Abstract
Background:
Mosquitoes transmit serious human diseases, causing millions of death every year. Vector control is facing a threat due to the emergence of resistance to synthetic insecticides. Insecticides of botanical origin may serve as suitable alternative biocontrol techniques in the future. Nine different locally available medicinally important plants suspected to posse larvicidal property were screened against fourth instar larvae of Aedes aegypti and Anopheles stephensi to a series of concentrations of the methanolic extracts.
Methods:
Susceptibility tests on Ae. aegypti and An. stephensi were conducted using standard WHO methods. The larvae of two mosquito species were exposed to methanolic extracts and mortality counts were made after 24 hours of exposure as per WHO method. Larvae of Ae. aegypti were more susceptible than that of An. stephensi.
Results:
Among the nine plant species tested, Annona reticulata leaf extract was more effective against Ae. aegypti larvae with LC50 and LC90 values of 95.24 and 262.64 ppm respectively and against An. stephensi larvae 262.71 and 636.94 ppm respectively. The least efficacy was in Cosmos bipinnatus with LC50 and LC90 values of 442.6 and 1225.93 ppm against Ae. aegypti and LC50 and LC90 values of 840.69 and 1334.01 ppm of Thespesia populnea against An. stephensi.
Conclusion:
The crude methanolic extract of the An. reticulata with good larvicidal efficacy could be considered for further characterization to control mosquito vectors instead of chemical insecticides. High efficacy found in An. reticulata extract will be considered for further studies to isolate the bioactive compound.
Keywords: Ae. aegypti, An. stephensi, Annona reticulata, Larvicide, Thespesia populnea
Introduction
Many new and re-emerging diseases are transmitted by Arthropod vectors (Brogdon and Mc Alister 1998). “Vector borne diseases account for around 17% of the estimated global burden of all infectious diseases” (WHO 2006). Over 350 species of mosquitoes are vectors of pathogens that cause diseases in humans and domesticated animals. More than fourteen mosquito genera are known to harbour arboviruses (Mattingly 1973). “These diseases contribute significantly to disease burden, death, poverty and social debility in tropical countries” (Yang et al. 2004). The proliferation of the diseases is not only due to the higher number of breeding places in urban agglomeration, but also due to increasing resistance of mosquitoes to current commercial insecticides such as organo-chlorides, organo-phosphates, carbamates, pyrethroids and also to biological insecticides (Goettel et al. 1992, Das and Amalraj 1997, Yadav et al. 1997).
Thus, mosquitoes are responsible for the transmission of more diseases than any other group of arthropods and play an important role as etiologic agents of devastating malaria, filariasis, dengue, yellow fever, Japanese encephalitis, chikungunya and other viral diseases. In addition, they also cause irritation to human by causing allergic responses that include local skin reactions as well as systemic reactions such as angioedema and urticaria (Peng et al. 1999). Aedes aegypti, a vector of yellow fever, dengue and chikungunya is widely distributed in the tropical and subtropical zones. About two-third of the world’s population, live in areas infested with dengue vectors, mainly Ae. aegypti (Hahn et al. 2001). Anopheles stephensi is the primary vector of malaria in India and other west Asian countries. Every year, an estimated 300–500 million new infections and 600,000 cases based on world malaria report 2013 (WHO 2013) deaths result from malaria worldwide.
In the past decade, chikungunya - a virus transmitted by Ae. spp. mosquitoes - has re-emerged in Africa, southern and southeastern Asia, and the Indian Ocean Islands as the cause of large outbreaks of human disease (Burt et al. 2012). Malaria is a protozoan infection of erythrocytes caused in human beings by five species of the genus Plasmodium (P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi). In most cases, malaria is transmitted via the bite of an infected female anopheline mosquito, but congenital malaria and acquisition through infected blood transfusion are well described (Falade et al. 2007). “More than 40 per cent of the world’s population-approximately 3 billion people are exposed to malaria in 108 endemic countries “(WHO 2009). About one million cases of malaria are reported in India every year.
Many approaches have been developed to control mosquito menace. One such approach to prevent mosquito borne disease is by employing larvicide. The current mosquito control approach is based on synthetic insecticides. Even though their effectiveness, they created many problems like insecticide resistance (Liu et al. 2005), pollution and toxic side effects on human beings (Lixin et al. 2006). This has necessitated the need for research and development of environmentally safe, biodegradable, indigenous method for vector control. Botanicals offer great promise as source of phyto-chemicals with proven potential as insecticides which can play an important role in the control of mosquitoes and in the interruption of disease transmission at individual as well at community level (Mittal and Subbarao 2003). Six plant families with several representative species, Asteracae, Cladophoraceae, Labiatae, Meliaceae, Oocystacae and Rutaceae appear to have the greatest potential for providing future mosquito control agents. Insecticides of plant origin do not cause toxicity to human and domestic animals and are easily biodegradable. In the present study leaf extract of the plant Passifloraceae, Annonaceae, Asteraceae, Lauraceae, Malvaceae, Lamiaceae was studied for its insecticidal activity against malaria and dengue vectors. The application of botanical derivatives against mosquito has been reviewed in detailed by Sukumar et al. (1991).
