Abstract
Background:
Geographic distribution of West Nile virus (WNV) is heterogeneous in Iran by a high circulation in the southern-western areas. The objective of our study was to determine environmental and climatic factors associated with the risk of WNV equine seropositivity in Iran.
Methods:
Serological data were obtained from a serosurvey conducted in equine population in 260 districts in Iran. The climate and environmental parameters included in the models were distance to the nearest wetland area, type of stable, Normalized Difference Vegetation Index (NDVI), annual mean temperature, humidity and precipitation.
Results:
The important risk factors included annual mean temperature, distance to wetlands, local and seasonal NDVI differences. The effect of local NDVI differences in spring was particularly notable. This was a normalized difference of average NDVI between two areas: a 5 km radius area centered on the stable and the 5–10 km surrounding area.
Conclusion:
The model indicated that local NDVI’s contrast during spring is a major risk factor of the transmission of West-Nile virus in Iran. This so-called oasis effect consistent with the seasonal production of vegetation in spring, and is associated to the attractiveness of the local NDVI environment for WNV vectors and hosts.
Keywords: West Nile Virus, Environment, Climate, NDVI, Wetland
Introduction
West Nile virus (WNV) is not restricted by international borders and involves all continents, revealing adaptation of the virus with different ecological zones. Mosquitoes and birds, as vector and reservoir respectively, play an important role in the virus circulation. Several studies have defined the potential importance of environmental factors on the WNV-vector-host interaction, the virus replication and on the population/habitat of the vector/reservoir (Bolling et al. 2005, Kinney et al. 2006).
Climate variation, as an environmental factor, has two different effects on ecological processes: (i) direct effects on virus/hosts/vectors, for example, by selecting the variants sensitive, or controlling the physiology of organism such as metabolic processes and reproduction (Gubler et al. 2001, Reiter 2001), and (ii) indirect effect on the habitats, the ecosystems and the relationships among the organisms (Stenseth et al. 2002). Effect of climatic factors on human WNV infection has been studied by analyzing a spectrum of weather factors for different states in the United States, and demonstrated a relation between these factors and incidence of human WNV cases (Soverow et al. 2009). Climate variations have changed the geographic and seasonal patterns of WNV with shifting toward new geographic areas and occurring earlier in the transmission season. Gibbs et al. demonstrated that temperature, housing density, urban/suburban land use, and physiographic region are important variables associated with the geographic distributions of WNV in Georgia. In addition, the models according climate and landscape change were used to predict the future expansion of WNV in North America (Gibbs et al. 2006, Chen et al. 2013).
Environmental factors such as elevation range, physiographic region, and percentage of vegetation cover have been evaluated to determine human WNV risk in Chicago (Ruiz et al. 2004). In western Kenya, mosquito larval presence was associated with lower elevations, greater wetness, short distances to water, and land use (Bian et al. 2006).
Among climatic factors, temperature is the most important extrinsic factor affecting the population and dynamic of virus/vector/hosts. Humidity is another factor, which has a direct effect on the survival of mosquitoes, by increasing vector flight and host-seeking behaviors. Survival rate might be reduced when hot weather is accompanied by low humidity. Rain provides the breeding sites for mosquitoes and helps create a humid environment, which prolongs the life of vectors. However, response to precipitation depends on the geographic location, season and the mosquito species (Reiter 2001, Ruiz et al. 2010).
Normalized difference vegetation index (NDVI) is a simple numerical indicator that can be used to assess vegetation density. As plants respond quickly to climate variations, NDVI is related to the climatic variations such as increase or decrease of precipitation, temperature and sunshine level (Stow et al. 2004). The first evidence of WN virus circulation in Iran, a country with climatic and environmental diversity, goes back in 1970s when existence of WNV antibodies among healthy human population has been demonstrated (Saidi S. 1976). After a several decades gap, in a study carried out among 1054 animal sera collected from different provinces, in 2008–2009, a relatively high circulation of the virus has been found in the country. The study showed a highly heterogeneous prevalence in different provinces, so that a higher prevalence was detected in the southwestern areas while lower prevalence was in the northern areas (Ahmadnejad et al. 2011). According to the last checklist, Iranian mosquitoes include 64 species and 3 subspecies classified in 7 genera and 12 unverified species. The most important vector of WNV, Culex spp, includes 19 species (Azari-Hamidian 2007). Although, there are many studies on Iranian mosquito fauna, there is no information about WNV vectors in the country.
