Abstract
Background:
Global findings indicate that incidence rate of cutaneous leishmaniasis (CL) has significantly increased during the past decade, as documented in many countries. This review was aimed to evaluate the trend of CL cases in terms of demographic and clinical characteristics during a decade after the earthquake (2003–2012) compared to the corresponding period before the earthquake in Bam (1993–2003).
Methods:
Direct smear preparations along with different intrinsic methods were used for detection and identification of the causative agents.
Results:
Overall, 20999 cases of CL have occurred during the last 20 years (1993–2012), 6731 cases before and 14268 cases after the earthquake (P< 0.001).
Conclusions:
Following a major earthquake, several risk factors could activate epidemics of cutaneous leishmaniasis in old foci and induce emerging foci in new areas.
Keywords: Cutaneous leishmaniasis, Disaster, Earthquake, Iran
Introduction
Leishmaniasis is estimated to cause the ninth largest disease burden in terms of morbidity and mortality among infectious diseases and is largely neglected in tropical and sub-tropical countries (WHO 2009). Cutaneous leishmaniasis (CL) represents an important public health concern of considerable magnitude in many parts of the world, especially in Africa, Indian Subcontinent, Eastern and Southern Mediterranean Regions and countries of the Middle East including Iran (WHO 2010). Both types of CL are present in different parts of Iran. Leishmania major, the causative agent of zoonotic CL (ZCL), and L. tropica, the cause of anthroponotic CL (ACL), are endemic in various districts of Iran (Shirzadi and Gouya 2012). Phlebotomus papatasi and P. sergenti are the principal sand fly vectors, respectively, for the aforementioned CL diseases (Nadim and Aflatoonian 1995, Rassi et al. 2008). They impose an enormous medical and socio-economic impact here, although they are a cause of global concern. CL has the potential to spread within the cities and across borders. ACL is prevalent in Kerman Province, particularly in the city of Bam (Sharifi et al. 2011a).
Global findings have shown that incidence rate of CL cases has significantly increased during the past decade (Alvar et al. 2012), as documented in Iran (Shirzadi and Gouya 2012) and across the world (Reyburn et al. 2003, Kolaczinski et al. 2004, WHO 2010, Ready 2011, Alvar et al. 2012, Ocampo et al. 2012).
Several contributing factors play an important role in increasing CL disease which include inadequate vector and reservoir control, urbanization, ecological changes, natural disasters, population movement, poor sanitation and garbage disposal system, human behavioral risk factors and resistance to standard drugs (Daszk et al. 2001, Desjeux 2001, Macpherson 2005, Croft et al. 2006). All of these changes can lead to favor epidemic, emergence and spread of CL.
Natural disasters including earthquakes, tsunamis, floods and cyclones can create suitable conditions for increased incidence of infectious diseases and cause epidemics (Dye and Wolpert 1988, Desjeux 2001, Bayramgurler et al. 2002, Smith 2005, Shultz et al. 2005, Sharifi et al. 2011b, Kouadio et al. 2012, Zhang et al. 2013). Iran is prone to natural disasters such as earthquake. Following natural disasters, particularly after earthquakes, various risk factors in favor of diseases will be created, which in turn provide a suitable condition for breeding the vector and thus transmission of the causative agent. Other confounding factors have contributed to the occurrence of new outbreaks and emergence of CL after disasters in several countries (Ashford 2000, Daszak et al. 2001, Patz et al. 2000, Fazaeli et al. 2009, Fakoorziba et al. 2010).
During the period after the earthquake, the population of Bam has significantly increased mainly due to various occupational opportunities related to the large-scale construction works and new project development, which caused massive movement of contractors and labor forces (Sharifi et al. 2011c). According to the official report, about 60,000 new comers, mostly males and from non-CL endemic regions (non-immune) arrived to the area and consequently resulted in increased incidence rate.
The aim of the present review was to evaluate trend of CL cases in terms of demographic and clinical characteristics during 10 years after the earthquake (2003–2012) compared to the corresponding period before the earthquake in Bam (1993–2003). Furthermore, we wanted to analyze the impact of control measures applied post disaster in Bam, which would serve as a model for planning future control strategies and public health measures not only in our country but also in other foci of endemic CL as well as epidemics.