In this regard, of late the researchers have shown more interest on plant derivatives as botanicals offer great promise as sources of molecules for the control of both agricultural pests and medically important insect species. Now a days, the increased use of phyto-chemicals for the control of these insects may be attributed to the fact that population throughout the world are aware of the danger inherent in conventional insecticides, particularly the detrimental effect on the environment (Pitasawat et al. 1998). It is in this regard, plant derived products have received increased attention from scientists and more than 2000 plant species are already known to have insecticidal properties (Balandrin et al. 1985, Sukumar et al. 1991). Natural insecticides such as pyrethrum, rotenone and nicotine among others have been extensively used until recently for insect control (Balandrin et al. 1985).
To search derivatives with insecticidal property from local plants, the present investigation has been made in the Vector Biology Research Lab, Department of Studies in Zoology at University of Mysore.
Materials and Methods
Aedes aegypti and An. stephensi larvae available at the mosquito colony maintained in Vector Biology Research Lab, Department of Studies in Zoology, University of Mysore by following standard rearing techniques. The larvae were reared in large enamel or plastic trays (30×24×5 cm) containing dechlorinated water and fed using finely powdered dog biscuits and dry yeast in the ratio of 2: 1.
Plant material and extraction
Nine plant species as listed in Table 1 were collected from in and around Mysore, Karnataka, from March 2012 to Jan 2013 and the leaves were shade dried, powdered manually and subjected to methanol solvent extraction in a Soxhlet apparatus until exhaustion, to obtain non-polar bioactive constituents. The pooled extract was evaporated in a rotory vacuum evaporator at 40 °C to dryness and stored at 4 °C in an air tight bottle for further analysis. This was later dissolved in acetone and employed to prepare different concentrations.
Table 1.
List of plant screened against Aedes aegypti and Anopheles stephensi
| No | Plants name | Family | Medicinal/toxic property |
|---|---|---|---|
| 1 | Passiflora foetida | Passifloraceae | Pitta, inflammation, insomnia, depression and anxiety disorders |
| 2 | Annona glabra | Annonaceae | Anticancer effects, substantial antimicrobial, antifungal and moderate insecticidal, sporicidal and cytotoxic |
| 3 | Cosmos bipinnatus | Asteraceae | Jaundice, intermittent fever and splenomegaly |
| 4 | Laurus nobilis | Lauraceae | rheumatism and dermatitis |
| 5 | Abutilon indicum | Malvaceae | Febrifuge, anthelmintic, antiemetic, anti-inflammatory, and in urinary and uterine discharges, piles, and lumbago |
| 6 | Gossypium herbaceum | Annonaceae | Febrifuge, anthelmintic, antiemetic, anti-inflammatory. |
| 7 | Annona reticulata | Annonaceae | Febrifuge, anthelmintic, antiemetic, anti-inflammatory, and in urinary and uterine discharges, piles, and lumbago |
| 8 | Hyptis suoveolens | Lamiaceae | Gastrointestinal infections, cramps, and pain, skin infections |
| 9 | Thespesia populnea | Malvaceae | Antifertility, antibacterial, antiinflammatory, antioxidant, purgative and hepatoprotective activity |
Larval bioassay
Bioassays on mosquito larvae were performed on late third or early fourth instars, according to standard guidelines of WHO (2005). The required quantity of plant extract of different concentrations was prepared in acetone as solvent. One ml of each of the concentration was mixed thoroughly with 249 ml of dechlorinated water in 500 ml glass beakers. Larvae were exposed in an ascending series of five concentrations according to log dose (Table 2). Parallel control tests were also maintained by adding 1ml of the solvent to 249 ml of dechlorinated water. Twenty five early fourth instar larvae were transferred to each of the beakers. A minimum of three replicates were kept for each concentration along with the control. Observation for the dead or moribund larvae was carried out after 24 h duration at 25±2 °C and 75±5 % of relative humidity (RH).
Table 2.
