Abstract
Background and Objectives:
The most prevalent and worldwide oral disease is dental caries that affects a significant proportion of the world population. There are some classical approaches for control, prevention and treatment of this pathologic condition; however, the results are still not completely successful. Therefore new methods are needed for better management of this important challenge. Chitosan is a natural and non-toxic polysaccharide with many biological applications, particularly as an antimicrobial agent. Chitosan nanoparticle is a bioactive and environment friendly material with unique physicochemical properties. The aim of the present study was to investigate the antimicrobial effect of chitosan and nano-chitosan on the most important cariogenic streptococci.
Materials and Methods:
For evaluation of antimicrobial effect of chitosan and nano-chitosan against oral streptococci broth micro-dilution method was carried out for four bacterial species; Streptococcus mutans, Streptococcus sobrinus, Streptococcus sanguis and Streptococcus salivarius. Also the effect of these materials on adhesion of above bacteria was evaluated. One-way ANOVA and post hoc Tukey test were used for statistical analysis.
Results:
The MICs of chitosan for S. mutans, S. sanguis, S. salivarius and S. sobrinus were 1.25, 1.25, 0.625 and 0.625 mg/mL, respectively. The MIC of chitosan nanoparticle for S. mutans, S. salivarius and S. sobrinus was 0.625 mg/mL and for S. sanguis was 0.312 mg/mL. Chitosan and chitosan nanoparticles at a concentration of 5 mg/mL also reduced biofilm formation of S. mutans up to 92.5% and 93.4%, respectively.
Conclusion:
The results of this study supported the use of chitosan and chitosan nanoparticles as antimicrobial agents against cariogenic Streptococci.
Keywords: Chitosan, Chitosan nanoparticles, Dental caries, Oral streptococci
INTRODUCTION
According to the World Health Organization reports, dental caries still remains a major health problem, especially among disadvantaged social groups worldwide (1). This disease affects both sexes, all races and age groups, and different social and economic classes. Dental caries causes pain and sadness, and it imposes a relatively high financial burden on families (2). There are about 700 different bacterial species in human oral microbiota. Cariogenic bacteria such as S. mutans and lactic acid bacterial species play an important role in the pathogenesis of dental caries. Both of them are able to grow in acidic conditions, such as lactic acid which is a characteristic organic product of sugar metabolism (3).
Antimicrobials such as ampicillin, chlorhexidine, quaternary ammonium compounds and metronidazole are used to prevent tooth decay, but they have several side effects such as tooth staining, diarrhea, increased calculus formation and changes in bowel flora (4). Therefore, new methods for prevention and probably better management of this important challenge are needed. In recent years, the use of natural nontoxic alternatives for the control and prevention of dental caries has emerged. Chitosan is a natural polymer with specific characteristics, including biodegradability, non-toxicity and antimicrobial activity, which has attracted great attention for some years (5). Prior studies have described the antimicrobial potential of chitin, chitosan and their derivatives during 1980–1990s (6). Some studies have shown that chitosan has antibacterial and anti-plaque actions and anti-adhesive properties against S. mutans and other streptococci (7–9). Chitosan nanoparticles have smaller size than chitosan and this property could make it unique. Nano-chitosan is a natural material with antibacterial effects (10–12) and gene transfer in artificial organs as controlled-release drug carrier; it also improves the strength and washability of textiles (13) and enhances the immunologic and protective efficacy of the DNA vaccine (14). Many studies have reported antibacterial characteristics of nano-chitosan (10, 12) but inadequate data are available on its effects against oral streptococci as the major cause of dental caries.
The aim of this study was to determine the anticariogenic effects of chitosan and nano-chitosan, especially its efficiency against oral streptococci.
MATERIALS AND METHODS
Test organisms.
The antibacterial activity of the chitosan and nano chitosan was evaluated against four strains of bacteria which was purchased from Iranian Research Organization for Science and Technology (IROST) including: Streptococcus mutans (ATCC 35668), Streptococcus sobrinus (ATCC 27607), Streptococcus sanguis (PTCC1449) and Streptococcus salivarius (PTCC 1448).
All microorganisms were grown in Tryptic Soy Broth (TSB), Blood Agar and Mitis Salivarius Agar (MSA) at 37 °C in a atmosphere containing 5% CO2. Then, growths were confirmed using Gram staining, catalase test, optochin and bacitracin tests. The bacteria were stored at −80 °C until used in the study.