Materials and Methods
Scope of this study
The review of literature was disappointing as there was no comprehensive study reported. We therefore looked at the operational aspects of ACL case-management, prevention and control measures that could be undertaken in disaster situation in an endemic focus of ACL. In addition, their possible impact on reduction of the CL disease was assessed. Therefore, in this review, epidemic of old foci and emergence of new foci in Bam district following the earthquake were reviewed. Since field trials of single and multiple doses of killed L. major plus BCG vaccine candidate against ACL (Sharifi et al. 1998a) had been conducted prior to the earthquake (1993–1998), we had very good information about the epidemiology of ACL, prior to the earthquake of 2003 which gave us good background information to compare the post-earthquake situation. The trained and knowledgeable team of personnel created for the vaccine studies, were the basis on which a CL clinic (CLC) for cutaneous leishmaniasis control was established which is still functioning. Therefore, in addition to using existing published studies, registered data on passive and active case-detections, were extracted, analyzed and presented.
Study area
This study was carried out in district of Bam, 180 km south to the city of Kerman, center of Kerman Province, southeast of Iran (Fig. 1). Bam is located 1000 m above the sea level along the Zagros mountain range. A massive earthquake struck the city of Bam and the neighboring areas on 26th December 2003. The affected population was 250,000, 100,000 in urban and 150,000 in rural areas. The magnitude of this earthquake was 6.6 on the Richter scale, leaving 30,000 death, 30,000 injured, 60,000 homeless and damaging over 90% of the city's infrastructures including medical and health facilities, causing l0 million tons of mud and rubbles (Abolghasemi et al. 2006).
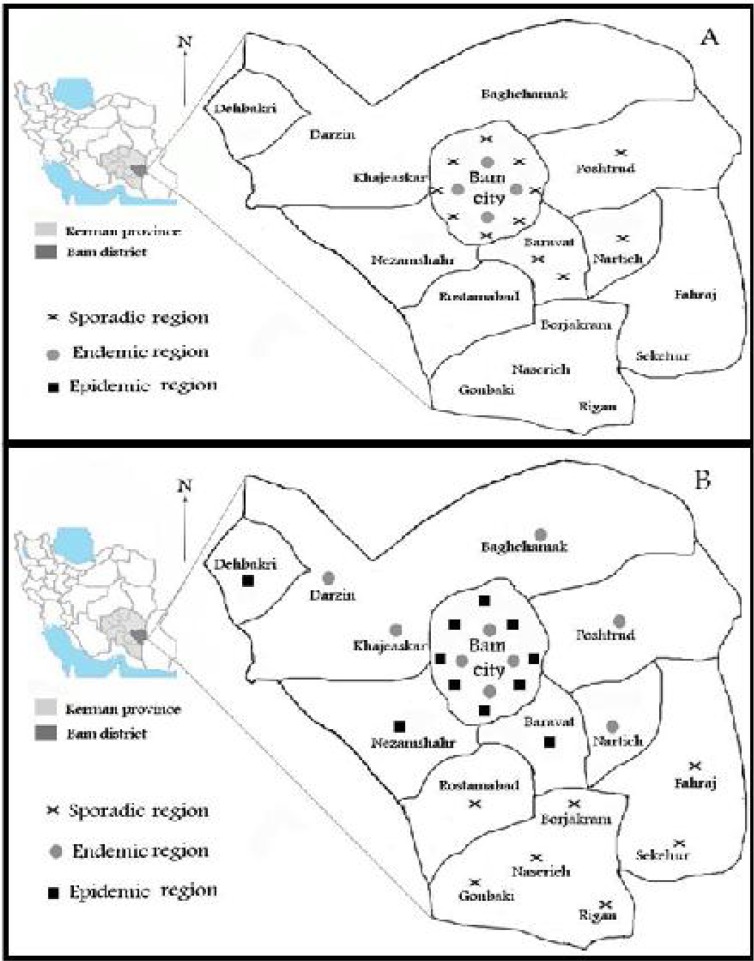
Fig. 1.
Distribution of cutaneous leishmaniasis before (A) and after (B) the earthquake in Bam District
Special attention was paid to ensure rapid recovery and rehabilitation of public health services. The city was divided to 12 zones by national and provincial health systems to facilitate accessibility of the population to essential health services and strengthen the medical supply chain. Simultaneously, a disease surveillance system was promptly established in the affected areas for delivering primary health care (PHC), even though the magnitude of destruction was enormous. However, it took two years to reconstruct the health and medical system following the earthquake. Approximately 30,000 people from the Province of Kerman and additional 30,000 individuals from other provinces of the country arrived at the city for various occupational purposes.
