37 °C. These sterilized materials were further used for the analyses (1).
Development of P. aeruginosa biofilms on different adsorbing surfaces.
P. aeruginosa were subjected to growth on two different adsorbing surface to check the ability of adherence on different inert support, namely a hydrophilic (dialysis membranes) and a hydrophobic (polystyrene plate, square glass and steel coupon) surfaces using liquid (TSB + 1% Glu + 0.6% YE) and solid medium (TSA + 1% Glu + 0.6% YE) for development of mature biofilm at 30 °C for 72 h. For all surfaces the activation/sterilization steps were followed as described above. The surfaces were laid for 12 h on the solid medium and were left at room temperature a process called as setting. Later 5000 times diluted (approximately 5 X 106 cfu/mL) sample was then inoculated on the respective surfaces under both solid and liquid conditions and the plates were incubated in an upright position for 72 h for 30 °C, which was proven to be best incubation time and temperature in this study. Upon incubation the dialysis membranes were fixed and processed for SEM studies. On the other hand polystyrene surface, square glass and steel coupon were then processed for crystal violet and ruthenium red staining to determine biofilm strength and EPS produced (1).
Microtiter plate biofilm assay.
P. aeruginosa pre-culture was prepared as described above. The assay method was adapted with the slight modification (9). The hydrophobic surfaces were sterilized. The pre-culture was diluted to 5000 times (approximately 5 X 106 cfu/mL) using respective freshly prepared media and vortexed for 5 seconds. For polystyrene surface, 200 μL of this dilution was then used to inoculate eight separate wells of a pre-sterilized polystyrene microtiter plate and eight wells of respective media were included as a control. To minimize evaporative loss and edge effects, the outermost rows and columns of each plate were filled with 150 μL of sterile water. The edges of the plate were then sealed with parafilm, and the plates were incubated for 72 h at different temperatures as mentioned above. Upon incubation the medium was decanted and unattached cells were removed by rinsing 3 times in 250 μL of 1 X PBS. Plates were then dried in an inverted position for 30 min at 42 °C. In case of square glass and steel coupon, inoculation was done as explained above; upon incubation unattached cells were removed by rinsing 3 times with 250 μL of isotonic PBS and dried for 30 min at 42 °C. Biofilm formed on these surfaces were scarped out (1cm × 1cm area) with the help of sterile blade and collected in a sterilized microfuge tubes. Biofilms were stained by adding 100 μL of aqueous 1% crystal violet solution to each well and were further incubated for 30 min at room temperature. Unbound dye was removed by rinsing 3 times in 250 μL of sterile water. The crystal violet was solubilized by adding 220 μL of 95% ethanol as de-staining solution and incubated at 4 °C for 45 min. The contents of each well (100 μL) were then transferred to a fresh sterile polystyrene microtiter plate and the optical density was read at 595 nm using a microplate reader. The readings were recorded and the graph is plotted. For comparable results, the experiment was performed in triplicates and the same was repeated for different medium and on different temperatures in three independent experiments, for the statistical correlation and interpretation of the results (1).
Quantification of exopolysaccharide (EPS) by ruthenium red microplate assay.
An overnight culture was prepared as described above. Cells (200 μL) were then transferred to seven wells of a pre-sterilized polystyrene microtiter plate and 100 μL of sterile medium was added to the outer well of each row of the microtiter plate as a blank. The plates were incubated for 72 h at different temperatures (4 °C, 18 °C, 25 °C, 30 °C and 37 °C); unattached cells were removed aseptically by pipetting and decanting. In case of square glass and steel coupon, inoculation was done as explained above. Briefly, an aqueous solution of ruthenium red (0.1 %, 200 μL) was added to each well of the plate and biofilms were stained for 45 min at room temperature. The liquid from each well was then carefully transferred to a fresh polystyrene microplate and the optical density was measured at 450 nm using microplate reader. The amount of dye bound by the biofilm in each well was determined by subtracting the OD 450 of the well from the average of the blank wells (1).
Compound microscopy (CM).
