Abstract
Background and Objectives:
Bacterial resistance to conventional antibiotics has become a widespread public health problem. The aim of this study was to investigate the influence of zinc oxide nanoparticles (ZnO NPs) on the antibacterial activity of several conventional antibiotics against Pseudomonas aeruginosa.
Materials and Methods:
ZnO NPs were prepared by solvothermal method and dispersed in glycerol with the help of ammonium citrate as a dispersant. The antibacterial effects of the resulting ZnO nanofluid, ceftazidime, tobramycin, and ciprofloxacin were investigated against two P. aeruginosa strains, including one clinical isolate and P. aeruginosa ATCC 9027 using microdilution method. For the evaluation of the combined effect of ZnO nanofluid and antibiotics, the fractional inhibitory concentration indices were calculated and isobolograms were plotted.
Results:
Clinical strain in comparison to standard strain of P. aeruginosa showed more resistance to ZnO nanofluid and the antibiotics. ZnO nanofluid acted synergistically with ceftazidime and tobramycin against both strains. Combination of ZnO nanofluid and ciprofloxacin displayed synergistic and partial synergistic activity against clinical and standard strains of P. aeruginosa, respectively.
Conclusion:
The results suggest that bacterial resistance to antimicrobials could be reduced by the synergistic action of ZnO NPs.
Keywords: Ceftazidime, Ciprofloxacin, Pseudomonas aeruginosa, Tobramycin, ZnO nanoparticles
INTRODUCTION
Pseudomonas aeruginosa is a frequent cause of nosocomial pneumonia, hospital-acquired urinary tract infections, wound infections, and blood stream infections (1, 2). P. aeruginosa is also responsible for opportunistic infections in immunocompromised patients (1). Three major classes of antibiotics are commonly used against this pathogen, including amino-glycosides (tobramycin), β-lactams (ceftazidime), and quinolones (ciprofloxacin) (3). Quinolones and β-lactams inhibit DNA gyrase and cell wall peptidoglycan-assembling transpeptidases, respectively, while aminoglycosides inhibit protein synthesis by binding to the 16S rRNA within the 30S ribosomal subunit (4–6). P. aeruginosa exhibits intrinsic resistance to many antibacterial agents, and moreover tends to acquire additional resistance during therapy (7). There are several mechanisms for antibiotic resistance, which include low intrinsic cell wall permeability, efflux systems, inactivation and modification of antibiotics, and changes in targets of antibiotics (4). A common approach to overcome antibiotic resistance is to use other compounds in combination with antibiotics (7).
Over the past decade, some of the nanometersized metal oxides were found to be cytotoxic against bacteria (12). Antibacterial effects of zinc oxide nanoparticles (ZnO NPs) on a large number of micro-organisms have been reported to be size-dependent (13–16). ZnO NPs are biosafe up to a certain amount, but may be hazardous at higher concentrations. Reddy et al. found no reduction in cell viability of human T cells following exposure to ZnO NPs at concentrations below 5 mM (17). Yang et al. also reported no hemolysis of red blood cells exposed to ZnO quantum dots in concentration above 1600 μg/ml (18). It is at present difficult to determine the threshold limits for various forms of ZnO due to a lack of sufficient data. The antibacterial mechanisms of ZnO NPs include binding to and damaging the bacterial membrane, penetrating into the bacterial, and generating reactive oxygen species (ROS) (19, 20).
In the present study, we investigated the influence of ZnO NPs on the antibacterial activity of tobramycin, ceftazidime, and ciprofloxacin against P. aeruginosa. The combination effects between ZnO nano-fluid and the antibiotics under investigation were also evaluated.
MATERIALS AND METHODS
The studies were performed on a standard strain of P. aeruginosa (ATCC 9027) and a clinical isolate of this bacterium (kindly supplied by Faculty of Veterinary Medicine, Ferdowsi University of Mashhad). Ceftazidime, tobramycin, and ciprofloxacin were purchased from Sigma.
Preparation and characterization of ZnO NPs.
ZnO NPs were synthesized by dissolving 0.001 mol of zinc acetate dehydrate in 920 ml of deionized water. Then, 80 ml of 0.02 M sodium hydroxide was added to the solution drop-wise to zinc acetate dehydrate solution under magnetic stirring at 0 °C. The formed transparent Zn(OH)42− solution was incubated into a water bath at 65 °C for 2 h and at room temperature for 3 days. Afterwards, ZnO NPs were separated from the suspension by centrifugation, washed several times by deionized water and ethanol and finally dried in a vacuum oven at 40 °C for 10 h (21). The X-ray diffraction (XRD) pattern of ZnO NPs was recorded using D8 Advance diffractometer (Bruker) with Cu Kα radiation (λ= 0.15406 nm). The average crystallite size can be calculated using Debye Scherrer equation:
where Dhkl is the crystallite size perpendicular to the normal line of (hkl) plane, k is a constant (0.9), λ is the wavelength of X-ray, βhkl is the full width at half maximum of the (hkl) diffraction peak and θhkl is the Bragg angle of (hkl) peak.