Cutaneous leishmaniasis clinic
In Bam, CL is a notified disease and its surveillance has long been integrated into the health system. The clinic was officially established in 2005 (two years after the earthquake) and used as a headquarters to coordinate and control various activities involved in the post-earthquake era including training personnel, health education, diagnosing and identifying cases, active and passive case-finding, implementing vector control measures, eliminating stray dogs, treating patients and referring non-healing cases to the provincial health clinic in Afzalipour University Hospital in Kerman, center of Kerman Province. A national control program was developed by National Center for Disease Control Office, Zoonoses Department, and several Medical Universities of Ministry of Health (MoH).
Case definition and identification
Skin scraping tissues were obtained from the periphery of lesions using a scalpel and sterile blade. The clinical materials were used for direct smear preparation and microscopic examination, culture and further identification by immunological methods, PCR or characterization by isoenzyme (Sharifi et al. 1997, Sharifi et al. 1998b, Sharifi et al. 2011a).
Personal protection measures
Dressing of lesions and stick insect repellents such as diethyl toluamide (Deet) to skin or clothing to reduce man-vector contact was applied. Face-to-face health education program of the population at risk and retraining of health personnel were in high priority. A large-scale deltamethrin-impregnated screens and curtains were assessed in high-risk areas in the city of Bam (Aflatoonian and Sharifi 2011, Noazin et al. 2013).
Environmental management
The activities included improving housing conditions, sanitation of temporary camps, land clearance around dwellings and insecticide spraying, particularly in high-risk areas, to eliminate potential sand fly breeding sites. About 10 million tons of mud and rubbles were removed.
Vector incrimination
So far, four main studies have been carried out on distribution of vectors and their implication in transmission of ACL in Bam before (Nadim et al. 1995) and after the earthquake (Aghasi and Sharifi 2003, Aghaei Afshar et al. 2011, Aghaei Afshar et al. 2013).
Treatment of patients
Pantavalent antimonials are the first choice medicines for treating all forms of leishmaniasis in global terms. In Bam, meglumine antimoniate (Glucantime) was given intramuscularly (IM), intralesionally or sometimes intravenously (IV). Combination therapy between meglumine antimoniate and cryotherapy (liquid nitrogen) or along with the second line drugs (allopurinol) was used mainly for treating ACL resistant forms (Esfandiarpour and Dabiri 2007, Sharifi et al. 2010).
Results
Epidemic of anthroponotic cutaneous leishmaniasis in the city of Bam
All the cases (20540 patients) were identified by direct smear preparation method and overall 150 random cases by intrinsic techniques including 14 cases by immunological and 9 patients by biochemical methods before the earthquake and 253 cases by molecular methods (conventional or nested-PCR) after the earthquake. Various methods revealed that all species (100%) were L. tropica after the earthquake, except for the cases identified before the earthquake which were predominantly L. tropica (90%) and was at low frequency (≤10%) L. major.
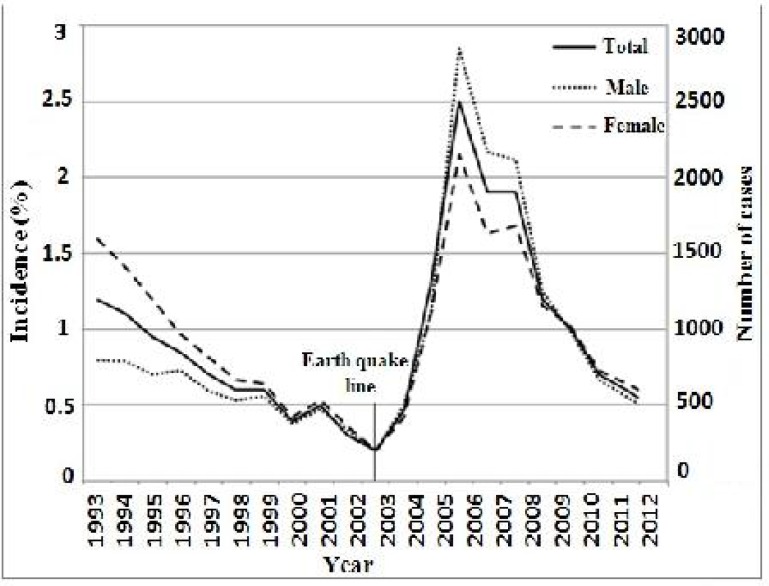
ACL due to L. tropica has been endemic in city of Bam since ancient times (Nadim and Aflatoonian 1995). According to the data gathered in the last 20 years (1993–2012), 20999 cases of ACL have occurred, 6731 cases before and 14268 cases after the earthquake (P< 0.001) during a similar time (Fig. 2). Over the same time, ACL incidence has steadily risen from 1.2% in 1993 to 2.5 % in the peak epidemic in 2006. There was a significant excess of incidence rate (P< 0.001) among the cases during the two times before and after the earthquake (Fig. 2).