A widely accepted method for the preservation and preparation of biological samples for microscopy was used to observe P. aeruginosa biofilm architecture (10) with some modifications. Biofilms were cultivated on dialysis membrane as described above. After incubation the membranes were processed for CM. Upon removal from the culture, membranes were rinsed by gentle repeated immersion in 15–20 mL sterile PBS for 15 – 30 s. Membranes were then placed in petriplates with 6.25 % glutaraldehyde in Sorenson buffer, pH 7.4 and fixed at 4 °C overnight. The fixing solution was drained and membranes were rinsed in excess of buffer. Images were captured at low 5× and high 100× magnification to observe biofilm before and after treatment with plant extracts.
Anti-biofilm activity of mature P. aeruginosa using herbal extracts: Selection and preparation of plant material.
Four herbs of commercial importance were collected during June-July 2012 from in and around Mysore District, Karnataka, India. The herbs were identified using expertise botanist from DOS in Botany, University of Mysore, Mysore, Karnataka and the same is deposited as the voucher specimen. These herbs were selected on the basis of their commercial importance as most of them are used in herb drug preparation in Ayurveda, due to their health benefits. The herbs used and their commercial importance are presented in Table 1. The fresh samples were initially washed, air-dried at room temperature and ground to a fine powder (approximately 60 mesh size) in a blender. Dichloro-methane/methanol (CH2CI2/MeOH, 1:1) extracts of the powdered herbs were prepared and were estimated for total phenolic content (11). Briefly, the total phenolic content of each of the herbal extracts was determined colorimetrically using the Folin–Ciocalteu method, a sample aliquot of 100 μL was added to 900 μL of water, 5 mL of 0.2 N Folin Ciocalteu re-agent and 4 mL of saturated sodium carbonate solution (100 g/L). The absorbance was measured at 765 nm after incubation for 2 h at room temperature. The total phenolic content was expressed as gallic acid equivalent (GAE) in milligrams per gram sample.
Table 1.
A brief description of the selected herbs for this study.
| Sl. No | Name of the herb & family | Compounds isolated from previous studies | Commercial importance |
|---|---|---|---|
| 1. | Aristolochia indica & Aristolochiaceae | Ishwarane, Aristolochene, Ishwarol, Sesquiterpene, Aristolochine alkine, Isoaristolochic acid, Allatonine, Aristolide, Cephadione and Aristolindiquinone | Antibacterial, Antifungal, Antidot, Antioxidant, Antispermatogenic, Anti-oestrogenic and Potent abortifacient |
| 2. | Justicia wynaadensis | Crystalline alkaloids and flavonoids | Antibacterial, Antifungal and Antioxidant |
| 3. | Gymnema sylvestre & Asclepiadaceae | Triterpene saponins, Gymnemic acids and Gymnemasaponins, Flavones, Anthraquinones, Hentri-acontane, pentatriacontane, α and β-chlorophylls, Phytin, Resins, d-quercitol, Tartaric acid, Formic acid, Butyric acid, Lupeol, β-Amyrin related glycosides and Stigmasterol. | Antibacterial, antifungal, Antioxidant, Antidiabetes, Anti effect on digestion, Urinary tract problems, Obesity, Hypoglycemia, Allergies, Anemia, Cholesterol, Hyperactivity, Antisweetener and Anti-inflammatory activities |
| 4. | Piper nigrum & Piperaceae | Volatile oil, Crystalline alkaloids, Piperine, Piperidine, Piperettine. Resin, Piperitine, Piperolein A, Piperolein B, Piperanin and Trichostachine | Antibacterial, Antifungal, Antioxidant, Aromatic stimulant, Stomachic in dyspepsia and Flatulence, Antiperiodic in malarial fever, Paraplegia and Arthritics, Anti-allergic, Phellandrene, Wisanine, dipentene and Sesquiterpenes |
Minimum inhibitory concentrations.