Preparation of stable ZnO nanofluid.
ZnO nano-fluid was prepared by dispersing ZnO NPs in glycerol as the base fluid with ammonium citrate as dispersant. The weight ratio of nanoparticles to ammonium citrate was kept 1:1 (21). The ZnO suspension was continuously stirred for a few hours to give a stable suspension with uniform dispersion of ZnO NPs. The particle size distribution (PDS) of the prepared ZnO NPs was examined by dynamic light scattering (DLS) from Malvern Zetasizer Nano-ZS instrument.
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) determination.
MIC was expressed as the lowest concentration of antibacterial agent that caused complete inhibition of growth during 24 hours (22). The MIC of ZnO nanofluid, ceftazidime, tobramycin, and ciprofloxacin were obtained by the broth microdilution method in a microplate. Serial two-fold dilutions of ZnO nanofluid and all the antibiotics with the following concentrations: ZnO nanofluid (5.8–375 μg/ml), ceftazidime (0.25–32 μg/ml), tobramycin (0.25–16 μg/ml), and ciprofloxacin (0.25–16 μg/ml) were prepared with nutrient broth medium (23, 24). A suspension of exponentially growing bacteria was added to each medium, to attain a final bacterial concentration of 105 CFU/ml. Samples were placed in 96-well microtiter plates and incubated for 24 hours at 37 °C with shaking about 120 rpm. Bacterial growth was monitored every 2 h by measuring the absorbance at 630 nm (OD630) until 24 h. The medium without antibacterial agents as positive control and medium without bacteria as negative control were included for each assay. For evaluation of antibacterial activity of ZnO NPs, antibacterial effect of glycerol and ammonium citrate was also assayed and considered as control. The antibacterial activity of the base fluid and dispersant was excluded from the antibacterial activity of ZnO nanofluid.
The MBC of ZnO nanofluid and the antibiotics were also determined. Briefly, 10 μl aliquots of each above sample, demonstrating no visible growth, were plated on culture agar plates. After 24 hours incubation at 37 °C, colony formation was investigated. MBC was defined as the lowest concentration of antibacterial agent that kills more than 99% of the bacteria (22).
After determining the MIC of ZnO NPs and antibiotics alone, we examined combinations of ZnO NPs with ceftazidime, tobramycin, and ciprofloxacin at subinhibitory concentrations. The following concentrations were tested: 1/16 × MIC – 1/2 × MIC for ZnO NPs and 1/8 × MIC – 1/2 × MIC for antibiotics (25, 26). The percentage of growth inhibition for both microorganisms in comparison with positive controls was determined using Eq. 1:
To avoid potential optical interference of the growing cultures caused by the light-scattering properties of the NPs, the same liquid medium without bacteria, but containing the same concentration of NPs was incubated under the same conditions and used as the blank control.
Fractional inhibitory concentration index (FICI) determination and isobologram analysis.
The combined action of ZnO nanofluid and each of the antibiotics was studied by checkerboard broth microdilution method (11). The MIC of antibiotics and ZnO nanofluid in combination was determined and the fractional inhibitory concentration index (FICI) was calculated using the following formula:
where MICA combination is the MIC of drug A in the presence of drug B and vice versa for MICB combination; MICA and MICB are MIC of A and B drugs alone, respectively. FICI was interpreted as follow: FICI ≤ 0.5 synergy, FICI > 0.5 and FICI < 1 partial synergy, FICI = 1 additive, FICI ≥ 2 and FICI < 4 indifferent, and FICI > 4 antagonism (3, 10). Moreover, the combination effect of ZnO nanofluid with the antibiotics was evaluated by isobologram analysis. Briefly, in a two-coordinate plot with one coordinate representing concentration of ZnO nanofluid and other representing concentration of the antibiotic (tobramycin, ceftazidime, and ciprofloxacin), MIC of ZnO nanofluid and the antibiotic are located on the x and y-axes, respectively. These two points are connected by the line of additively. The combination concentration of ZnO nanofluid and antibiotic that caused complete inhibition of bacterial growth, are pointed in the plot. As this point is located below, on, or above the additively line indicates synergy, additively or antagonism, respectively (27).
Data Analysis.
The results of percentage of growth inhibition of the tested antibiotics alone and in combination with ZnO NPs are expressed as mean ± standard error mean (SEM). Every experiment was repeated at least three independent times. All data were analyzed and compared utilizing one-way ANOVA Tukey test (SPSS software) and differences with p <0.05 were considered significant. The obtained FICI values for each combination were compared through Student’s t test (28).