Preparation of chitosan and chitosan nanoparticles solution.
Low molecular weight chitosan (>85% deacetylated) was purchased from Sigma-Aldrich (USA). Chitosan solution was prepared by dispersing it in 0.25% acetic acid solution (Merck, Germany).
Nanochitosan was prepared by Laboratory of Tarbiat Modarres University, Noor, Iran (15). Nanochitosan was dissolved in 1% acetic acid and for complete dissolution of its particles kept overnight under magnetic stirring then with distilled water to obtain the desired volume.
Determination of antibacterial activity.
Bacterial suspensions with 1×108 bacteria were prepared from fresh cultures of bacteria in tryptic soy agar; (TSA; Quelab, Montreal, Canada) using sterile swabs they were cultured on the Muller-Hinton agar medium with 5% defibrinated sheep blood. Then, 6-mm-diameter wells were punched over the agar plates using sterile Pasteur pipettes. The bottoms of the wells were sealed by pouring a drop of molten MHA. Then 50 μL of the chitosan and nano-chitosan solutions were poured into the wells. These plates were kept at a temperature of 4 °C until the materials in the wells were completely diffused into the agar, and the plates were incubated anaerobically at 37 °C for 24 h. The diameter of the inhibition zone was measured (16).
Determination of MIC and MBC.
For determination of minimum inhibitory concentration of chitosan and nano-chitosan solutions, micro-dilution broth method was used. Overnight, the streptococci cultures in tryptic soy broth (TSB; Quelab, Montreal, Canada) were adjusted to 106 colony-forming units (CFU/mL). Two-fold serial dilutions of chitosan and nano-chitosan solutions were prepared in broth to give the final concentration of 0.039–5 mg/mL. In sterile 96-well plates, 100 μL of each dilution of chitosan and nano-chitosan solutions were placed into the well containing 100 μL of bacterial suspension. Triplicate samples were performed for each test concentration (17). Wells containing culture medium and bacteria were used as negative control. Turbidity measurements were made for all the wells after 24 h of incubation at 37 °C under anaerobic conditions. The growth of Streptococci was measured at 630 nm using a micro-plate reader (AWARENESS, Technology INC, Stat fax 2100).
The MBC (minimum bactericidal concentration) was defined as the lowest concentration of test compounds that did prevent any visible bacterial growth on the tryptic soy agar (TSA) plate after 24 h incubation at 37 °C under anaerobic condition (18).
Determination of anti-adhesion effect of chitosan and nanochitosan solution.
To measure the anti-adhesion effect of chitosan and nano-chitosan solutions, the micro-titer plate method used as described previously by Di Giulio et al, with modifications (19). Briefly, overnight growth of bacteria in TSB with 1% sucrose were adjusted with the similar media to reach a concentration of 106 CFU/mL. Then 10 μL of different dilutions (serial dilutions) prepared from each dilution of chitosan and nano-chitosan were added to 1 mL of each bacterial suspension. Then 200 μL of them were transferred into each well of 96-well polystyrene plate. Blank wells contained only buffer and control wells contained bacteria without treatment. After 24 h of incubation at 37 °C at 5% CO2, the wells were washed three times with 200 μL of sterile phosphate-buffered saline (PBS) to remove unattached cells. Then the adherent bacteria were stained for 5 min with 200 μL of 2% crystal violet. The stain was rinsed off by placing the plates under running tap water.
The level of formation of biofilm was evaluated after adding 200 μl of 33% (v/v) glacial acetic acid per well. This was done via measuring the absorbance of the solution at 492 nm by an ELISA reader. The anti-adhesion activity of chitosan and chitosan nanoparticle was determined and compared with the control.
Measurement of reduction percentage was calculated using equation:
B = absorbance of blank, C = absorbance of control and T = absorbance of test (20).
Statistical analysis.
The data were statistically analyzed by One way ANOVA and Tukey’s post hoc tests on Graf Pad Prism5 software (GraphPad Software Inc., CA, USA). A value of P < 0.05 was considered statistically significant.
RESULTS
Measurement of inhibition zone diameter.