Fig. 2.
Distribution and incidence of anthroponotic cutaneous leishmaniosis cases in Bam before and after the earthquake by year (1993–2012)
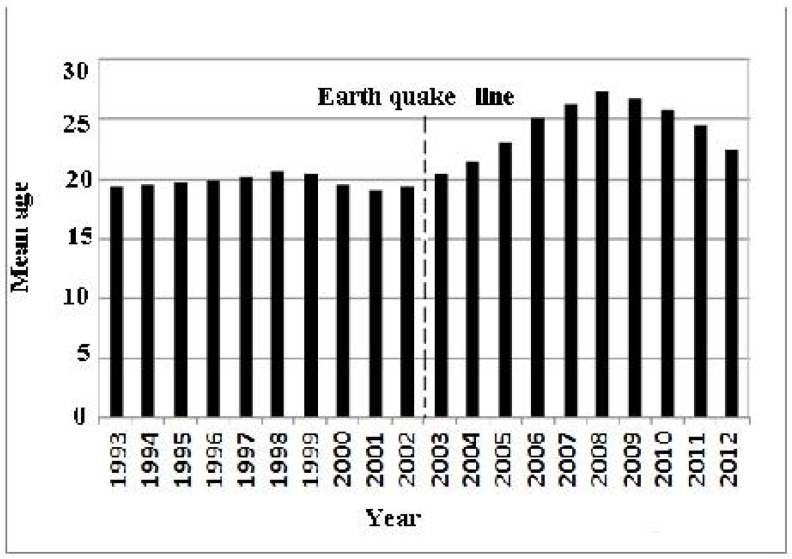
Most of the cases occurred in females (53.4%) and then in males (46.6%) 10 years before the earthquake. In contrast, proportion of females (43.7%) to males (56.3%) was reversing (Fig. 2) 10 years after the earthquake (P< 0.001). The majority of CL cases was recorded in individuals of <20 years old 10 years before the earthquake (mean age, 17 years), whereas this proportion changed in the corresponding period after the earthquake (Fig. 3) and there was a gradual shift of infection toward older individuals (mean age of 25 years).
Fig. 3.
Mean age distribution of anthroponotic cutaneous leishmaniosis cases in Bam before and after the earthquake by year (1993–2012)
Anatomical distributions of lesions were considerably different during these two time periods, face (60%) was the most site of involvement in the 10 years before the earthquake while location of involvement significantly changed to hands (59%) in the years after the earthquake (P< 0.001). Severity of diseases in terms of the number of lesions significantly changed before and after the earthquake (P< 0.05) (Sharifi et al. 2014).
Duration of the CL disease sharply decreased from 10.8 months to 6.1 months (P< 0.001) in the years after the earthquake. Majority of the cases originally came from four old zones before the earthquake, whereas in the period after the earthquake, the cases were distributed throughout the 12 zones within the city of Bam (Fig. 1).
Epidemic of anthroponotic cutaneous leishmaniasis in adjacent districts
A newly established focus of ACL with low endemicity occurred following the earthquake in the rural areas of Mohammadabad County, Jiroft District, adjacent to Dehbakri County, bordering Bam District (Poursmaelian et al. 2010). Originally, seven cases sporadically emerged between 1997 and 2002 prior to the earthquake. The cases sharply increased to 181 patients after 2003 to peak in 2006 and become endemic, thereafter. The overall prevalence rate was 5.3%, 6.2% in females and 4.5% in males, resulting in a significant gender difference (P< 0.05). Most of the lesions were single and on the face (47%) followed by hands and legs (39%). Nested-PCR showed L. tropica, the only causative parasite.
Emergence of anthroponotic cutaneous leishmaniasis following the earthquake southern villages of Bam
A newly emerging focus of ACL was identified in southern villages (Nazamshahr County) of Bam District (Aflatoonian et al. 2013). Overall, 1.2% of the inhabitants were infected (0.5% active and 0.7% scars) and females were more significantly infected (1.7%) than males (0.8%) (P= 0.003). All the age groups were equally affected. Most of the lesions were on the face and majority of them had single lesion. Most of the cases appeared from 2006 to 2008 during the CL epidemic in the city of Bam. PCR indicated L. tropica as the causative agent.