To confirm the antimicrobial activity of the selected herbs, the extracts were initially tested on planktonic P. aeruginosa culture using the modified p-iodonitrotetrazolium violet (INT) microplate, minimum inhibitory concentration (MIC) assay (12). The extracts were dissolved in sterile water and tested using the same range of concentrations (0.05–10 mg/mL). The positive control for P. aeruginosa was ciprofloxacin. The negative control was distilled water. The MIC procedure was carried out by aliquoting one hundred microlitres (100 μL) of sterile distilled water into all the wells of the microtitre plate. The prepared extracts were then pipetted into the first well in each column in triplicate. Serial dilutions were performed in decreasing concentrations down the columns. Following the serial dilutions, 100 μL of the standard culture (1.0×106 cfu/mL) was added to all the wells. The plates were sealed with sterile adhesive tape and incubated at 37 °C for 24 h for P. aeruginosa. Following incubation, the MIC assay was determined. To visualize the bacterial growth, 40 μL of INT (0.04 mg/mL) was added to each well and the plates incubated at room temperature for 6 h. The plates were then examined for colour changes and the MIC was indicated by the first clear well (lowest concentration having no microbial growth) in a column.
Inhibition of biofilm formation.
To prevent initial cell attachment, 100 μL of plant extracts were aliquoted into wells of a 96 well microtitre plate. Standardized cultures (1.0×106 cfu/mL) of P. aeruginosa was then added (100 μL) into the wells and incubated for 4 h at 30 °C without shaking. The final concentrations of the extracts ranging from 10 to 40 mg/mL were used, while ciprofloxacin at a concentration of 0.0025 mg/mL (MIC value) was used as the positive control for P. aeruginosa. Distilled water was used as the negative control. Following incubation, the biofilm biomass was assayed using above mentioned crystal violet (CV) staining assay and percentage inhibition determined using the following equation (9).
Inhibition of preformed biofilms.
To test the ability of the extracts to prevent biofilm development, biofilms were pre-formed in 96 well microtitre plates by aliquoting 100 μL of standardized P. aeruginosa (1.0×106 cfu/mL) into the wells and incubated for 4 h at 37 °C. Following incubation, 100 μL of each of the plant extract that showed some degree of inhibiting cell attachment was added to a final concentration of 10 to 40 mg/mL in the wells. Ciprofloxacin at a concentration of 0.0025 mg/mL (MIC value) was used as the positive controls. Distilled water was used as the negative control. The plates were further incubated for 72 h at 30 °C. Following incubation with the extracts, the crystal violet assay was performed to assay for biofilm biomass as described above.
Statistical analysis.
All experiments were carried out in triplicates and repeated in three independent sets of experiments. Data were shown as mean + standard deviation (SD). SPSS 10.0.5 version for windows (SPSS software Inc., USA) computer programme was used for statistical analysis. The significance of differences in biofilm formation was assessed by ANOVA, followed by Post hoc comparison test. Correlations between quantitative properties were evaluated by calculating the Duncan and Dunnett’s coefficient. Statistical significance value set at P<0.05, n=3.
RESULTS
Growth of P. aeruginosa in different media with respect to time and temperature.
The bacterial growth at 37 °C was monitored for 8 h in media with different composition, as described above. The TSB + 1.0% Glu + 0.6% YE yielded a significant growth in bacterial counts, hence were selected for our further experiments (Fig. 1). P. aeruginosa with TSB + 1.0% Glu + 0.6% YE was incubated at different temperature (4 °C, 18 °C, 25 °C, 30 °C, 37 °C) and monitored for 8 h, It was observed that, growth was slow at 25 °C, optimal at 30–37 °C, and there was no growth was noted at 4 °C (Fig. 2A). In further experiment, it was observed that P. aeruginosa, grew rapidly and multiplied depending on the nutritional supplement and reached stationary phase at 72 h (Fig. 2B).
Fig. 1.
Growth curve of P. aeruginosa placktonic culture in TSB, TSB + 0.5% Glu, TSB + 1.0% Glu, TSB + 0.5% Glu + 0.6 YE, TSB + 1.0% Glu + 0.6% YE, TSB + 0.6% YE as a medium at 37 °C. Values represent mean ± SD (n=6).
Fig. 2.
(2A) Assessment of P. aeruginosa biofilm at different incubation periods; (2B) Evaluation of Cell density and Biofilm strength at different temperatures; (2C) Exopolysaccaride formed at different temperatures. Values represent mean ± SD (n=6).