The means of three measurements of inhibition zone diameter of chitosan and chitosan nanoparticle for Streptococcus mutans, S. salivarius, S. sobrinus and S. sanguis are summarized in Table 1. The greatest zones of inhibition by the chitosan and chitosan nanoparticle were found at a dose of 5 mg/mL and a decrease in concentration resulted in a decrease in the zone of growth inhibition. The lowest zones of inhibition by the chitosan and chitosan nanoparticle were found at a dose of 1.25 mg/mL (P<0.05). The diameter of inhibition zone of chitosan nanoparticles was significantly more than that of chitosan with all the above-mentioned bacterial species.
Table 1.
The mean of diameters of inhibition zones of chitosan and nanochitosan against four oral streptococci
| concentration mg/ml | Zone of inhibition (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Streptococcus salivarius | Streptococcus sanguis | Streptococcus sobrinus | Streptococcus mutans | |||||
| chitosan | nanochitosan | chitosan | nanochitosan | chitosan | nanochitosan | chitosan | nanochitosan | |
| 5 | 12.167 | 14.5 | 13.5 | 15.67 | 12 | 14 | 12.5 | 15.16 |
| 2.5 | 10.167 | 11.8 | 10.5 | 12.25 | 10 | 12 | 10.5 | 12 |
| 1.25 | 9 | 10.67 | 8.5 | 10.5 | 8.5 | 9.5 | 8.5 | 10 |
Determination of MIC and MBC.
MIC and MBC of chitosan and chitosan nanoparticles were determined, which are presented in Table 2. MIC of chitosan for S. mutans and S. sanguis was 1.25 mg/mL, with 0.625 mg/mL for S. sobrinus and S. salivarius. MIC of chitosan nanoparticle for S. mutans, S. sobrinus and S. salivarius was 0.625 mg/mL, with 0.312 mg/mL for S. sanguis, which was significantly less than that for the other three bacteria (P<0.05). MIC of nano-chitosan for S. mutans and S. sanguis was less than that of chitosan (P<0.05), indicating higher antibacterial activity. In relation to S. sobrinus and S. salivarius there were no significant differences between the MIC of chitosan and the MIC of chitosan nanoparticles (P>0.05). MBC of chitosan nanoparticle only for S. mutans was less than that of chitosan (P<0.05).
Table 2.
Values of MIC and MBC for the four groups of oral streptocci bacteria.
| Bacteria | MBC**(mg/ml) | MIC*(mg/ml) | ||
|---|---|---|---|---|
| chitosan | nanochitosan | chitosan | nanochitosan | |
| Streptococcus mutans | 1.25 | .625 | 2.5 | 1.25 |
| Streptococcus sobrinus | .625 | .625 | 2.5 | 2.5 |
| Streptococcus sanguis | 1.25 | .312 | 1.25 | 1.25 |
| Streptococcus salivarius | .625 | .625 | 1.25 | 2.5 |
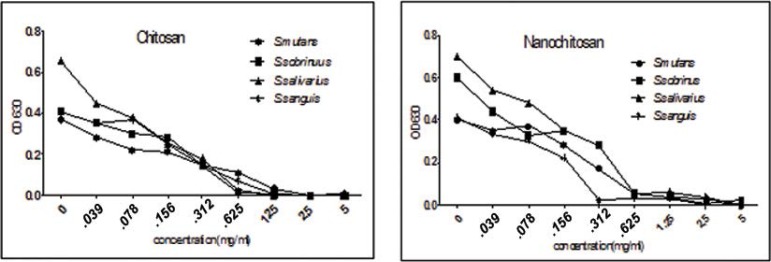
The inhibitory effects of different concentrations of chitosan and chitosan nanoparticle on the growth of the tested streptococci are shown in Fig. 1, which shows that in 1.25–5 mg/mL concentrations of chitosan, the growth of S. mutans and S. sanguis decreased >90% and in 0.625–5 mg/mL of chitosan the growth of S. sobrinus and S. salivarius decreased >90%. In addition, in 0.625–5 mg/mL concentrations of chitosan nanoparticles the growth of S. mutans, S. sobrinus and S. salivarius and in its 0.312–5 mg/mL concentration, the growth of S. sanguis decreased >90%. Decreases in concentrations of chitosan and chitosan nanoparticles resulted in increases in OD, indicating an increase in bacterial growth (P<0.05).
Fig. 1.
The effects various concentrations of chitosan and nano chitosan on the growth of four oral streptococci
Determination of anti-adhesion effect of chitosan and nanochitosan solutions.