RESULTS AND DISCUSSION
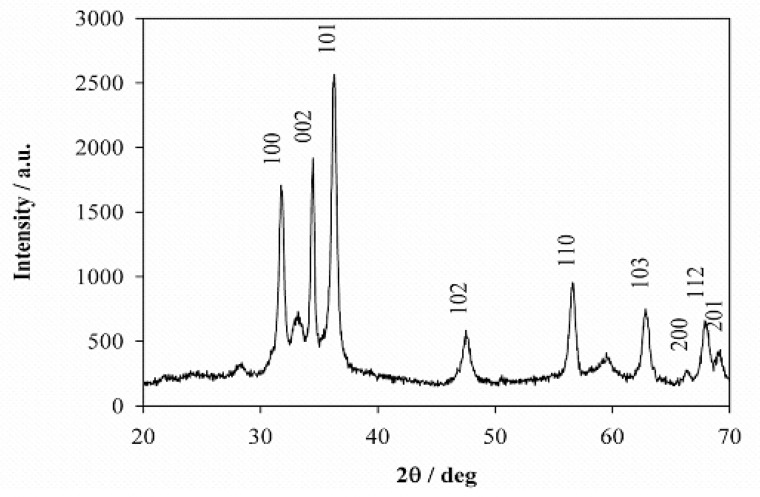
Characterization of the ZnO NPs. The XRD pattern of ZnO NPs is shown in Fig. 1 and confirmed that ZnO NPs are crystalline. A number of strong Bragg reflections can be seen which correspond to the (100), (002), (101), (102), (110), (103), (200), (112), and (201) reflections of wurtzite hexagonal phase of ZnO (29). The diffraction peaks match well with those in the JCPDS card (Joint Committee on Powder Diffraction Standards, Card No. 89–1397). The crystallite size of the ZnO NPs estimated using Scherrer formula (Eq. 5) was 20.2 nm.
Fig. 1.
Powder X-ray diffraction of the ZnO nanostructures
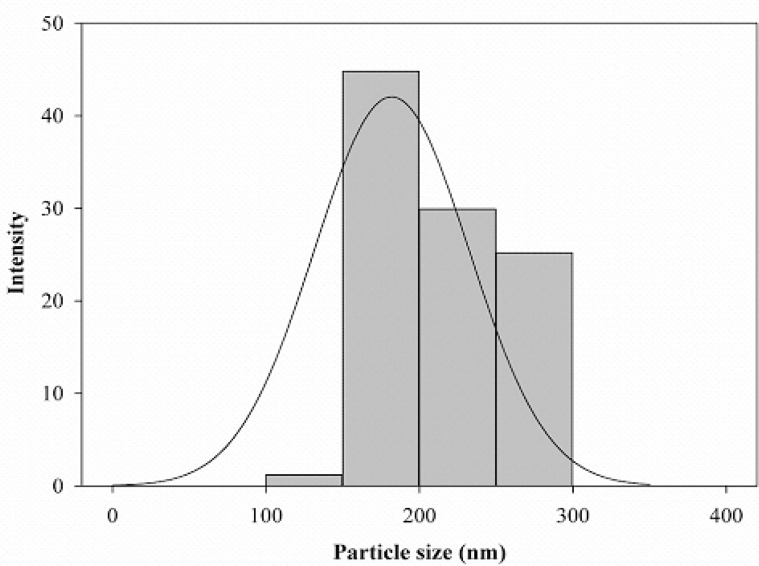
The average particle size determined by DLS was 181.9 nm (Fig. 2). Clearly, the mean particle size obtained by DLS is greater than of the actual particle size since the hydrodynamic radius is probed with DLS.
Fig. 2.
Particle size distribution of ZnO NPs
MIC and MBC of ZnO NPs, tobramycin, ceftazidime, and ciprofloxacin.
The antimicrobial activity of ZnO NPs and antibiotics alone and in combination were tested at different concentrations against standard and clinical P. aeruginosa strains in nutrient broth medium. The results showed that P. aeruginosa ATCC9027 was more susceptible to ZnO NPs than the clinical isolate of this bacterium with MIC values of 93.7 and 375 μg/ml for standard and clinical strains, respectively. The MBC/MIC values of ZnO NPs against the standard and clinical strains were, respectively, 1 and 2. The MIC values for ceftazidime against standard and clinical isolates of P. aeruginosa were 0.5 to 8 μg/ml, for tobramycin 0.5 to 2 μg/ml, and for ciprofloxacin 0.0625 to 0.5 μg/ml. The MBC values of these three antibiotics were equal with the MIC values of them. These findings show that mechanism of antibacterial activity of ZnO NPs, tobramycin, ceftazidime, and ciprofloxacin against both strains is bactericidal (22). As shown in Tables 1 and 2, growth of standard and clinical strains of P. aeruginosa decreased as the concentration of ZnO NPs and the tested antibiotics increased.