Biofilm formation with respect to adhesive capacity on different surfaces.
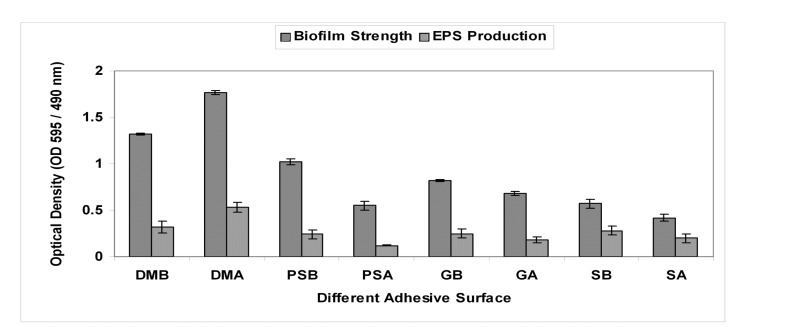
Biofilm formation of P. aeruginosa on steel surface, glass, polystyrene, with TSB + 1% Glu + 0.6% YE, TSB + 1% Glu + 0.6% YE + agar as a medium was quantified after incubation for 72 h at 30 °C respectively. Fig. 3. shows EPS strength (OD 450 nm) and biofilm strength (OD 595 nm) on Dialysis membrane on Broth (DMB) and agar (DMA), polystyrene on broth surfaces (PSB) and agar (PSA), glass on broth (GB), glass on agar surfaces (GA), steel on broth (SB) and steel on agar surfaces (SA) respectively after incubation period of 72 h. After incubation time both the number of adherent cell (OD 595 nm) and EPS strength (OD 450 nm) were highest on polystyrene when compared to glass and steel surfaces, which had high biofilm formation.
Fig. 3.
Biofilm strength and EPS strength on Dialysis membrane on Broth (DMB) and agar (DMA), polystyrene on broth surfaces (PSB) and agar (PSA), glass on broth (GB), glass on agar surfaces (GA), steel on broth (SB) and steel on agar surfaces (SA) respectively after incubation period of 72 h. Values represent mean ± SD (n=6).
Microscopic analysis of P. aeruginosa biofilm grown on cellulose dialysis membrane.
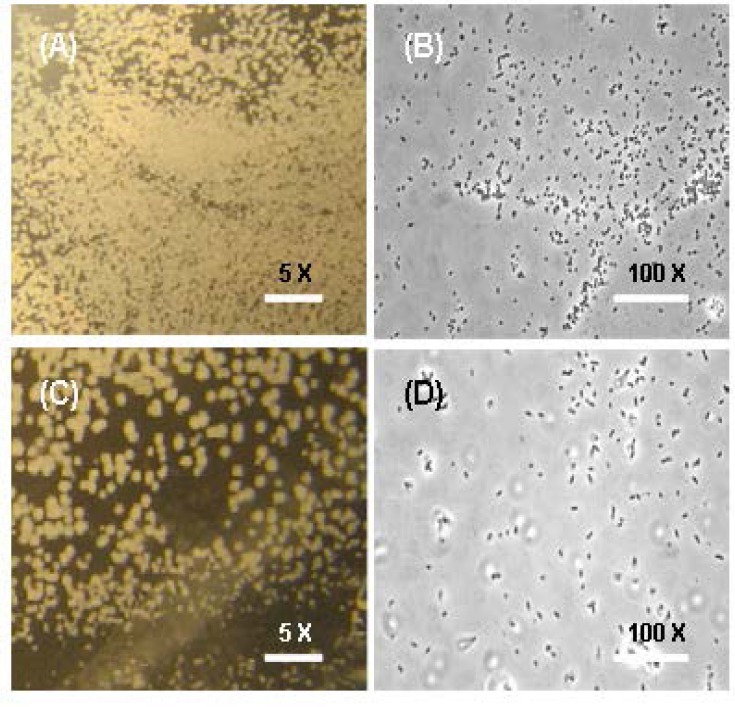
In order to see the dependency of incubation time on biofilm formation on hydrophilic surface, mainly dialysis membrane were used. The medium were maintained in solid condition by supplementing 15% per litre of the TSB + 1% Glu + 0.6% YE (TSA), which would mimic industrial surfaces, sheets of cellulose dialysis membrane, which were inoculated with highly diluted bacterial suspensions and incubated on TSA for 72 h at a temperature of 30 °C. The membranes were inspected by compound microscopy for bacterial growth and extracellular polymer formation which is been depicted in Fig. 4.