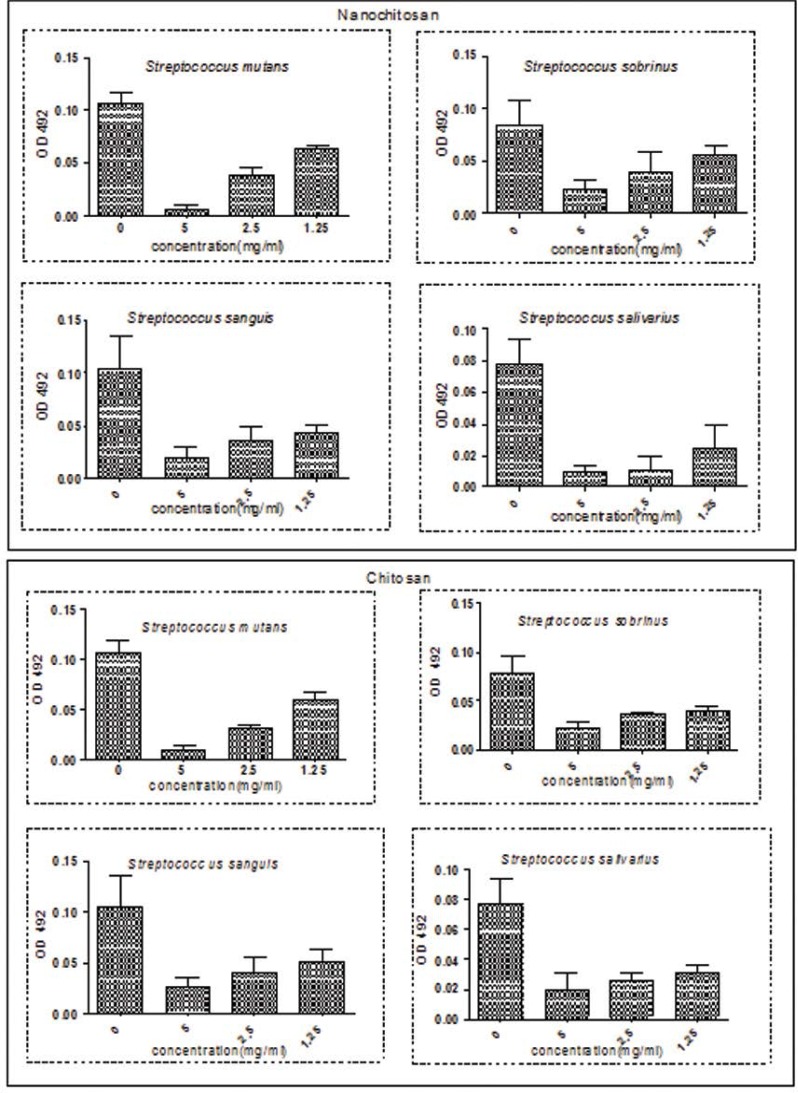
The anti-adhesion effect of chitosan and nano-chitosan was evaluated for each tested Streptococcus species and the results are presented in Table 3 and Fig 2. The percentage of adherence reduction decreased with a decrease in the concentration of chitosan and chitosan nanoparticles from 5 to 1.25 mg/mL (P<0.05). The maximum effect of chitosan and nano-chitosan was observed on S. mutans with 92.5% and 93.4% reductions in adherence, respectively, at a concentration of 5 mg/mL (P<0.05). There were no significant differences in adherence levels between the study groups (P>0.05).
Table 3.
Percentage of adherance reduction of various concentrations of chitosan and nanochitosan on oral streptococci
| Percentage of adherance reduction | ||||||||
|---|---|---|---|---|---|---|---|---|
| various concentrations of material mg/ml | Streptococcus salivarius | Streptococcus sanguis | Streptococcus Sobrinus | Streptococcus mutans | ||||
| chitosan | nanochitosan | chitosan | nanochitosan | chitosan | nanochitosan | chitosan | nanochitosan | |
| 5 | 92.5 | 93.4 | 72.1 | 72.6 | 74.2 | 78.9 | 74.3 | 88.4 |
| 2.5 | 71.2 | 64.4 | 54.4 | 52.3 | 61.9 | 64.7 | 67.9 | 87.1 |
| 1.25 | 46.7 | 41.1 | 49.3 | 34.5 | 50.4 | 59.04 | 60.2 | 67.9 |
Fig 2.