Table 1.
The percentage of P. aeruginosa ATCC 9027 growth inhibition in the presence of the tested antibiotics alone and in combination with ZnO NPs at subinhibitory concentrations (1/2 and 1/4 × MIC)
| Antibiotic Concentration (μg/ml) |
ZnO NPs Concentration (μg/ml) | ||
|---|---|---|---|
| 0 | 23.4 | 46.8 | |
| Ceftazidime | |||
| 0 | 0 | 23.29 ± 0.02 | 47.41 ± 0.28 |
| 0.0625 | 47.44 ± 1.74 | 50.94 ± 0.54 | 100 |
| 0.125 | 57.63 ± 2.53 | 59.27 ± 1.86 | 100 |
| 0.25 | 70.81 ± 1.94 | 100 | 100 |
| Tobramycin | |||
| 0 | 0 | 23.29 ± 0.04 | 47.41 ± 0.48 |
| 0.0625 | 50.82 ± 0.59 | 57.84 ± 2.83 | 100 |
| 0.125 | 59.83 ± 0.77 | 68.72 ± 1.51 | 100 |
| 0.25 | 68.63 ± 2.36 | 100 | 100 |
| Ciprofloxacin | |||
| 0 | 0 | 23.75 ± 4.64 | 47.14 ± 3.82 |
| 0.0078 | 32.91 ± 1.93 | 36.74 ± 6.52 | 100 |
| 0.0156 | 54.62 ± 0.54 | 55.11 ± 4.49 | 100 |
| 0.0312 | 66.80 ± 0.94 | 64.87 ± 2.91 | 100 |
ZnO NPs: ZnO nanoparticles
Table 2.
The percentage of clinical isolate of P.aeruginosa growth inhibition in the presence of the tested antibiotics alone and in combination with ZnO NPs at subinhibitory concentrations (1/2 – 1/16 × MIC)
| Antibiotic Concentration (μg/ml) | ZnO NPs Concentration (μg/ml) |
||||
|---|---|---|---|---|---|
| 0 | 23.4 | 46.8 | 93.7 | 187.5 | |
| Ceftazidime | |||||
| 0 | 0 | 32.48 ± 2.08 | 50.57 ± 3.38 | 76.78 ± 3.03 | 93.29 ± 0.82 |
| 1 | 12.29 ± 2.83 | 46.52 ± 0.47 | 95.49 ± 1.48 | 98.32 ± 0.32 | 99.8 ± 0.75 |
| 2 | 22.96 ± 1.12 | 93.57 ± 1.60 | 98.12 ± 0.12 | 98.88 ± 0.11 | 100 |
| 4 | 47.52 ± 7.69 | 99.20 ± 0.46 | 100 | 100 | 100 |
| Tobramycin | |||||
| 0 | 0 | 42.44 ± 1.59 | 57.54 ± 0.67 | 79.24 ± 1.61 | 93.15 ± 0.60 |
| 0.25 | 7.68 ± 2.76 | 81.03 ± 3.07 | 92.24 ± 1.95 | 97.12 ± 0.24 | 97.12 ± 0.24 |
| 0.5 | 25.10 ± 3.24 | 85.45 ± 1.46 | 96.51 ± 0.80 | 100 | 100 |
| 1 | 62.31 ± 3.03 | 92.03 ± 0.82 | 100 | 100 | 100 |
| Ciprofloxacin | |||||
| 0 | 0 | 45.01 ± 2.64 | 54.05 ± 1.45 | 75.87 ± 2.37 | 94.81 ± 1.65 |
| 0.0625 | 42.51 ± 3.48 | 74.66 ± 1.16 | 94.15 ± 0.82 | 98.59 ± 0.09 | 98.57 ± 0.25 |
| 0.125 | 78.40 ± 6.10 | 94.66 ± 0.50 | 100 | 100 | 100 |
| 0.25 | 96.96 ± 0.87 | 100 | 100 | 100 | 100 |
ZnO NPs: ZnO nanoparticles
FICI and isobologram analysis.
The synergistic effect between ZnO nanofluid and the antibiotics were evaluated using the checkerboard assay and FIC index was calculated. FICI is an indicator of degree of interaction between ZnO nanofluid along with tobramycin, ceftazidime, and ciprofloxacin for standard and clinical P. aeruginosa strains (Table 3). The FICI of the combination of ZnO nanofluid plus ceftazidime or tobramycin was calculated as 0.375, showing synergistic interaction of ZnO nanofluid with these antibiotics against the two strains under investigation. The combination of ZnO nanofluid with ciprofloxacin against the standard and clinical strains is estimated as partial synergism and synergism, respectively.
Table 3.