Fig. 4.
Compound Microscopy (CM) of P. aeruginosa biofilm formed on cellulose dialysis membranes (DM) on TSB supplemented with 1% glucose and 0.6% yeast extract grown at 30°C with magnification of 5 × and 100 × before (A, B) and after treatment (C, D) with J. wynaadensis respectively.
Analysis of antibiofilm activity by plant extracts.
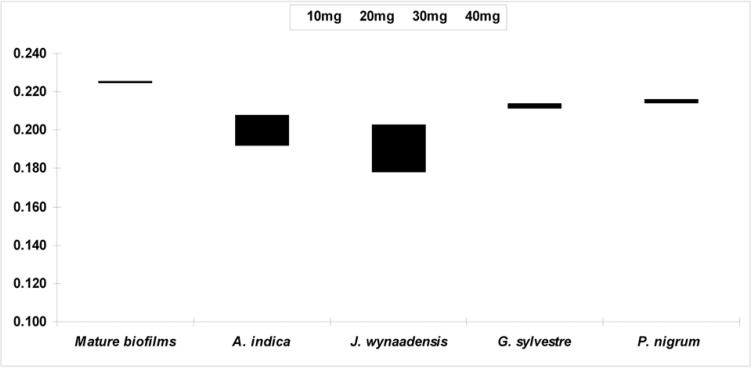
The extracts were prepared and tested for antibacterial activity, Justicia wynaadensis leaf (MIC 5.2 mg/mL) and Aristolochia indica (Eswari) root (MIC 8.7 mg/mL) extracts showed significant antibacterial activity against P. aeruginosa than compared to White piper nigrum (Pepper) and Gymnemas sylvestre (Gurmar) leaf both more than 10 mg/mL respectively and hence considered practically insignificant. Further, when tested Justicia wynaadensis leaves extract showed best anti-biofilm activity, when compared to other extracts (Fig. 5) with 0.225 OD for mature biofilms reduced to 0.178 OD by J. wynaadensis and 0.192 OD by A. indica respectively.
Fig. 5.
Graphical representation of Antibiofilm activity by Justicia wynaadensis leaf, Aristolochia indica (Eswari) root extract and other extracts compared to normal biofilm growth. Values represent mean ± SD (n=6).
DISCUSSION
Pseudomonas aeruginosa is a notorious opportunistic, nosocomial and metabolic versatility pathogenic bacterium and is responsible for serious health complications. P. aeruginosa can colonize on various surfaces by forming a biofilm in which bacterial cells stick together and are embedded within a self-produced extracellular polysaccharide matrix (2, 3). Biofilm cells of P. aeruginosa are reported to be more resistant to antibiotics and biocides than planktonic cells, which often cause difficulties in eradicating them from individuals infected with the bacterium (4). A means to control biofilm growth to more effectively treat P. aeruginosa infections is thus needed, which requires the better understanding of the growth patterns of the bacterium in free-floating and sessile form. According to the United States Pharmacopeial Convention, Tryptic Soy broth yields good growth of P. aeruginosa. It contains enzymatic digest of casein, enzymatic digest of soybean meal, sodium chloride, di-potassium phosphate and dextrose. In addition, composition like glucose and yeast extract induces the excellent growth of the bacterium. The results from physic-chemical parameters indicate that, TSB + 1.0% Glu + 0.6% YE yielded a significant growth in vitro, as these mesophiles have been adapted to thrive in temperature close to that of their host, depending on nutrient supplement they grow robustly. It is usually observed that mesophilic bacterium prefer to grow at normal human body temperature, thus facilitating pathogenesis (1).