The effects various concentrations of chitosan and nano chitosan on adherence of four studied oral streptococci
As shown in Fig. 2, the different dilutions (1.25–5) of chitosan and chitosan nanoparticles significantly reduced biofilm formation compared to the control group (p <0.05).
DISCUSSION
Chitosan as a natural nontoxic biopolymer produced by partial deacetylation of chitin has numerous applications in food, pharmaceutical and chemical industries (21,16). In dentistry research, chitosan has been used due to its antibacterial characteristics for prevention of dental caries (22). Chitosan at nano-size has superior activities, including antimicrobial effects, drug, gene and/or vaccine delivery systems, and anti-tumor effect (10, 21, 23).
In this study, antimicrobial activity of chitosan and chitosan nanoparticles on four strains of cariogenic streptococci, including S. mutans, S. sobrinus, S. sanguis and S. salivarius was evaluated. The results showed that these substances have bacteriostatic or bactericidal and anti-adhesion effects, and they can reduce biofilm/plaque formation in vitro. In this study the effects of 1.25 mg/mL concentration of chitosan on growth reduction of >90% of S. mutans and S. sanguis and its 0.625 mg/mL concentration on growth reduction of >90% of S. sobrinus and S. salivarius were observed. Costa et al. (24) showed that chitosan mouthwash effectively reduced viable counts of Streptococcus spp and Enterococcus spp and it was safe with lower cytotoxicity than a commercial mouthwash.
In addition, it has been shown that the 0.626 mg/mL concentration of chitosan nanoparticles is able to decrease S. mutans, S. sobrinus and S. salivarius growth up to >90% and its 0.312 mg/mL concentration can reduce S. sanguis growth up to >90%.
In this study, the decreasing effect of chitosan and chitosan nanoparticles on adhesion of oral Streptococci was observed; therefore, chitosan decreased 92.5% of S. mutans adhesion and >70% that of S. sobrinus, S. sanguis and S. salivarius. Costa et al (25) compared mouthwashes containing chitosan with two commercial mouthwashes and found that only the chitosan mouthwash was capable of interfering with adherence, biofilm formation and mature biofilm of S. mutans, L. acidophilus, E. faecium, C. albicans and P. intermedia. In this study, antibacterial effect of different dilutions of chitosan against oral Streptococci was compared and it was found that with increasing the concentration of chitosan, anti-adhesion effect on tested bacteria increased, and 5 mg/mL concentration was found the most effective. These findings are consistent with the results of previous studies.
Sano et al. (26) reported that chitosan has an anti-plaque effect on S. sobrinus with saliva-treated hydroxyapatite and in another study (27) showed that chitosan rinse was effective in reducing plaque formation and S. mutans counts in saliva compared to the placebo rinse. Hayashi et al. (28) studied the impact of chewing gum containing chitosan on cariogenic bacteria and reported that oral bacteria in the group consuming chitosan chewing gum significantly decreased compared with the control group, and the count of S. mutans decreased 1 h after chewing the gum. Costa et al (29) reported that chitosan is capable of 94% reduction in S. mutans mature biofilms. Furthermore, it was shown that chitosan nanoparticle decreased 93.4%, 72.6%, 78.9% and 88.4% of adhesiveness of S. mutans, S. sobrinus, S. sanguis and S. salivarius, respectively, and a decrease in chitosan nanoparticle concentration resulted in an increase in their adhesion. Paz et al. (30) showed that chitosan nanoparticles prepared from low-MW chitosan induced >95% damage to S. mutans biofilms. Furthermore, Neilands et al. (31) reported that chitosan nanoparticles have the ability to hinder acid tolerance response (ATR) of adhered S. mutans. Several mechanisms are suggested for antimicrobial properties of chitosan, one of which is interaction between polycationic nature of chitosan with anion site in microbial cell membranes proteins, resulting in the leakage of some intracellular constituents (22, 32).
Comparison of chitosan and chitosan nanoparticles showed that inhibition zone for nano-chitosan was significantly higher than that of chitosan (P<0.05). Nano-chitosan has smaller size in comparison to chitosan and exhibits higher affinity for bacterial cells, which is probably responsible for its higher antibacterial activity (10). The MIC of nano-chitosan for S. mutans and S. sanguis and MBC of chitosan nanoparticle for S. mutans were less than those of chitosan (P<0.05). However, chitosan nanoparticles did not exhibit higher anti-adhesion property than chitosan on S. mutans, S. sobrinus and S. sanguis at a concentration of 5 mg/mL (P>0.05). Since there are few studies in this regard, further studies are needed to explain the findings exactly.