This study addressed the dynamic and the spatial patterns of WNV transmission by analyzing the geographical and ecology parameters affect the WNV circulation in the infected areas in Iran.
Materials and Methods
Study area
Iran is bordered by the Gulf of Oman, the Persian Gulf, and the Caspian Sea. It has arid or semiarid climates mostly characterized by low rainfall and high potential evapotranspiration and its location cause it to receive less than a third of the world average precipitation. The complex physical conditions of Iran including topography and landscape have created a diverse climate pattern, so that it led to the formation of different ecological zones with various species of plants and animals (Heshmati 2007).
Furthermore, the climate is influenced by Caspian Sea in north, coastal areas of south of the country, Mediterranean area and Red sea. Alborz and Zagros mountains also play an important role in determining the nonuniform spatial and temporal distribution of precipitation in the whole country.
Iran is located at high latitudes rendering vast differences between seasons in the country. Temperature in Iran is intense function of altitude, latitude and moisture content of the atmosphere. The summer is extremely hot with temperatures in the interior; over 55 °C has been recorded in some places. Humidity prevents temperature fluctuations in the north and south of the country (Alijani 1995).
The rainy period in most of the country is from November to May followed by dry period between May and October with rare precipitation. The average annual rainfall of the country is about 240 mm with maximum amounts in the Caspian Sea plains.
Collection and processing of data
Serological data:
Equine data and their geographic coordinates were obtained from a previous seroprevalence study. The sera (n= 1054) were collected from 260 stables located within 27 of 30 provinces in the country. Diagnosis of WNV antibodies in the sera was performed by using plaque reduction neutralization test (PRNT) (Fig. 1). Antibodies to WNV were detected in 249 (23.7%) of the samples (Ahmadnejad et al. 2011).
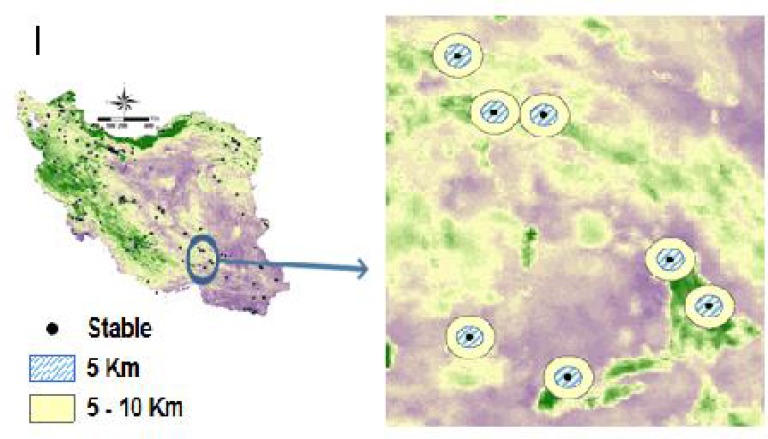
Fig. 1.
The studied NDVI indicators for the sampling stables
Landscape and Climatic data
Climatic data:
We analyzed three climatic factors such as rainfall, temperature and relative humidity, which are very important factors to affect the mosquito breeding activities.
Mean monthly temperature, precipitation and relative humidity data of 10-year period (1996–2005) from 103 stations were obtained from Iran Meteorological Organization (IRIMO). Numerical layers of sampling places and meteo-stations were inputted into ArcMap 9.3 software. The stations and their nearest sampling places were defined by Thiessen method (Fortes et al. 2005). Mean yearly data was used for analysis between WNV prevalence and climatic parameters. Average temperature and precipitation is given in degrees Celicius and millimeter, respectively. Relative humidity is expressed as a percentage.
Normalized Difference Vegetation Index (NDVI) is a simple numerical indicator that can be used to analyze remote sensing measurements and assess vegetation density and whether the target being observed contains live green vegetation or not (Brown et al. 2008).
NDVI time series data were acquired by Advanced Very High Resolution Radiomete (AVHRR) sensor onboard the National Oceanic and Atmospheric Administrationr satellite (NOAA).