Depending on the suitable surface, previous reports (13), observed a less number of cells in smooth steel and polystyrene surfaces, however they did find rougher matt had 1.4 times more microorganism than smooth surface, because rough surface have more surface area and provide more shielding from smooth surface promoting growth of the bacterium. A number of workers have examined the adherence of Pseudomonas species to substrata, such as polystyrene (14), glass (15) and stainless steel (16). It is usually found that bacteria prefer to attach to low-energy hydrophobic surfaces such as polystyrene rather than to high-energy, hydrophilic surfaces such as glass. Adhesion and colonization are prerequisites for the establishment of bacterial pathogenesis. The prevention of adhesion is an attractive target for the development of new therapies in the prevention of infection. Bacteria have developed a multiplicity of adhesion mechanisms, but our ability to rationally design effective anti-adhesives is critically affected by the limitations of our knowledge of the bacterial function in relation to different surfaces. The potential for the future development of phytochemical-based anti-adhesives have to be demonstrated by a significant number of in vitro and in vivo studies. At 30 °C multi-layered aggregates of bacteria connected by a loose mesh of fibrils were observed. Micrograph shows an overview of a homogenous film of P. aeruginosa biofilm at magnification (100 x). There was a significant difference in the architecture of the aggregates during adhesion to the membrane before and after treatment of the plant extracts. Similar observations have been described in previous studies (1, 17).
Further, to test the antibacterial and antibiofilm efficacy, the extracts of Justicia wynaadensis leaf, Aristolochia indica (Eswari) root, White piper nigrum (Pepper) seed and Gymnemas sylvestre (Gurmar) leaf were prepared. Justicia wynaadensis leaf extract exhibited significant activity indicating, P. aeruginosa biofilms can be combated with naturally available plant extracts which will probably affect the bacterial cell membrane or/both exopolymeric substances as depicted in Fig. 5, thus exemplifying the fact that the pathogenesis and associated biofilm formation can be minimized (bacteriostatic) and/or avoided (bactericidal) with phytochemical principles. However, our results correspond many similar studies on P. aeruginosa biofilms (18–21), suggesting the difficultly in completely inhibiting the biofilms formation than compared to planktonic forms of the same strains/species, hence making the study more complicated and repeated microbial relapse makes the problem much worse. In summary, our data confirms that the P. aeruginosa is very well developed in Tryptic Soy broth with glucose and yeast extract as an additional constituent with incubation period 72 h at 30 – 37 °C. Further, P. aeruginosa is developed in food processing equipments like steel, glass and polystyrene (similar properties like plastic). Our study most importantly shows that, dense biofilms were formed in hydrophilic cellulose dialysis membrane which is helpful to analyze the biofilms formation. Anti-biofilm activity against P. aeruginosa is potentially inhibited by Justicia wynaadensis followed by Aristolochia indica extracts. Finally to conclude, our study helps in better understanding of P. aeruginosa biofilm formation and associated pathogenesis, which can be helpful in understanding the development on food processing surfaces. Further, the plant extract will be explored for purified molecules which can exhibit anti-quromone activity (20, 21), so as to be used as a potent sanitizer against the P. aeruginosa infection and other similar related organisms, ultimately taking care of food safety and hygiene.
ACKNOWLEDGEMENTS
The authors greatly acknowledge The President (Sri. Vasudeva Murthy) and Management of Mahajana Education Society (MES), The Principal (Prof. K.V. Prabhakara), Director (Prof. C.K. Renukarya), PBMM Post-Graduate Centre Mysore, India, for providing the infrastructure and funds to execute this research. FZ sincerely thank Dr. Shubha Gopal, Associate Professor, Department of Studies in Microbiology, University of Mysore, Mysore and Prof. Juergen Kreft, Professor-Extraordinary, University of Wuerzburg, Biozentrum, Germany for introducing the subject and for their mentorship. DBL thank Jain University for the constant encouragement and support given to progress in research.