Concentration and Mortality of Nine plant species against Aedes aegypti and Anopheles stephensi
| No | Plant Name | Aedes aegypti | Anopheles stephensi | ||
|---|---|---|---|---|---|
| Concentration(ppm) | Mortality (%) | Concentration(ppm) | Mortality (%) | ||
| 1 | Passiflora foetida | 100 | 14 | 200 | 12 |
| 150 | 36 | 350 | 30 | ||
| 200 | 56 | 500 | 54 | ||
| 250 | 78 | 650 | 74 | ||
| 300 | 96 | 800 | 90 | ||
| 2 | Annona glabra | 100 | 16 | 50 | 10 |
| 150 | 34 | 200 | 28 | ||
| 250 | 60 | 400 | 48 | ||
| 400 | 74 | 550 | 70 | ||
| 600 | 90 | 700 | 92 | ||
| 3 | Cosmos bipinnatus | 150 | 10 | 50 | 12 |
| 300 | 28 | 200 | 30 | ||
| 450 | 52 | 350 | 52 | ||
| 600 | 64 | 500 | 78 | ||
| 750 | 76 | 750 | 90 | ||
| 4 | Laurus nobilis | 100 | 12 | 100 | 14 |
| 250 | 28 | 250 | 36 | ||
| 350 | 58 | 400 | 52 | ||
| 500 | 78 | 750 | 74 | ||
| 700 | 88 | 900 | 92 | ||
| 5 | Abutilon indicum | 100 | 12 | 150 | 16 |
| 300 | 28 | 300 | 36 | ||
| 450 | 62 | 450 | 50 | ||
| 550 | 78 | 700 | 76 | ||
| 750 | 92 | 750 | 90 | ||
| 6 | Gossypium herbaceum | 150 | 10 | 100 | 08 |
| 200 | 34 | 250 | 30 | ||
| 250 | 62 | 400 | 48 | ||
| 300 | 76 | 550 | 74 | ||
| 350 | 90 | 800 | 88 | ||
| 7 | Annona reticulata | 30 | 12 | 100 | 12 |
| 100 | 40 | 200 | 30 | ||
| 150 | 68 | 300 | 52 | ||
| 200 | 84 | 400 | 70 | ||
| 250 | 96 | 500 | 90 | ||
| 8 | Hyptis suoveolens | 150 | 12 | 150 | 08 |
| 250 | 32 | 300 | 32 | ||
| 350 | 48 | 450 | 50 | ||
| 400 | 66 | 600 | 76 | ||
| 550 | 82 | 750 | 94 | ||
| 9 | Thespesia populnea | 100 | 10 | 500 | 10 |
| 250 | 28 | 700 | 30 | ||
| 400 | 48 | 900 | 52 | ||
| 550 | 68 | 1100 | 78 | ||
| 700 | 90 | 1300 | 92 | ||
Data analysis
Larval mortality counts were adjusted for the mortality in control, if any, by employing Abbott’s formula (Abbott 1975) to give an estimate of the plant extract attributable mortality. The corrected mortality data were subjected to regression analysis of probit mortality on log dosage (Finney 1971). The significant difference in LC50 is based on the non-overlapping of 95 % Fiducial limits.
Results
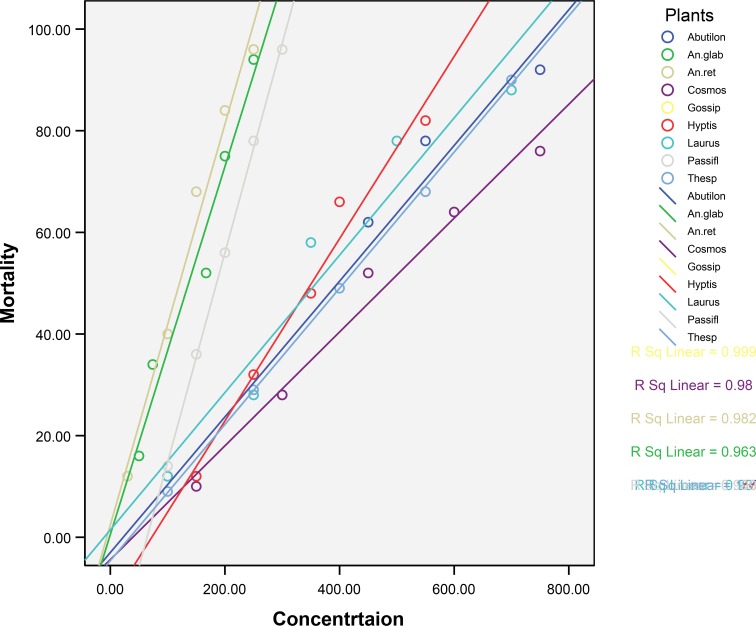
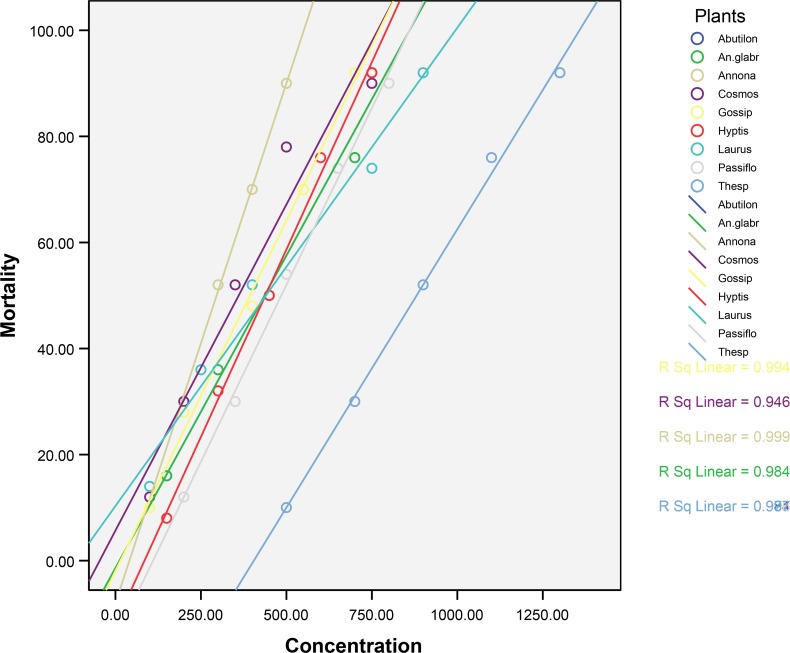
Table 2 provides the mortality rate of mosquito larvae against different concentrations of methanol extracts. Table 3 and 4 provide the efficacy of methanol extracts tested against the dengue vector Ae. aegypti and malarial vector An. stephensi larvae. Fig. 1 and 2 show the log dose-probit mortality responses of all plant extracts. Though the plants exhibited larvicidal activity against the two mosquito species, out of the nine plants