In conclusion, chitosan and nano-chitosan showed anti-growth and anti-adherence effects against cariogenic bacteria in vitro. The results indicated high potential of chitosan and nano-chitosan as anticariogenic agents, suggesting their potential application as dental biomaterials for prevention of dental caries.
REFERENCES
- 1. Petersen PE, Lennon MA. Effective use of fluorides for the prevention of dental caries in the 21st century: the WHO approach. Community Dent Oral Epidemiol 2004; 32: 319– 321. [DOI] [PubMed] [Google Scholar]
- 2. Moses J, Rangeeth BN, Gurunathan D. Prevalence of dental caries, socio-economic old school going children of chidambaram status and treatment needs among 5 to 15 year old school going children of chidambaram. J Clinical Diagnosis Res 2011; 5: 146– 151. [Google Scholar]
- 3. Karpinski TM, Szkaradkiewicz AK. Microbiology of dental caries. J Biol Earth Sci 2013; 3: M21– M24. [Google Scholar]
- 4. Bernardes WA, Lucarini R, Tozatti MG, Souza MGM, Andrade Silva ML, da Silva Filho AA, et al. Antimicrobial activity of Rosmarinus officinalis against oral pathogens: relevance of carnosic acid and carnosol. Chem Biodivers 2010; 7: 1835– 1840. [DOI] [PubMed] [Google Scholar]
- 5. Kong M, Chen. X.G, Xing K, Park H.J: Antimicrobial properties of chitosan and mode of action: A state of the art review. Int J Food Microbiol 2010; 144 : 51– 63. [DOI] [PubMed] [Google Scholar]
- 6. Goy RC, de Britto D, Assis OBG. A Review of the antimicrobial activity of chitosan. Ciência & Tecnologia 2009; 19: 241– 247. [Google Scholar]
- 7. Bae K, Jun EJ, Lee SM, Paik DI, Kim JB. Effect of water-soluble reduced chitosan on Streptococcus mutans, plaque regrowth and biofilm vitality. Clin Oral Investig 2006; 10: 102– 107. [DOI] [PubMed] [Google Scholar]
- 8. Sano H, Shibasaki K-I, Matsukubo T, Takaesu Y. Effect of molecular mass and degree of deacetylation of chitosan on adsorption of Streptococcus sobrinus 6715 to saliva treated hydroxyapatite. The Bulletin of Tokyo Dental College 2002; 43: 75– 82. [DOI] [PubMed] [Google Scholar]
- 9. Sano H, Shibasaki K-I, Matsukubo T, Takaesu Y. Effect of chitosan rinsing on reduction of dental plaque formation. The Bulletin of Tokyo Dental College 2003; 44: 9– 1 [DOI] [PubMed] [Google Scholar]
- 10. Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr Res 2004; 339: 2693– 2700. [DOI] [PubMed] [Google Scholar]
- 11. Du WL, Niub SS, Xua YL, Xu ZR. Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohydr Polym 2009; 75: 385– 389 [Google Scholar]
- 12. de Paz LECv, Resin A, Howard KA, Sutherland DS, Wejse PL. Antimicrobial effect of chitosan nanoparticle on Streptococcus mutans biofilm. Appl Environ Microbiol 2011; 77: 3892– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yang HC, Wang WH, Huang KS, Hon MH. Preparation and application of nanochitosan to finishing treatment with anti-microbial and anti-shrinking properties. Carbohydr Polym 2009; 79: 176 179. [Google Scholar]
- 14. Feng G, Jiang Q, Xia M, Lu Y, Qiu W, Zhao D. Enhanced immune response and protective effects of nano-chitosan-based DNA vaccine encoding T cell epitopes of esat-6 and FL against Mycobacterium Tuberculosis infection. PLoS ONE 2013; 8( 4) : e61135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Heidari A, Younesi H, Mehraban Z, Heikkinen H. Selective adsorption of Pb(II), Cd(II), and Ni(II) ions from aqueous solution using chitosan–MAA nanoparticles. Int J Biol Macromol 2013; 61: 251– 263. [DOI] [PubMed] [Google Scholar]
- 16. Islam M, Masum S, Mahbub KR, Haque Z. Antibacterial activity of crab-chitosan against Staphylococcus aureus and Escherichia coli. J Adv Scient Res 2011; 2: 63– 66. [Google Scholar]