Northwestern villages of Bam
Another epidemic of ACL due to L. tropica occurred in rural areas of Dehbakri County, North West of Bam District (Sharifi et al. 2011c). Again, all the age groups were affected although patients ≤10 years of age showed the highest rate of infection (P= 0.0001). The overall prevalence rate was 5.3%, 6.3% in females and 4.3% in males. Out of 204 cases, 1.8% had active sores and 3.5% showed scars with a significant difference between the genders (P= 0.005). Of the lesions, 47% were on the face and 77.9% had one lesion. The incidence gradually increased 2004–2005 but grew exponentially 2006–2008.
Vector susceptibility test
Baseline susceptibility tests were carried out on field-collected strains of P. papatasi and P. sergenti and tested using WHO impregnated papers with DDT 4% and deltamethrin 0.05% in the endemic focus of disease in Dehbakri County during 2010. LT50 values of DDT4% and deltamethrin 0.05% against P. papatasi were 20.6 and 13.6 min, respectively. The same data for P. sergenti ranged between 21.8 and 17.7 min (Aghaei Afshar et al. 2011). The impact and outcome indicators following residual spraying with deltamethrin were assessed in a new focus of CL in Dahbakri (Bam). Incidence of the disease was significantly decreased (3.7 vs. 8.5). There was a significant difference between the treatment and control areas on blood fed and gravity of sand flies. The results clearly indicated that indoor residual spraying caused a significant decline of density, blood fed and gravity of vectors, resulting in a sharp reduction of disease incidence (Aghaei Afshar et al. 2013).
Cost of treatment and prevention
In a descriptive cross-sectional survey, data about treatment and prevention costs of 5320 CL patients during the two years of epidemic (2006 and 2007) were analyzed (Aflatoonian et al. 2009). The per capita treatment cost was estimated to be US$ 48, which showed a significant difference in terms of cost effectiveness as compared with the patients in other CL endemic countries. It seems that the implementation of preventive measures had a negligible effect on the ACL control strategy (Aflatoonian et al. 2010). The authors concluded that establishment of CLC in Bam reduced the disease costs and provided an efficient, free and easy accessible service for the patients after the earthquake. This experience could be a suitable model to be used in a similar disaster situation in other parts of the world.
Discussion
Global data indicate that CL is spreading and extending its range to new foci, especially after major natural and anthropogenic environmental changes (Daszak et al. 2001, Alvar et al. 2012). Such changes can activate various risk factors and lead to alterations in the density and range of vector and reservoir population, thereby enhancing accessibility of humans to infected sand fly bites (Patz et al. 2000, Desjeux 2001). The review of data indicate that majority of the CL cases occurred in male individuals of higher ages in the period after the earthquake. The reason was mainly due to arrival of sensitive male labor forces to the city of Bam for various positions, which was created after the earthquake (Sharifi et al. 2011b). Similar risk factors have contributed major roles to the emerging epidemics of CL or other infectious diseases in Iran and abroad (Dye and Wolpert 1988, Bayramgurler et al. 2002, Shultz et al. 2005, Smith 2005, Fazaeli 2009, Fakoorziba et al. 2010, Kouadio et al. 2012, Zhang et al. 2013).
Despite implementation of comprehensive control measures ACL occurred in several localities within the district of Bam. However, such control approaches have not adequately been evaluated. Although, large impregnated curtains and screens were assessed in several places within the city of Bam. Significant effectiveness of these control measures was observed. In addition, the impact and outcome indicators following application of residual spraying with deltamethrin were evaluated in a new emerging focus of ACL in Dahbakri County, North-West of Bam district. The incidence rate was significantly decreased (Aghaei Afshar et al. 2013). Most of the cases occurred in non-indigenous individuals who arrived to the affected areas from neighboring districts and various provinces of the country, after the earthquake. Majority of whom were non-immune labors and contractors who traveled from non-CL endemic areas to the city of Bam where CL infection was endemic (Fig. 1). The incidence of CL was in order of 3-fold in non-local population during the peak epidemic (2006) compared to the indigenous residents (Aflatoonian and Sharifi 2010). While the incidence of disease was relatively stable in local residents, it rose gradually from 2005 to 2007 in the city of Bam (Sharifi et al. 2011b).