Fractional inhibitory concentration index (FICI) of the tested antibiotics alone and in combination with ZnO NPs against P. aeruginosa ATCC 9027 and clinical isolate of P.aeruginosa as determined by checkerboard method
| Strain | MIC in combination | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ZnO NPs + CAZ | ZnO NPs + CIP | ZnO NPs + TOB | |||||||
| ZnO NPs (μg/ml) | CAZ (μg/ml) | FICI | ZnO NPs (μg/ml) | CIP (μg/ml) | FICI | ZnO NPs (μg/ml) | TOB (μg/ml) | FICI | |
| Clinical isolate of P.aeruginosa | 93.7 | 2 | 0.5 | 23.4 | 0.125 | 0.3125 | 46.8 | 0.5 | 0.375 |
| P.aeruginosa ATCC 9027 | 23.4 | 0.0625 | 0.375 | 46.8 | 0.0078 | 0.625 | 23.4 | 0.0625 | 0.375 |
ZnO NPs: ZnO nanoparticles, CAZ: Ceftazidime, CIP: Ciprofloxacin, TOB: Tobramycin, FICI: Fractional inhibitory concentration index
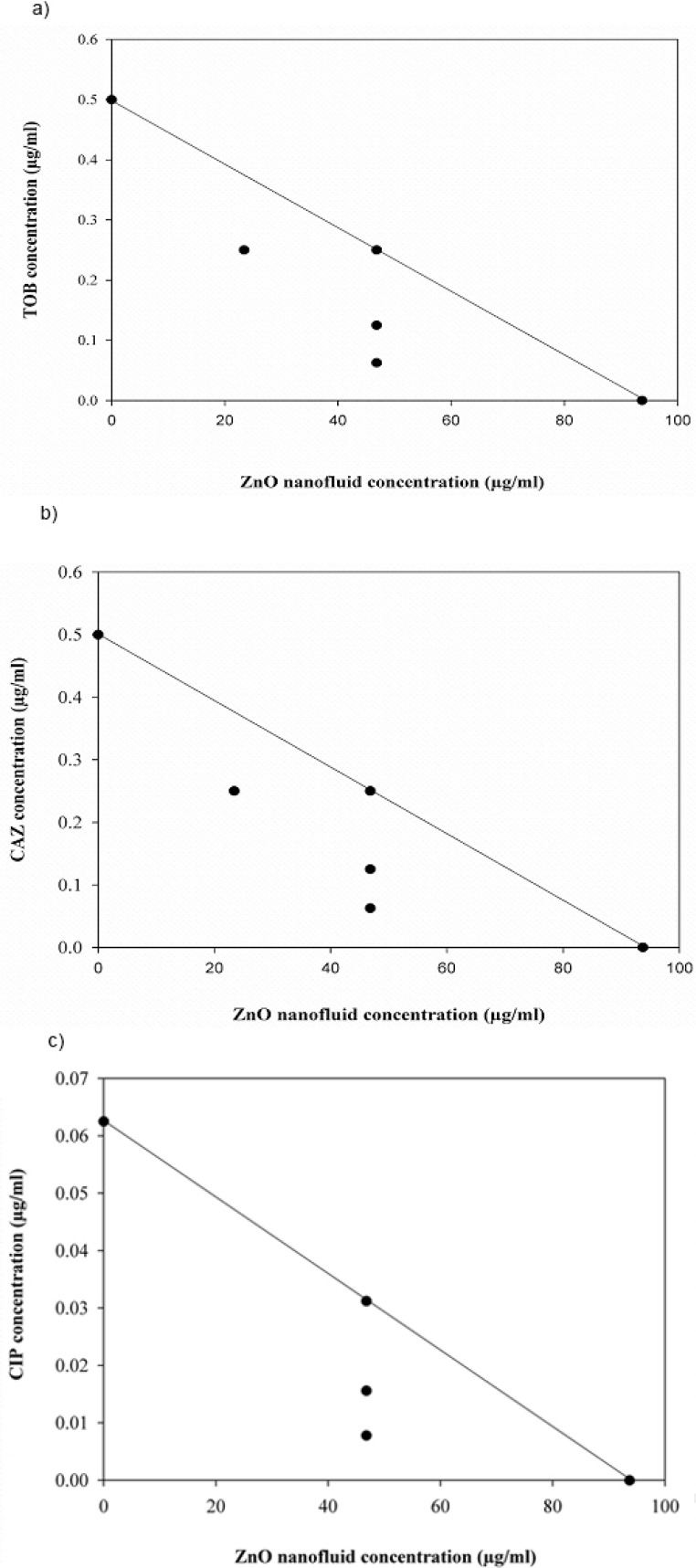
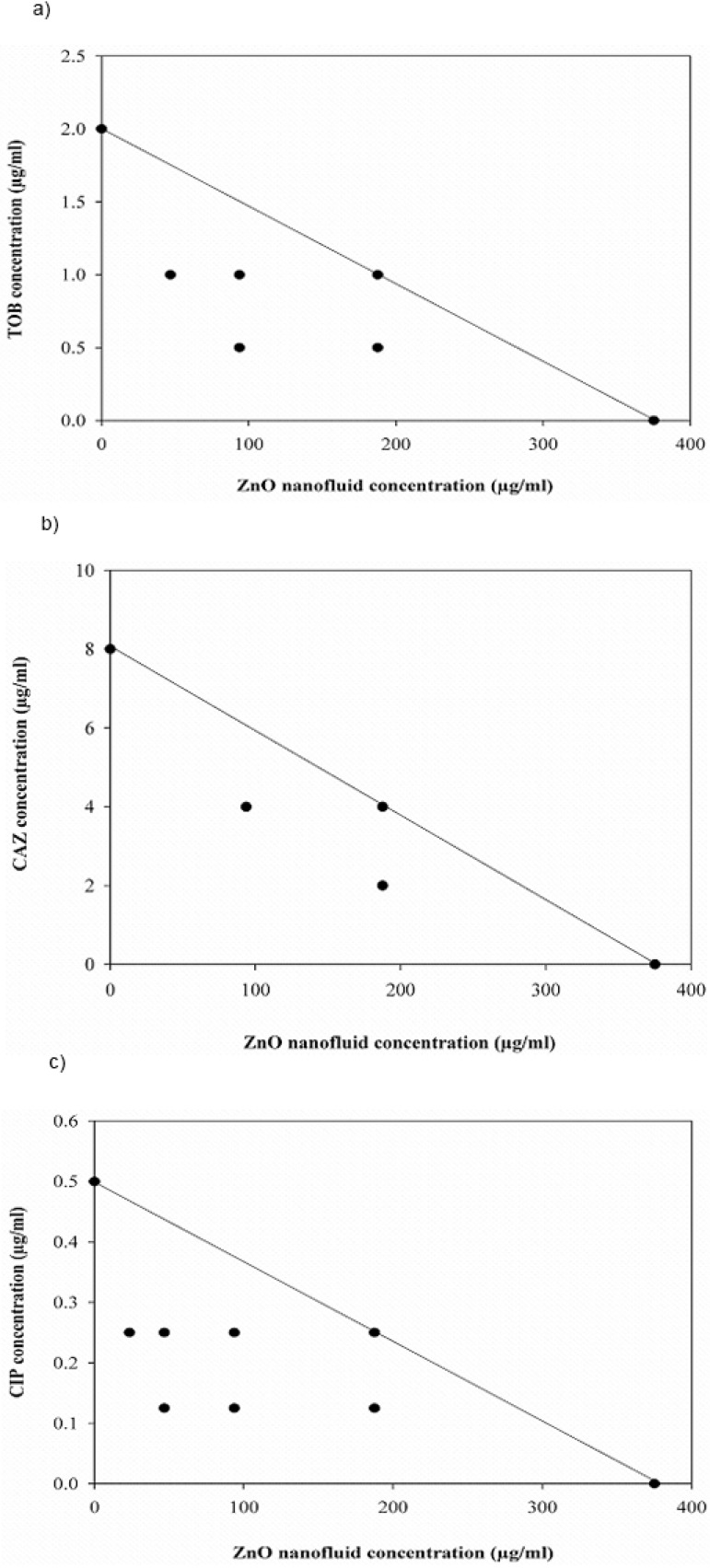
Combination effect between ZnO nanofluid and the antibiotics under investigation against both P. aeruginosa strains was also evaluated by isobologram analysis (Fig. 3 and 4). The points of combination concentrations of the two antibacterial agents in each assay are located on or below the additively line, showing additively or synergy effect, respectively.
Fig. 3.
Isobologram analysis for the combinations of ZnO nanofluid and antibiotics against P. aeruginosa ATCC 9027. (a) tobramycin (TOB), (b) ceftazidime (CAZ) and (c) ciprofloxacin (CIP)
Fig. 4.
Isobologram analysis for the combinations of ZnO nanofluid and antibiotics against clinical isolate of P. aeruginosa. (a) tobramycin (TOB), (b) ceftazidime (CAZ) and (c) ciprofloxacin (CIP)
Our cytotoxic analysis showed that combined use of ZnO nanofluid with the antibiotics potentiated their antibacterial activity in both test strains. Permiabilizing agents are reported to have the capability of increasing the susceptibility of P. aeruginosa to some antibiotics (30). A mechanism of antibacterial activity of ZnO NPs was reported to damage to the cell membrane and leak out contents of bacterial cell (31, 32). Therefore, it is probable that ZnO NPs by increasing the permeability of cell membrane may cause the enhancement of antibiotic efficacy. Another explanation for these findings would be that ZnO NPs may interfere with pumping activity of efflux systems (33).