REFERENCES
- 1. Zameer F, Gopal S, Krohne G, Kreft J. Development of a biofilm model for Listeria monocytogenes. World J Microbiol Biotechnol 2009; 26: 1143– 1147. [Google Scholar]
- 2. Smith AW. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv Drug Deliv Rev 2005; 57: 1539– 1550. [DOI] [PubMed] [Google Scholar]
- 3. Drenkard E, Ausubel FM. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 2002; 416: 740– 743. [DOI] [PubMed] [Google Scholar]
- 4. Chmielewsky RAN, Frank JF. Biofilm formation and control in food processing facilities. Compr Rev Food Sci Food Saf 2003; 2: 22– 32. [DOI] [PubMed] [Google Scholar]
- 5. Lyczak JB, Cannon CL, Pier GB. Establisment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect 2000; 2: 1051– 1060. [DOI] [PubMed] [Google Scholar]
- 6. Stover C K, Pham XQ, Erwin AL. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000; 406: 959– 964. [DOI] [PubMed] [Google Scholar]
- 7. Van Houdt R, Michiels CW. Biofilm formation and the food industry, a focus on the bacterial outer surface. J Appl Microbiol 2010; 109: 1117– 31. [DOI] [PubMed] [Google Scholar]
- 8. Parsek MR, Fuqua C. Biofilms 2003: emerging themes and challenges in studies of surface-associated microbial life. J Bacteriol 2004; 186: 4427– 4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Djordjevic D, Wiedmann M, McLandsborough LA. Microtiter plate assay for assessment of Listeria monocytogenes biofilm formation. Appl Environ Microbiol 2002; 68: 2950– 2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kalmokoff ML, Austin JW, Wan XD, Sanders G, Banerjee S, Farber JM. Adsorption, attachment and biofilm formation among isolates of Listeria monocytogenes using model conditions. J Appl Microbiol 2001; 91: 725– 734. [DOI] [PubMed] [Google Scholar]
- 11. Meghashri S, Vijay Kumar H, Gopal S. Antioxidant properties of a novel flavonoid from leaves of L. aspera. Food Chem 2010; 122: 105– 110. [Google Scholar]
- 12. Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 1998; 64: 711– 3. [DOI] [PubMed] [Google Scholar]
- 13. Vanhaecke E, Remon JP, Moors M, Raes F, De Rudder D, Van Peteghem A. kinetics of Pseudomonas aeruginosa adhesion to 304 and 316-L stainless steel: role of cell surface hydrophobicity. Appl Environ Microbiol 1990; 56: 788– 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fletcher M, Marshall KC. Bubble contact angle method for evaluating substratum interfacial characteristics and its relevance to bacterial attachment. Appl Environ Microbiol 1982; 44: 184– 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marshall KC, Stout R, Mitchell R. Mechanism of the initial events in the sorption of marine bacteria to surfaces. J Gen Microbiol 1971; 68: 337– 348. [Google Scholar]
- 16. Stanley PM. Factors affecting the irreversible attachment of Pseudomonas aeruginosa to stainless steel. Can J Microbiol 1983; 29: 1493– 1499. [DOI] [PubMed] [Google Scholar]
- 17. Zameer F, Kreft J, Gopal S. Interaction of the dual species biofilms of Listeria monocytogenes and Staphylococcus epidermidis. J Food Saf 2010; 30: 954– 968. [Google Scholar]
- 18. Borges A, Saavedra MJ, Simões M. The activity of ferulic and gallic acids in biofilm prevention and control of pathogenic bacteria. Biofouling 2012; 28: 755– 767. [DOI] [PubMed] [Google Scholar]
- 19. Plyuta V, Zaitseva J, Lobakova E, Zagoskina N, Kuznetsov A, Khmel I. Effect of plant phenolic compounds on biofilm formation by Pseudomonas aeruginosa. APMIS 2013; 121: 1073– 1081. [DOI] [PubMed] [Google Scholar]
- 20. Kim Y, Lee J, Kim S, Baek K, Lee J. Cinnamon bark oil and its components inhibit biofilm formation and toxin production. Int J Food Microbiol 2015. 16; 195C: 30– 39. [DOI] [PubMed] [Google Scholar]
- 21. Kim HS, Lee SH, Byun Y, Park HD. 6-Gingerol reduces Pseudomonas aeruginosa biofilm formation and virulence via quorum sensing inhibition. Sci Rep 2015; 5: 8656– 8667. [DOI] [PMC free article] [PubMed] [Google Scholar]