The images have been extracted from http://www.class.ngdc.noaa.gov based on AVHRR level 1B and resolution of 1.1 km. As satellite images cannot be directly combined with other geographic information system, so geometric correction is necessary to bring the images from ground or slant range geometry into a map reference, and for this purpose, we used ENVI 4.3 software. Composites were used to calculate NDVI values using ERDAS 8.7 software and using near infrared (NIR) and red bands as follows:
NDVI raster files were imported into a Geographic Information System (GIS, ESRI1, ArcGISTM 9.3). Buffers of 5 and 5–10 km around each sampled point were created with the GIS software and mean NDVI was extracted within each circular buffer. These values were exported into a dbase IV table for further analysis.
Three NDVI indicators were used for analysis,
Annual mean NDVI,
Seasonal NDVI differences, which is normalized differences of average NDVI for successive seasons in a 5km radius area around each stable and,
Local NDVI differences, which is normalized differences of average NDVI between two areas: a 5 km radius area centered on each stable and the 5–10 km surrounding area (Fig. 1).
Dispersal of adult mosquitoes is an important factor in the spatial scale of transmission of vector-borne pathogens. The buffers of 5 and 5–10 km were chosen based on estimates of dispersal distances of Culex mosquitoes and their blood-sucking activities on which birds (infected) at the first and then other hosts, such as equines. We assume that to bite a bird, the maximum estimated dispersal of a female mosquito is about 2.5km and similarly in another host (2.5×2=5km). The second buffer area was chosen by the same radius of 5km buffer. Moreover, NDVI contrast between two buffers could be an indicator of localized mosquitoes as well as birds (Venkatesan and Rasgon 2010, Hamer et al. 2014).
Distance to the nearest Wetland area
Iranian wetlands possess a great diversity and provide a habitat for more than 140 migratory and sedentary bird species. Among 42 kinds of wetlands in the world characterized by the Ramsar Convention, all, except one, are found in Iran, indicating an immense variety of Iranian wetlands. Due to the extension of the country, there are a large number of wetlands with a broad range of size: area between a few to 500000 hectares (Bagherzadeh-Karimi and Rouhani-Rankouhi 2007).
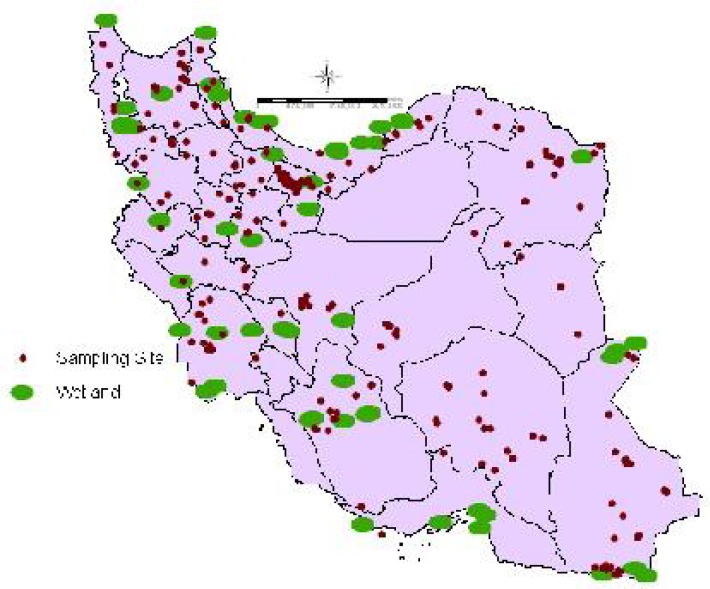
The data on wetlands was acquired from Iran Environment protection organization. For this study, we collected the data of 60 important wetlands area, of which 22 are Ramsar sites. The area of these wetlands extends around 2954970.20 hectares (Fig. 2). To define proximity to the wetlands, the shape files of wetlands and sampling sites of animals was imported into ArcMap version 9.3. Using the ‘Euclidean Distance’ function, from the spatial analyst extension, nearest wetland and its distance to each sampling place was calculated.
Fig. 2.
Important studied wetlands and sampling sites
Statistical analysis
Univariate analyses were performed in order to test the relationship between the outcome variable and each explanatory variable. Finally, logistic regression analyses were performed in order to identify which variables were involved in the stables seropositivity.