screened using methanol as solvent, the Annona reticulata (Annonaceae) was found to possess better larvicidal activity against Ae. aegypti followed by An. stephensi. The high percentage of mortality with low concentration was recorded of in the species against Ae. aegypti has been with LC50 and LC90 values being 95.24 and 262.64 ppm respectively (Table 3). Likewise LC50 and LC90 values against An. stephensi are 262.71 and 636.94 ppm respectively (Table 4). The 95% Fiducial Limits (FL) of An. reticulata for LC50 is 46.83–139.69 and 95% FL for LC90 is 172.23–931.30 and the slope is 2.90±0.55 against Ae. aegypti. Similarly, the 95% FL for LC50 is 197.21–137.54 and for LC90 it is 457.41-1434.98 with the slope being 3.33±0.49 against An. stephensi. Among these two mosquito species, susceptibility of Ae. aegypti was found to be significantly more than that of An. stephensi. Further, the larvicidal efficacy of An. reticulata was found to be significant by more compared to other plants (P< 0.05). The figure depicts the log dose-probit mortality responses and slope regression lines of tested plants.
Table 3.
Efficacy of methanolic extracts of nine plant species against Aedes aegypti
| No | Plant name | LC50 (ppm) | 95% FL | LC90 (ppm) | 95% FL | Slope±SE | Heterogeneity (df) | Regression equation |
|---|---|---|---|---|---|---|---|---|
| 1 | Annona reticulata* | 95.24 | 46.83–139.69 | 262.64 | 172.23–931.30 | 2.90±0.55 | 5.20(3) | Y=2.90X±0.75 |
| 2 | Annona glabra | 114.47 | 54.99–191.18 | 320.82 | 191.84–3738.15 | 2.86±0.64 | 6.98(3) | Y=2.86X±0.89 |
| 3 | Passiflora foetida | 172.31 | 143.20–200.36 | 300.99 | 96.24–242.06 | 5.29±0.70 | 2.73(3) | Y=5.29X±6.83 |
| 4 | Gossypium herbaceum | 216.23 | 194.57–239.21 | 620 | 524.18–778.30 | 2.79±0.24 | 1(3) | Y=2.79X±1.53 |
| 5 | Laurus nobilis | 302.66 | 207.85–409.02 | 813.94 | 555.81–2193.14 | 2.98±0.47 | 3.35(3) | Y=2.98X±2.39 |
| 6 | Hyptis suaveolens | 327.18 | 303.55–352.76 | 720.37 | 624.61–880.33 | 3.73±0.35 | 1(3) | Y=3.73X±4.40 |
| 7 | Abutilon indicum | 332.98 | 123.63–546.59 | 903.76 | 549.46–692.12 | 2.95±0.71 | 7.40(3) | Y=2.95X±2.45 |
| 8 | Thespesia populnea | 353.07 | 236.81–498.69 | 969.48 | 635.18–3376.91 | 2.92±0.50 | 3.79(3) | Y=2.92X±2.44 |
| 9 | Cosmos bipinnatus | 442.60 | 402.11–488.40 | 1225.93 | 1013.27–2710 | 2.89±0.28 | 1(3) | Y=2.89X±2.66 |
Note: LC50 - median lethal concentration, FL - fiducial limits; LC90 - lethal concentration, df - degree of freedom.
Difference in LC50 from the extracts of other eight plants is significant based on non-overlapping 95% fiducial limits (P< 0.05).
Table 4.