Conclusions
The findings presented herein strongly suggested that, following the earthquake, major risk factors were created which were associated with epidemics and emergences of CL in Bam district and adjacent areas. Such precipitating determinants included suitable environmental conditions, poor hygienic conditions, individual and behavioral changes and arrival of non-immune population. These factors could activate epidemics of old foci and induce new emerging foci. Epidemiological and clinical features of the disease in terms of gender, age, location, severity and duration of the lesions significantly changed after the earthquake. Proper surveillance, followed by prompt diagnosis and subsequent treatment, should be extended to all areas with high risk in order to prevent and control epidemics and/or emergences of ACL, particularly in endemic foci after a major natural disaster.
Acknowledgements
We thank all the partners and authors who contributed information and added to the scientific merits of this work. We would also like to thank the personnel in Bam Health System, cutaneous leishmaniasis clinic and Kerman Leishmaniasis Research Center for their extensive work during the last two decades. The authors declare that there is no conflict of interests.
References
- Abolghasemi H, Radfar MH, Khatami M, Saghafi Nia M, Amid A, Briggs S. ( 2006) International medical response to a natural disaster: lessons learned from the Bam earthquake experience. Prehosp Disaster Med. 21: 141– 147. [DOI] [PubMed] [Google Scholar]
- Aflatoonian MR, Sharifi I, Fekri AR. ( 2009) Evaluation of the cost-effectiveness of cutaneous leishmaniasis treatment after the earthquake. J Kerman Univ Med Sci. 4: 365– 373. [Google Scholar]
- Aflatoonian MR, Sharifi I, Abbasi R, Ranjbar L. ( 2010) To evaluate the cost of prevention on incidence of cutaneous leishmaniasis due to earthquake in Bam. Iran J Epidemiol. 6: 32– 38. [Google Scholar]
- Aflatoonian MR, Sharifi I. ( 2010) prevalence rate of cutaneous leishmaniasis in Bam district during 20 years (1988–2007). J Kerman Univ Med Sci. 17 ( 4): 297– 306. [Google Scholar]
- Aflatoonian MR, Sharifi I. ( 2011) The epidemiology of cutaneous leishmaniasis in the city and suburb of Bam in 2010: active case-finding, treatment and health education of the school children. Iran J Epidemiol. 7: 52– 57. [Google Scholar]
- Aflatoonian MR, Sharifi I, Poursmaelian S, Hakimi-Parizi M, Ziaali N. ( 2013) The emergence of anthroponotic cutaneous leishmaniasis following the earthquake in southern villages of Bam district, southeastern Iran, 2010. J Arthropod-Borne Dis. 7: 8– 14. [PMC free article] [PubMed] [Google Scholar]
- Aghasi M, Sharifi I. ( 2003) Survey of the fauna and monthly activity of the sand fly as the vector of the cutaneous leishmaniasis in the city of Bam. J Kerman Univ Med Sci. 10: 85– 91. [Google Scholar]
- Aghae Afshar A, Rassi Y, Sharifi I, Abai MR, Oshaghi MA, Yaghoobi-Ershadi MR, Vatandoost H. ( 2011) Susceptibility status of Phlebotomus papatasi and P. sergenti (Diptera: Psychodidae) to DDT and Deltamethrin in a focus of cutaneous leishmaniasis after earthquake strike in Bam, Iran. Iran J Arthropod-Borne Dis. 5: 32– 41. [PMC free article] [PubMed] [Google Scholar]
- Aghaei Afshar A, Vatandoost H, Sharifi I, Rassi Y, Abai MR, Oshaghi MA, Yaghoobi-Ershadi MR, Rafizadeh S. ( 2013) First determination of impact and outcome indicators following indoor residual spraying (IRS) with deltamethrin in a new focus of anthroponotic cutaneous leishmaniasis (ACL) in Iran. Asian Pac J Trop Dis. 3: 5– 9. [Google Scholar]
- Akhavan AA, Yaghoobi-Ershadi MR, Hasibi F, Jafari R, Abdoli H, Arandian MH, Soleimani H, Zahraei-Ramazani AR, Mohebali M, Hajjaran H. ( 2007) Emergence of cutaneous leishmaniasis due to Leishmania major in a new focus of southern Iran. Iran J Arthropod-Borne Dis. 1: 1– 8. [Google Scholar]