Explanatory variables used in logistic regression modeling included annual average temperature, relative humidity and precipitation, distance to the nearest wetland, annual average NDVI, Normalized seasonal differences of NDVI, normalized local differences of NDVI and the type of stable (farm or club) (Table 1). Normalized seasonal differences of NDVI for two successive seasons S1 and S2 and for stable S was calculated as: (NDVIS,S2–NDVIS,S1)–(1/N) Σ(NDVI*,S2–NDVI*,S1), where NDVIx,y is the average NDVI value observed in a 5km radius area around stable x during season y, and N is the number of stables.
Table 1.
Multivariate logistic model of the presence of anti-WNV seropositive animals in Iranian stables, 2008–2009
| Variable | Odds-ratio (95% CI) | p | |
|---|---|---|---|
| Type of stable (village of club) | NS2 | 0.07 | |
| Annual mean temperature | Δ=5°C | 2.03 (1.10–3.92) | 0.03 |
| Annual mean %humidity1 | <38% | Reference | |
| 38–46% | NS | 0.12 | |
| 46–52% | NS | 0.27 | |
| >52% | NS | 0.34 | |
| Annual mean precipitation1 | <154 mm | Reference | |
| 154–247 mm | NS | 0.84 | |
| 247–327 mm | NS | 0.67 | |
| >327 mm | NS | 0.71 | |
| Distance to the nearest wetland area | Δ=10 km | 0.73 (0.54–0.99) | 0.05 |
| Annual mean NDVI | NS | 0.77 | |
| Seasonal NDVI differences | Δspring-summer>0 | 2.37 (1.07–5.41) | 0.04 |
| Δsummer-autumn>0 | NS | 0.96 | |
| Δautumn-winter>0 | NS | 0.38 | |
| Δwinter-spring>0 | NS | 0.92 | |
| Local NDVI differences | spring>0 | 4.26 (1.07–17.98) | 0.04 |
| summer>0 | NS | 0.11 | |
| autumn>0 | NS | 0.35 | |
| winter>0 | NS | 0.23 | |
Classes bounds based on distribution quantiles
NS= Non-significant
Normalized local differences of NDVI were calculated between two areas; a 5 km radius area centered on the stable and the 5–10 km surrounding area. For stable x and season y, value was calculated as: (NDVIx,y,0–5km–NDVIx,y,5–10km)–(1/N) Σ(NDVIx,*,0–5km–NDVIx,*,5–10km), where NDVIx,y,z is the average NDVI value observed around stable x during season y in area z, and N is the number of stables.
As the previous study showed a high effect of the type of stables on the seropositivity (Ahmadnejad et al. 2011), we included this variable in the present analyses.
Accuracy (percent of testing sites correctly classified), sensitivity (percent of positive testing sites correctly classified), and specificity (percent of negative testing sites correctly classified) were computed for the model. In addition, the area under the receiver operating characteristic curve (AUC ROC) was calculated as indices of the fit of the model.
All the statistical analyses were conducted using R 2.10.1, and maps were produced with a Geographic Information System (ESRI1, ArcGISTM 9.0).
Results
The stables with at least one positive animal considered as positive stable and 108 of 260 stables (41.5%) were positive for WNV infection.
In the training set, on univariate analysis, all the variables were associated with the presence of WNV antibody (P< 0.05).
The odds ratio and 95% confidence intervals were calculated for seropositivity and all risk factors. Multivariate logistic regression analyses revealed a strong relation between WNV antibody prevalence and annual mean temperature, distance to the nearest wetland, seasonal NDVI differences and Local NDVI differences variables (Table 1).
The average minimum and maximum annual temperature were 5 °C and 27.7 °C, respectively. Annual mean temperature for infected and uninfected places was 19.97 °C (95% CI 18.97–20.97) and 15.58 (95% CI 15.04–16.11), respectively.
The average minimum and maximum annual humidity were 25.6% and 83.8%, respectively. Annual mean humidity for infected places was 44.57% (95% CI 42.07–47.06) and for uninfected places was 47.94% (95% CI 45.98–49.89).
The average minimum and maximum annual precipitation were 50.8 mm and 1759.1 mm, respectively. Annual mean precipitation for infected places was 258.74 mm (95% CI 210.58–306.91) and for uninfected places was 299.18 mm (95% CI 266.83–331.53).