Metal ions may play a very important role in the mechanism of action of these antibiotics (34–36). The interaction of several metal ions with quinolones is reported (37, 38). For example, carbonyl group, carboxylic oxygens, and fluore atom in ciprofloxacin may interact with Zn atom in ZnO NPs. These interactions may stabilize ciprofloxacin-ZnO NPs system. Ionic interaction between protonated nitrogen atoms in ciprofloxacin and hydroxylated surface of ZnO NPs may also stabilize ciprofloxacin-ZnO NPs system (33). Anacona et al. showed that the copper (II) and zinc (II) complexes of ciprofloxacin had higher antibacterial activity than ciprofloxacin against P. aeruginosa (39).
In this study, it is probable that ciprofloxacin may form complex with Zn2+ ions released from the surface of ZnO NPs and increase its antibacterial activity. ZnO NPs may also inhibit the efflux transporters and thereby increase the efficacy of antibiotics against P. aeruginosa.
CONCLUSION
In the present paper, we report for the first time the combination effect of ZnO nanofluid with antibiotics tobramycin, ceftazidime, or ciprofloxacin against P. aeruginosa. Our findings suggest that application of ZnO nanofluid combined with these three antibiotics can enhance their antibacterial effects.
ACKNOWLEDGEMENT
This project was supported by Ferdowsi University of Mashhad, grant number 3/21611.
REFERENCES
- 1. Van Delden C, Iglewski BH. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis 1998; 4: 551– 560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feltman H, Schulert G, Khan S, Jain M, Peterson L, Hauser AR. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology 2001; 147: 2659– 2669. [DOI] [PubMed] [Google Scholar]
- 3. Dundar D, Otkun M. In-vitro efficacy of synergistic antibiotic combinations in multidrug resistant Pseudomonas aeruginosa strains. Yonsei Med J 2010; 51: 111– 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lambert PA. Mechanisms of antibiotic resistance in Pseudomonas aeruginosa. J R Soc Med 2002; 95: 22– 26. [PMC free article] [PubMed] [Google Scholar]
- 5. Durante-Mangoni E, Grammatikos A, Utili R, Falagas ME. Do we still need the aminoglycosides? Int J Anti-microb Agents 2009; 33: 201– 205. [DOI] [PubMed] [Google Scholar]
- 6. Jeong N, Kim JY, Park SC, Lee JK, Gopal R, Yoo S, et al. Antibiotic and synergistic effect of Leu-Lys rich peptide against antibiotic resistant microorganisms isolated from patients with cholelithiasis. Biochem Biophys Res Commun 2010; 399: 581– 586. [DOI] [PubMed] [Google Scholar]
- 7. Park HR, Kim TH, Bark KM. Physicochemical properties of quinolone antibiotics in various environments. Eur J Med Chem 2002; 37: 443– 460. [DOI] [PubMed] [Google Scholar]
- 8. Fish DN, Choi MK, Jung R. Synergic activity of cephalosporins plus fluoroquinolones against Pseudomonas aeruginosa with resistance to one or both drugs. J Antimicrob Chemother 2002; 50: 1045– 1049. [DOI] [PubMed] [Google Scholar]
- 9. Prinsloo A, Van Straten AMS, Weldhagen GF. Antibiotic synergy profiles of multidrug-resistant Pseudomonas aeruginosa in a nosocomial environment. South Afr J Epidemiol Infect 2008; 23: 7– 9. [Google Scholar]
- 10. Tin S, Sakharkar KR, Lim CS, Sakharkarm MK. Activity of chitosans in combination with antibiotics in Pseudomonas aeruginosa. Int J Biol Sci 2009; 5: 153– 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jayaraman P, Sakharkar MK, Lim CS, Tang TH, Sakharkar KR. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int J Biol Sci 2010; 6: 556– 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor E, Webster TJ. Reducing infections through nanotechnology and nanoparticles. Int J Nanomed 2011; 6: 1463– 1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett 2008; 279: 71– 76. [DOI] [PubMed] [Google Scholar]
- 14. Jalal R, Goharshadi EK, Abareshi M, Moosavi M, Yousefi A, Nancarrow P. ZnO nanofluid: green synthesis, characterization, and antibacterial activity. Mater Chem Phys 2010; 121: 198– 201. [Google Scholar]
- 15. Siddique S, Shah ZH, Shahid S, Yasmin F. Preparation, characterization and antibacterial activity of ZnO nanoparticles on broad spectrum of microorganisms. Acta Chim Slov 2013; 60: 660– 665. [PubMed] [Google Scholar]
- 16. Shi LE, Li ZH, Zheng W, Zhao YF, Jin YF, Tang ZX. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: a review. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2014; 31: 173– 186. [DOI] [PubMed] [Google Scholar]