Efficacy of methanolic extracts of nine plant species against Anopheles stephensi
| No | Plant name | LC50 (ppm) | 95% FL | LC90 (ppm) | 95% FL | Slope±SE | Heterogeneity (df) | Regression equation |
|---|---|---|---|---|---|---|---|---|
| 1 | Annona reticulata* | 262.71 | 197.21–337.54 | 636.94 | 457.41–1434.98 | 3.33±0.49 | 2.89(3) | Y=3.33X±3.06 |
| 2 | Cosmos bipinnatus | 258.39 | 105.36–471.41 | 1048.00 | 544.56–1326.18 | 2.10±0.45 | 5.74(3) | Y=2.10X±0.68 |
| 3 | Gossypium herbaceum | 298.80 | 62.13–768.79 | 1221.72 | 564.60–1130.12 | 2.09±0.55 | 8.40(3) | Y=2.09X±0.18 |
| 4 | Laurus nobilis | 336.57 | 222.28–475.98 | 1197.75 | 757.60–3516.32 | 2.32±0.33 | 2.82(3) | Y=2.32X±0.87 |
| 5 | Abutilon indicum | 372.81 | 231.41–532.21 | 1000.95 | 655.62–4054.35 | 2.98±0.56 | 4.51(3) | Y=2.98X±2.68 |
| 6 | Hyptis suaveolens | 391.66 | 361.46–422.54 | 843.60 | 748.86–986.10 | 3.84±0.31 | 1(3) | Y=3.84X±4.97 |
| 7 | Passiflora foetida | 441.01 | 409.18–473.62 | 922.27 | 820.34–1077.59 | 3.99±0.33 | 1(3) | Y=3.99X±5.57 |
| 8 | Annona glabra | 448.78 | 338.06–570.24 | 900.18 | 675.62–1951.54 | 4.23±0.69 | 3.94(3) | Y=4.23X±6.24 |
| 9 | Thespesia populnea | 840.69 | 801.16–880.57 | 1344.01 | 1249.14–1478.27 | 6.28±0.50 | 1(3) | Y=6.28X±13.39 |
Note: LC50 median lethal concentration, FL, fiducial limits, LC90 lethal concentration, df, degree of freedom.
Difference in LC50 from the extracts of other eight plants is significant based on non-overlapping 95 % fiducial limits (P< 0.05).
Fig. 1.
Regression graph showing mortality of methanolic extracts of different plant species on Aedes aegypti
Fig. 2.
Regression graph showing mortality of methanolic extracts of different plant species on Anopheles Stephensi
Discussion
The results of the larvicidal bioassay employing different plant extracts against two different mosquito species (Table 3, 4) indicate significant larvicidal activity (P< 0.05) with methanol extract. The biological activity of these plant extract may be due to various compounds such as, phenolics, terpenoids, flavonoids and alkaloids (Gohil et al. 2010). Such compounds may jointly or independently contribute to produce toxic activity against the mosquito species.
Environmental safety of an insecticide is of paramount importance while employing against pests and vectors. An insecticide need not cause high mortality on target organisms in order to be acceptable (Kabaru and Gichia 2001). Resistance to insecticides dates back to the beginning of application of chemicals, since DDT was initially introduced for mosquito control in 1946 and just in one year, the first case of DDT resistance occurred in Ae. tritaeniorhynchus and Ae. sollicitans (Hemingway and Ranson 2000). More than 500 species of arthropods are reported to be resistant to various insecticides (Shelton et al. 2007). In this regard, phyto-chemicals may serve as suitable alternatives to synthetic insecticides in future, as they are relatively safe, inexpensive and are readily available throughout the world. According to Bowers et al. (1995), the screening of locally available medicinal plants for mosquito control will be cost effective, reduce dependence on expensive imported products and stimulate local efforts to enhance public health. It is in this regard, the present study adds to our knowledge on the efficacy of the locally available medicinal plants.
An earlier report points that, ethanolic extract of Annona squamosa leaf has the most promising larvicidal activity against Cx. quinquefasciatus larvae (Das et al. 2007). The larvicidal and growth regulating activities of Annona squamosa and Syzygium cumini two related species have been reported against An. stephensi and other mosquitoes (Saxena et al. 1993 and Kaushik and Saini 2008). The significant activity demonstrated by extracts of A. squamosa and A. senegalensis suggest that the two plants may have strong killing effects against insects particularly mosquitoes, hence giving a promising source of larvicidal agents (Magadula et al. 2009). Previously, a collection of A. squamosa plant materials from Brazil indicated larvicidal effect against Ae. albopictus and Culex quinquefasciatus (Das et al. 2007) and against An. stephensi (Saxena et al. 1993). However, no reports on the larvicidal efficacy are available on An. reticulata. By comparing our results with the earlier studies, it is evident that the methanolic extracts of An. reticulata leaf have promising larvicidal activity against two mosquito species. It shows the LC50 value of 95.24 against Ae. aegypti and 262.71 against An. stephensi (Tables 3, 4).