- 17. Tsai T-H, Tsai T-H, Chien Y-C, Lee C-W, Tsai P-J. In vitro antimicrobial activities against cariogenic streptococci and their antioxidant capacities: A comparative study of green tea versus different herbs. Food Chemistry 2008; 110: 859– 864. [DOI] [PubMed] [Google Scholar]
- 18. Prakatthagomol w, Sirithunyalug J, Okonogi S. Comparison of antibacterial activity against food-borne bacteria of Alpinia galanga, Curcuma longa and Zingiber cassumunar. CMU J Nat Sci 2012; 11: 177– 185. [Google Scholar]
- 19. Di Giulio M, Di Bartolomeo S, Di Campli E, Sancilio S, Marsich E, Travan A, et al. The Effect of a silver nanoparticle polysaccharide system on streptococcal and saliva-derived biofilms. Int J Mol Sci 2013; 14: 13615– 1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abidi SH, Ahmed K, Sherwani SK, Kazmi SU. Reduction and removal of Pseudomonas aeruginosa biofilm by natural agents. Int J Chem Pharm Sci 2014; 5: 28– 34. [Google Scholar]
- 21. Vaezifar S, Razavi S, Golozar MA, Karbasi S, Morshed M, Kamali M. Effects of aome parameters on particle Size distribution of chitosan nanoparticles prepared by ionic gelation method. J Clust Sci 2013; 24: 891– 903. [Google Scholar]
- 22. Kim JS, Shin DH. Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin. Restor Dent Endod 2013; 38: 36– 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qi LF, Xu ZR, Li Y, Jiang X, Han XY. In vitro effects of chitosan nanoparticles on proliferation of human gastric carcinoma cell line MGC803 cells. World J Gastroenterol 2005; 11: 5136– 5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Costa EM, Silva S, Costa M, Pereira M, Campos D.A, et al. Chitosan mouthwash: Toxicity and in vivo validation. Carbohydr Polym 2014; 111: 385– 392. [DOI] [PubMed] [Google Scholar]
- 25. Costa EM, Silva S, Madureira AR, Cardelle-Cobas A, Tavaria FK, Pintado MM. A comprehensive study into the impact of a chitosan mouthwash upon oral microorganism’s biofilm formation in vitro. Carbohydr Polym 2014; 101: 1081– 1086 [DOI] [PubMed] [Google Scholar]
- 26. Sano H, Shibasaki K-I, Matsukubo T, Takaesu Y. Effect of molecular mass and degree of deacetylation of chitosan on adsorption of Streptococcus sobrinus 6715 to saliva treated hydroxyapatite. Bull Tokyo Dent Coll 2002; 43: 75– 82. [DOI] [PubMed] [Google Scholar]
- 27. Sano H, Shibasaki K-I, Matsukubo T, Takaesu Y. Effect of chitosan rinsing on reduction of dental plaque formation. Bull Tokyo Dent Coll 2003; 44: 9– 16. [DOI] [PubMed] [Google Scholar]
- 28. Hayashi Y, Ohara N, Ganno T, Yamaguchi K, Ishizaki T, Nakamura T, Sato M. Chewing chitosan-containing gum effectively inhibits the growth of cariogenic bacteria. Arch Oral Biol 2007; 52: 290– 4. [DOI] [PubMed] [Google Scholar]
- 29. Costa EM, Silva S, Tavaria F.K, Pintado MM. Study of the effects of chitosan upon Streptococcus mutans adherence and biofilm formation. Anaerobe 2013; 20: 27– 31. [DOI] [PubMed] [Google Scholar]
- 30. Chávez de Pazde Paz LE, Resin A, Howard KA, Sutherland DS, Wejse PL. Antimicrobial effect of chitosan nanoparticle on Streptococcus mutans biofilm. Appl Environ Microbiol 2011; 77: 3892– 3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neilands J, Sutherland D, Resin A, Wejse PL, Cavez de Paz LE. Chitosan nanoparticles affect the acid tolerance response in adhered cells of Streptococcus mutans. Caries Res 2011; 45: 501– 505. [DOI] [PubMed] [Google Scholar]
- 32. Shahidi F, Arachchi JKV, Jeon YJ. Food applications of chitin and chitosans. Trends Food Sci Technol 1999; 10 : 37– 51. [Google Scholar]