- 17. Reddy KM, Feris K, Bell J, Hanley C, Punnoose A. Selective toxicity of zinc oxide nanoparticles to pro-karyotic and eukaryotic systems. Appl Phys Lett 2007; 90: 2139021– 2139023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang L, Lan J, Xu Z, Chen T, Zhao T, Cheng T, et al. Toxicity and biodistribution of aqueous synthesized ZnS and ZnO quantum dots in mice. Nanotoxicology 2014; 8: 107– 116. [DOI] [PubMed] [Google Scholar]
- 19. Applerot G, Lipovsky A, Dror R, Perkas N, Nitzan Y, Lubart R, et al. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Adv Funct Mater 2009; 19: 842– 852. [Google Scholar]
- 20. Li M, Zhu L, Lin D. Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and influence of medium components. Environ Sci Technol 2011; 45: 1977– 1983. [DOI] [PubMed] [Google Scholar]
- 21. Moosavi M, Goharshadi EK, Youssefi A. Fabrication, characterization, and measurement of some physicochemical properties of ZnO nanofluids. Int J Heat Fluid Flow 2010; 31: 599– 605. [Google Scholar]
- 22. Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of gram-positive bacterial infections. Clin Infect Dis 2004; 38: 864– 870. [DOI] [PubMed] [Google Scholar]
- 23. Padmavathy N, Vijayaraghavan R, Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci Technol Adv Mater 2008; 9:035004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raghupathi KR, Koodali RT, Manna AC, Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011; 27: 4020– 4028. [DOI] [PubMed] [Google Scholar]
- 25. Cappelletty DM, Rybak MJ. Comparison of methodologies for synergism testing of drug combinations against resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother 1996; 40: 677– 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. den Hollander JG, Horrevorts AM, van Goor ML, Verbrugh HA, Aouton JW. Synergism between tobramycin and ceftazidime against a resistant Pseudomonas aeruginosa strain, tested in an in vitro pharmacokinetic model. Antimicrob Agents Chemother 1997; 41: 95– 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhao L, Au JL, Wientjes MG. Comparison of methods for evaluating drug-drug interaction. Front Biosci (Elite Ed.) 2010; 2: 241– 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Landau S, Everitt BS. (2004). A Handbook of Statistical Analyses using SPSS. Vol. 1 Chapman & Hall/CRC Press; Boca Raton, FL. [Google Scholar]
- 29. Yang L, Wang G, Tang C, Wang H, Zhang L. Synthesis and photoluminescence of corn-like ZnO nano-structures under solvothermal-assisted heat treatment. Chem Phys Lett 2005; 409: 337– 341. [Google Scholar]
- 30. Ayres H, Furr J, Russell A. Effect of permeabilizers on antibiotic sensitivity of Pseudomonas aeruginosa. Lett Appl Microbiol 1999; 28: 13– 16. [DOI] [PubMed] [Google Scholar]
- 31. Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fiévet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett 2006; 6: 866– 870. [DOI] [PubMed] [Google Scholar]
- 32. Tam KH, Djurišić AB, Chan CMN, Xi YY, Tse CW, Leung YH, et al. Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Films 2008; 516: 6167– 6174. [Google Scholar]
- 33. Banoee M, Seif S, Nazari ZE, Jafari-Fesharaki P, Shahverdi HR, Moballegh A, et al. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J Biomed Mater Res B Appl Biomater 2010; 93: 557– 561. [DOI] [PubMed] [Google Scholar]
- 34. Drevenšek P, Turel I, Poklar Ulrih N. Influence of copper (II) and magnesium (II) ions on the ciprofloxacin binding to DNA. J Inorg Biochem 2003; 96: 407– 415. [DOI] [PubMed] [Google Scholar]
- 35. Xiao DR, Wang EB, An HY, Su ZM, Li YG, Gao L, et al. Rationally designed, polymeric, extended metal–ciprofloxacin complexes. Chemistry 2005; 11: 6673– 6686. [DOI] [PubMed] [Google Scholar]
- 36. Xiao DR, Wang EB, An HY, Li YG, Xu L. Syntheses and structures of three unprecedented metal-ciprofloxacin complexes with helical character. Cryst Growth Des 2007; 7: 506– 512. [Google Scholar]
- 37. Drevenšek P, Ulrih NP, Majerle A, Turel I. Synthesis, characterization and DNA binding of magnesium–ciprofloxacin (cfH) complex [[Mg(cf)2]•2.5H2O. J Inorg Biochem 2006; 100: 1705– 1713. [DOI] [PubMed] [Google Scholar]
- 38. Al-Mustafa J, Taha ZA. Thermodynamics of the complexation of ciprofloxacin with calcium and magnesium perchlorate. Thermochim Acta 2011; 52: 9– 13. [Google Scholar]
- 39. Anacona JR, Toledo C. Synthesis and antibacterial activity of metal complexes of ciprofloxacin. Transition Met Chem (Dordrecht Neth) 2001; 26: 228– 231. [Google Scholar]