Karunamoorthi and Ilango (2010) have reported that the LC50 and LC90 values of methanol leaf extracts of Croton macrostachyus (C. macrostachyus) were 89.25 and 224.98 ppm, respectively against late third instar larvae of malaria vector, An. arabiensis (An. arabiensis). The crude leaf extract of Azadirachta indica with different solvents, viz. benzene, chloroform, ethyl acetate and methanol were tested for larvicidal activity against An. stephensi. The LC50 values were 19.25, 27.26, 23.26 and 15.03, respectively. Kamaraj et al. (2009) have reported that the peel methanol extract C. sinensis, leaf and flower ethyl acetate extracts of Ocimum canum against larvae of An. stephensi (LC50 =95.74, 101.53, 28.96, LC90=303.20, 492.43 and 168.05 ppm) respectively. The highest larval mortality was found in methanol extract of O. canum against the larvae of Ae. aegypti (LC50=99.42, 94.43 and 81.56 ppm) and against Cx. quinquefasciatus (LC50= 44.54, 73.40 and 38.30 ppm), respectively (Kamaraj et al. 2008). An. stephensi and Ae. aegypti. Chowdhury et al. (2009) have reported that the chloroform and methanol extracts of mature leaves of Solanum villosum showed the LC50 value for all instars between 24.20 and 33.73 mg/l after 24 h and between 23.47 and 30.63 mg/l after 48 h of exposure period against An. subpictus. Govindarajan (2010) evaluated larvicidal activity of crude extract of Sida acuta against three important mosquitoes with LC50 values ranging between 38 and 48 mg/l. The crude extract had strong repellent action against the three species of mosquitoes as it provided 100 per cent protection against An. stephensi for 180 min followed by Ae. aegypti (150 min) and Cx. quinquefasciatus (120 min).
Dua et al. (2009) showed that the LC50 values of the neem oil were 1.6 and 1.7 ppm while LC90 values were 3.4 and 3.7 ppm against An. stephensi and Ae. aegypti respectively. Shivakumar and Kataria (2011) have reported that An. showed a high susceptibility to very low concentrations of Azadirachta indica (1–5 ppm), whereas in the case of Ae. aegypti, the diagnostic concentration is slightly higher at 10ppm. The larvicidal action on An. stephensi, at a concentration of 3ppm resulted in 100% mortality within 72 h and a concentration of 15 ppm produces 90% mortality within 24 h of the treatment. In the present investigation out of the nine plants screened using methanol as solvent, Annona reticulata (Annonaceae) was found to possess better larvicidal activity against early fourth instar larvae of Ae. aegypti followed by An. stephensi with LC50 and LC90 values being 95.24 and 262.64 ppm respectively (Table 3). Likewise LC50 and LC90 values against An. stephensi are 262.71 and 636.94 ppm respectively (Table 4).
Annona muricata was an active larvicide with a 48-hour LC50 value of 67.4 μg/mL. (Jacobson 1958). He has further reported similar results for the seed extracts of another Annona species, namely A. cherimola, A. glabra and A. squamosa, which had lethal effects on larvae of Ae. sp. Its potential as a larvicidal plant was further supported by the results of a recent study by Satoto (1993), who has found that A. squamosa seed was one of the most effective larvicides against both Culex tritaeniorhynchus and Ae. aegypti.
Earlier studies further indicated that crude extracts of leaves of Annona reticulata when evaluated for in vitro anthelmintic activity on Indian adult earth worms Eisinia fetida, a dose dependent inhibition of spontaneous motility (paralysis) of the worms was noticed (Sonal et al. 2011). There are no reports available on the toxicity of Annona reticulata against mosquito larvae. The present studies add that, apart from all medicinal property viz, antimicrobial, antitumoric, anthelmintic, antibacterial, cytotoxic etc, mosquito larvicidal property also present in this plant. Therefore, the methanolic extracts of this plant inhibiting the development of larval growth, indicates hopes for further characterization of the active compound in our lab.
Conclusion
The results from this study indicate that phyto-products isolated from the plants are more advisable to use for control of mosquito borne diseases. These plants are remarkably economical and ecofriendly with more larvicidal properties. Among the plants screened An. reticulata showed high larvicidal efficacy against two mosquito species. Hence, An. reticulata will be selected for further chemical isolation of the active ingredient in future studies. It could be considered as a potent resource for controlling mosquito larvae. Such practice would not only reduce the disadvantages of insecticides on the environment but also promote sustainable utilization of locally available bio-resource by rural communities.
Acknowledgement
The authors are thankful to the Chairman, Department of Studies in Zoology, University of Mysore, Mansagangothri, Mysore for the facilities provided. The authors declare that there is no conflict of interests.