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J, Boer M. ( 2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford RW. ( 2000) The leishmaniasis as emerging and reemerging zoonoses. Int J Parasitol. 30: 1269– 1281. [DOI] [PubMed] [Google Scholar]
- Bayramgürler D, Bilen N, Namli S, Altinaş L, Apaydin R. ( 2002) The effects of 17 August Marmara earthquake on patient admittances to our dermatology department. J Eur Acad Dermatol Venereol. 16: 249– 245. [DOI] [PubMed] [Google Scholar]
- Croft SL, Sunder S, Fairlamb AH. ( 2006) Drug resistance in leishmaniasis. Clin Microbiol Rev. 19: 111– 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daszak P, Cunningham AA, Hyatt AD. ( 2001) Anthropogenic environmental changes and the emergence of infectious diseases in wildlife. Acta Trop. 78: 103– 116. [DOI] [PubMed] [Google Scholar]
- Desjeux P. ( 2001) The increase in risk factors for the leishmaniasis worldwide. Trans Roy Soc Trop Med Hyg. 95: 239– 243. [DOI] [PubMed] [Google Scholar]
- Dye C, Wolpert DM. ( 1988) Earthquakes, influenza and cycles of Indian kalaazar. Trans Roy Soc Trop Med Hyg. 82: 843– 850. [DOI] [PubMed] [Google Scholar]
- Esfandiarpour I, Dabiri SH. ( 2007) Treatment of cutaneous leishmaniasis recidivans with a combination of allopurinol and meglumine antimoniate: a clinical and histologic study. Int J Dermatol. 46: 848– 852. [DOI] [PubMed] [Google Scholar]
- Fakoorziba MR, Baseri A, Eghbal F, Rezaee S, Azizi K, Moemenbellah-Fard ( 2011) Post-earthquake outbreak of cutaneous leishmaniasis in a rural region of southern Iran. Ann Trop Med Parasitol. 105: 217– 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazaeli A, Fouladi B, Sharifi I. ( 2009) Emergence of cutaneous leishmaniasis in a border area at south-east of Iran: an epidemiological survey. J Vector Borne Dis. 46: 36– 42. [PubMed] [Google Scholar]
- Kolaczinski J, Brooker S, Reyburn H, Rowland M. ( 2004) Epidemiology of anthroponotic cutaneous leishmaniasis in Afghan refugee camps in northwest Pakistan. Trans Roy Soc Trop Med Hyg. 98: 373– 378. [DOI] [PubMed] [Google Scholar]
- Kouadio Ik, Aljunid S, Kamigaki T, Hammad K, Oshitani H. ( 2012) Infectious diseases following natural disasters: prevention and control measures. Expert Rev Anti Infect Ther. 10: 95– 104. [DOI] [PubMed] [Google Scholar]
- Macpherson CNL. ( 2005) Human behaviour and the epidemiology of parasitic zoonoses. Int J Parasitol. 35: 1319– 1331. [DOI] [PubMed] [Google Scholar]
- Nadim A, Aflatoonian MR. ( 1995) Anthroponotic cutaneous leishmaniasis in Bam, southeast Iran. Iran J Publ Health. 24: 15– 24. [Google Scholar]
- Nadim A, Motabar M, Houshmand B, Keyghobadi K, Aflatoonian MR. ( 1995) Evaluation of pyrethroid impregnated bednets for control of anthroponotic cutaneous leishmaniasis in Bam (Islamic Republic of Iran). World Health Organization /Leish/95.37; Geneva. [Google Scholar]
- Noazin S, Shirzadi MR, Kermanizadeh A, Yaghoobi-Ershadi MR, Sharifi I. ( 2013) Effect of large-scale installation of deltamethrin-impregnated screens and curtains in Bam, a major focus of anthroponotic cutaneous leishmaniasis in Iran. Trans Roy Soc Trop Med Hyg. 107( 7): 444– 450. [DOI] [PubMed] [Google Scholar]
- Ocampo CB, Ferro MC, Cadena H, Gongora R, Pérez M, Valderrama-Ardila CH. ( 2012) Environmental factors associated with American cutaneous leishmaniasis in a new Andean focus in Colombia. Trop Med Int Health. 17: 1309– 1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA, Graczyk TK, Geller N, Vittor AY. ( 2000) Effects of environmental changes on emerging parasitic diseases. Int J Parasitol. 30: 1395– 1405. [DOI] [PubMed] [Google Scholar]
- Poursmaelian S, Mirzaei M, Sharifi I, Zarean M. ( 2010) The prevalence of cutaneous leishmaniasis in the city and suburb of Mohammadabad, Jiroft district and identification of parasite species by nested-PCR, 2008. J Kerman Univ Med Sci. 18: 218– 227. [Google Scholar]
- Rassi Y, Abai MR, Javadian E, Rafizadeh S, Imamian H, Mohebali M, Fateh M, Hajjaran H, Ismaili K. ( 2008) Molecular data on vectors and reservoir hosts of zoonotic cutaneous leishmaniasis in central Iran. Bull Soc Path Exot. 101: 425– 428. [PubMed] [Google Scholar]
- Ready PD. ( 2011) Leishmaniasis emergence in Europe (a review article). Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19505. [PubMed]
- Reyburn H, Rowland M, Mohsen M, Khan B, Davies C. ( 2003) The prolonged epidemic of anthroponotic cutaneous leishmaniasis in Kabul, Afghanistan: bringing down the neighborhood. Trans Roy Soc Trop Med Hyg. 97: 170– 176. [DOI] [PubMed] [Google Scholar]
- Sharifi I, Ardehali S, Motazadian H, Aflatoonian MR. ( 1997) Identification and characterization of Leishmania isolates in school children in Bam, southeastern Iran. Iran J Med Sci. 22: 82– 88. [Google Scholar]
- Sharifi I, Fekri AR, Aflatoonian MR, Khamesipour A, Nadim A, Mousavi MR, Momeni AZ, Dowlati Y, Godal T, Zicker F, Smith PG, Modabber F. ( 1998a) Randomized vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 351: 1540– 1544. [DOI] [PubMed] [Google Scholar]
- Sharifi I, Fekri AR, Aflatoonian MR, Khamesipour A, Mahboudi F, Dowlati Y, Nadim A, Modabber F. ( 2010) Leishmaniasis recidivans among school children in Bam, South-east Iran, 1994–2006. Int J Dermatol. 49: 557– 561. [DOI] [PubMed] [Google Scholar]
- Sharifi F, Sharifi I, Zarean M, Hakimi Parizi M, Aflatoonian MR, Fasihi Harandi M, Zahmatkesh R, Mashayekhi M, Kermanizadeh AR. ( 2011a) Spatial distribution and molecular identification of Leishmania species from endemic foci of south-eastern Iran. Iran J Parasitol. 7: 45– 52. [PMC free article] [PubMed] [Google Scholar]
- Sharifi I, Nakhaei N, Aflatoonian MR, Hakimi Parizi M, Fekri AR, Safizadeh H, Shirzadi MR, Gooya MM, Khamesi-pour A, Nadim A. ( 2011b) Cutaneous leishmaniasis in Bam: a comparative evaluation of pre- and post-earthquake years (1999–2008). Iran J Public Health. 40: 49– 56. [PMC free article] [PubMed] [Google Scholar]
- Sharifi I, Poursmaelian S, Aflatoonian MR, Ardakani RF, Mirzaei M, Fekri AR, Khamesipour A, Parizi MH, Harandi MF. ( 2011c) Emergence of a new focus of anthroponotic cutaneous leishmaniasis due to Leishmania tropica in rural communities of Bam district after the earthquake, Iran. Trop Med Int Health. 16: 510– 513. [DOI] [PubMed] [Google Scholar]
- Sharifi I, Aflatoonian MR, Aflatoonian B, Kermanizadeh A. ( 2014) The severity of cutaneous leishmaniasis before and after the earthquake in Bam, southeastern Iran. J Parasit Dis. (DOI 10.1007/s12639-014-0435-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirzadi MR, Gouya MM. ( 2012) National Guidelines for Cutaneous Leishmaniasis Surveillance in Iran. Ministry of Health and Medical Education, Zoonoses Control Department, Tehran Iran. [Google Scholar]
- Shultz JM, Russell J, Espinel Z. ( 2005) Epidemiology of tropical cyclones: the dynamics of disaster, disease, and development. Epidemiol Rev. 27: 21– 35. [DOI] [PubMed] [Google Scholar]
- Smith AW. ( 2005) Tsunami in south Asia: what is the risk of post-disaster infectious disease outbreaks. Ann Acad Med Singapore. 34: 625– 631. [PubMed] [Google Scholar]
- World Health Organization ( 2009) Leishmaniasis: background information. A brief history of the disease. Available at: www.who.int/leishmaniasis/en.
- World Health Organization ( 2010) Control of the leishmaniasis. Report of a Meeting of the WHO Export Committee on the Control of Leishmaniasis, WHO Technical Report Series 949, Geneva. [Google Scholar]
- Zhang S, Lu Z, Liu H, Xiao Xindong, Zhao Zongguo, Bao Genshu, Han Jian, Jing Tao, Chen Gen. ( 2013) Incidence of Japanese encephalitis visceral leishmaniasis and malaria before and after the Wechuan earthquake, in China. Acta Trop. 1: 85– 89. [DOI] [PubMed] [Google Scholar]