References
- Abbott WS. ( 1925) A method for computing the effectiveness of an insecticide. J Econ Entomol. 18: 265– 267. [Google Scholar]
- Balandrin MF, Klocke JA, Wurtele ES, Bollinger WH. ( 1985) Natural plant chemicals: sources of industrial and medicinal materials. Science. 228: 1154– 1160. [DOI] [PubMed] [Google Scholar]
- Bowers WS, Sener B, Evans PH, Erdogani FB. ( 1995) Activity of Turkish medicinal plants against mosquitos Ae. aegypti and An. gambiae. Insect Sci. 16: 339– 342. [Google Scholar]
- Brogdon WF, Mc Allister JC. ( 1998) Insecticide resistance and Vector Control. Emerg Insect Dis. 4( 4): 605– 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. ( 2012) Chikungunya: a re-emerging virus. Lancet. 379: 662– 671. [DOI] [PubMed] [Google Scholar]
- Chowdhury N, Chatterjee SK, Laskar S, Chandra G. ( 2009) Larvicidal. activity of Solanum villosum Mill (Solanaceae: Solanales) leaves to An. subpictus Grassi (Diptera: Culicidae) with effect on non-target Chironomus circumdatus Kieffer (Diptera: Chironomidae). J Pest Sci. 82: 13– 18. [Google Scholar]
- Das PK, Amalraj D. ( 1997) Biological control of malarial vectors. Ind J Med Res. 106: 174– 197. [PubMed] [Google Scholar]
- Das NG, Goswami D, Rabha B. ( 2007) Preliminary evaluation of mosquito larvicidal efficacy of plant extracts. J Vector Borne Dis. 44: 145– 148. [PubMed] [Google Scholar]
- Dua KV, Pandey AC, Raghavendra K, Gupta A, Sharma T, Dash AP. ( 2009) Larvicidal activity of neem oil (Azadirachta indica) formulation against mosquitoes. Malar J. 8: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falade C, Mokuolu O, Okafor H, Orogade A, Falade A, Adedoyin O. ( 2007) Epidemiology of congenital malaria in Nigeria: a multi-centre study. Trop Med Int Health. 12: 1279– 1287. [DOI] [PubMed] [Google Scholar]
- Finney DJ. ( 1971) Probit analysis. 3rd ed Cambridge University Press, Cambridge. [Google Scholar]
- Goettel MS, Toohey MK, Ram RC, Pillae JS. ( 1992) Field evaluation of Bti against Ae. vigilax and Culex sitiens in Fiji. Mosq News. 42: 277– 278. [Google Scholar]
- Gohil MV, Agrawal SK, Saxena AK, Garg D, Gopimohan C, Bhutani KK. ( 2010) Synthesis, biological evaluation and molecular docking of aryl hydrazines and hydrazides for anticancer activity. Indian J Exp Biol. 48: 265– 268. [PubMed] [Google Scholar]
- Govindarajan M. ( 2010) Larvicidal and repellent activities of Sida acuta Burm. F (Family: Malvaceae) against three important vector mosquitoes. Asian Pac J Trop Med. 3: 691– 695. [Google Scholar]
- Hahn CS, French OG, Foley P, Martin EN, Taylor RP. ( 2001) Biospecific monoclonal antibodies of dengue virus to erythrocytes in a monkey model of positive veremia. J Immunol. 66: 1057– 1065. [DOI] [PubMed] [Google Scholar]
- Hemingway J, Ranson H. ( 2000) Insecticide resistance in insect vectors of human disease. Ann Rev Entomol. 45: 371– 391. [DOI] [PubMed] [Google Scholar]
- Jacobson M. ( 1958) Insecticides from Plants. A Review of the Literature, 1954–1971. Agriculture Handbook, USDA, Washington, DC, pp. 1941– 1952. [Google Scholar]
- Kamaraj C, Rahuman AA, Bagavan A. ( 2008) Screening for antifeedant and larvicidal activity of plant extracts against Helicoverpa armigera Hübner, Sylepta derogata F and An. stephensi Liston. Parasitol Res. 103: 1361– 1368. [DOI] [PubMed] [Google Scholar]
- Kamaraj C, Rahuman AA, Bagavan A. ( 2008) Antifeedant and larvicidal effects of plant extracts against Spodoptera litura F, Ae. aegypti L and Culex quinquefasciatus Say. Parasitol Res. 1032: 325– 331. [DOI] [PubMed] [Google Scholar]
- Karunamoorthi K, Ilango K. ( 2010) Larvicidal activity of Cymbopogon citratus (DC) Stapf and Croton macrostachyus Del. against An. arabiensis Patton, a potent malaria vector. Eur Rev Med Pharmacol Sci. 14( 1): 57– 62. [PubMed] [Google Scholar]
- Kaushik R, Saini P. ( 2008) Bioefficacy of Syzygium cumini (Jamun) leaf extracts against larval stages of Ae. aegypti (Diptera: Culicidae). Indian J Environ Sci. 12( 2): 99– 102. [Google Scholar]
- Kabaru JM, Gichia L. ( 2001) Insecticidal activity of extracts derived from different plants of the mangrove tree Rhizophora mucronata (Rhizophoraceae) lam. against three arthropods. Afr J Sci Tech (AJST), Sci. Eng. Ser. 2: 44– 49. [Google Scholar]
- Liu H, Xu L, Zhang, Liu N. ( 2005) Chlorpyrifos resistance in mosquito Culex quinquefasciatus. J Med Entomol. 42( 5): 815– 820. [DOI] [PubMed] [Google Scholar]
- Lixin S, Huiquin D, Chongxia G, Jin Q, Jing S, Lei M, Changliang Z. ( 2006) Larvicidal activity of extracts of Ginko biloba Exocarp for three different strains of Culex pipiens Pallens. J Med Entomol. 43( 2): 258– 261. [DOI] [PubMed] [Google Scholar]
- Mattingly PF. ( 1973) Culicidae (Mosquitoes). In: Insects and Other Arthropods of Medical Importance. Smith KGV. (Ed). Trustees of the British museum (Natural history), London, pp. 37– 107. [Google Scholar]
- Mittal PK, Subbarao SK. ( 2003) Prospect of using herbal product in the control of mosquito vectors. ICMR Bulletin. 33 ( 1): 0377– 4910. [Google Scholar]
- Peng Z, Yang J, Wangn H, Simons FER. ( 1999) Production and characterization of monoclonal antibodies to two new mosquito Ae. aegypti salivary proteins. Insect Biochem Mol Biol. 29: 909– 914. [DOI] [PubMed] [Google Scholar]
- Pitasawat B, Choochote W, Kanjanapothi D, Panthong A, Jitpakdi A, Chaithong U. ( 1998) Screening for larvicidal activity of ten carminative plants. Southeast Asian J Trop Med Public Health. 29: 660– 662. [PubMed] [Google Scholar]
- Satoto TBT. ( 1993) A laboratory study of the biological effects of some medicinal plants on Culex tritaeniorhynchus sp. [MS thesis in Tropical Medicine]. Faculty of Graduate Studies, Mahidol University, Bangkok. [Google Scholar]
- Saxena RC, Harshan V, Saxena A, Sukumaran P, Sharma MC, Lakshanakumar M. ( 1993) Larvicidal and chemosterilant activity of Annona squamosa alkaloids against An. stephensi. J Am Mosq Control Assoc. 9( 1): 84– 87. [PubMed] [Google Scholar]
- Shelton AM, Roush RT, Wang P, Zhao JZ. ( 2007) Resistance to insect pathogens and strategies to manage resistance: An update. In: Field manual of techniques in invertebrate pathology. Lacey LA, Kaya HK. (Eds). 2nd Edn Kluwer Academic, Dortrecht, The Netherlands, pp. 793– 811. [Google Scholar]
- Shivakumar MS, Kataria R. ( 2011) Comparative efficacy of Azadirachtin on the larval population of Culex quinquefasciatus, An. stephensi, and Ae. aegypti (Diptera: Culicidae) in Gujarat, India. Int J Pharma Bio Sci. 2( 1): 42– 47. [Google Scholar]
- Sonal B, Trupti T, Ganesh J. ( 2011) In vitro anthelmintic activity of Annona reticulata against Eisinia fetida. Int J Res Pharm Sci. 2( 4): 569– 570. [Google Scholar]
- Sukumar K, Perich MJ, Boobar LR. ( 1991) Botanical derivatives in mosquito control: a review. J Am Mosq Control Assoc. 7( 2): 210– 237. [PubMed] [Google Scholar]
- WHO ( 2005) Guidelines for laboratory and field testing of mosquito larvicides. Available at: WHO/CDS/WHOPES/GCDPP/2005.
- WHO ( 2006) Pesticides and their application for the control of vectors and pests of public health importance. 6th Ed World Health Organization. Available at: WHO/CDS/NTD/WHOPES/GCDPP/2006.1. [Google Scholar]
- World Health Organization ( 2009) World malaria report 2009. Accessed on February 16, 2010. Available at: http://www.who.int/malaria/world_malaria_report_2009/en/index.html.
- World Health Organization ( 2013) World malaria report 2013. The report was launched at the National Press Club in Washington, DC on 11th December 2013. Available at: www.who.int/iris/bitstream/10665/97008/1/9789241564694_eng.pdf.
- Yadav RS, Sharma VP, Upadhyay AK. ( 1997) Field trial of Bacillus sphaericus (serotype H5a, 5b) against filariasis and Japanese encephalitis vectors in India. J Am Mosq Control Assoc. 13: 158– 163. [PubMed] [Google Scholar]
- Yang YC, Lee EH, Lee HS, Lee DK, Ahn YG. ( 2004) Repellency of aromatic medicinal plant extracts and a steam distillate to Ae. aegypti. J Am Mosq Control Assoc. 20( 2): 146– 149. [PubMed] [Google Scholar]