The risk of being WNV seropositive increased for stables located in the area with higher temperature, as 5 °C increase in mean annual temperature was associated with a statistically significant 100% higher prevalence of WNV infection.
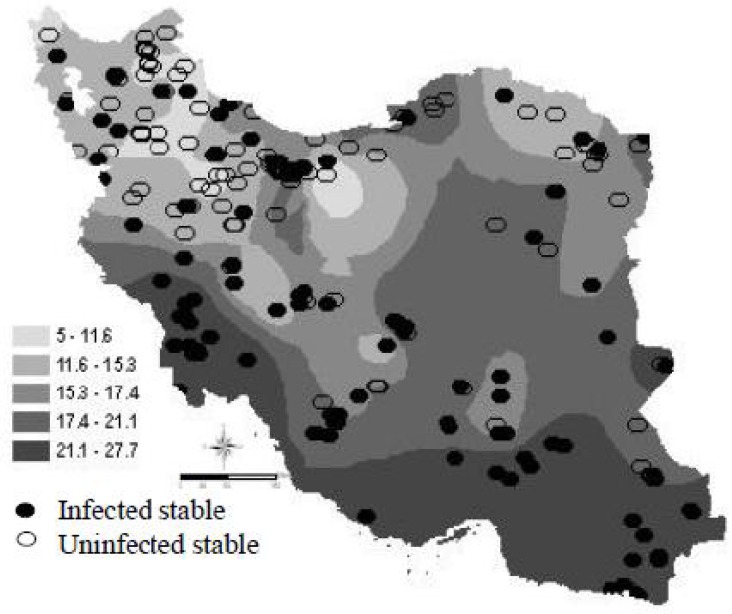
Fig. 3 Fig. 3 shows the aggregation of infected stables within the area with higher temperature.
Fig. 3.
Annual mean temperature and geographical distribution of WNV infected and uninfected stables in Iran
No significant association was observed between type of stable, annual mean humidity, precipitation, annual mean NDVI and seropositivity. It is notable that type of stable is different from their location, as the former more is indicating the management of the stable and the latter indicating geographic location.
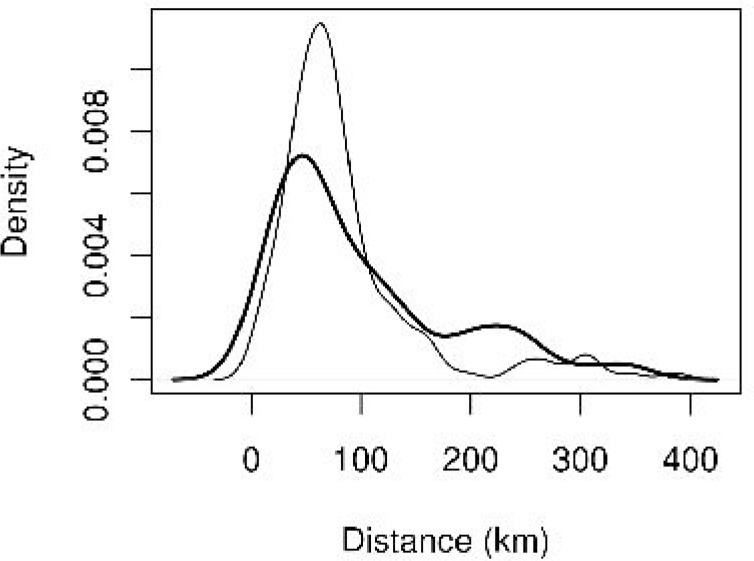
There was a significant negative correlation between distance to the nearest wetland and seropositivity of stables. The risk of being WNV seropositive for stables increased (27%) with 10km decrease of distance from the nearest wetland area (Fig. 4).
Fig. 4.
Distribution of positive stables (thick line) and of negative stables (thin line) according to the distance to the nearest wetland
A logistic regression model showed an association between seasonal/local NDVI and seropositivity. A higher significant difference between NDVI of spring and summer within a 5 km radius area around the stable increased the seropositivity four times higher.
The difference of NDVI between two areas, a 5 km radius area centered on the stable and the 5–10 km surrounding area, in spring for infected stables was four times higher that of uninfected stables.
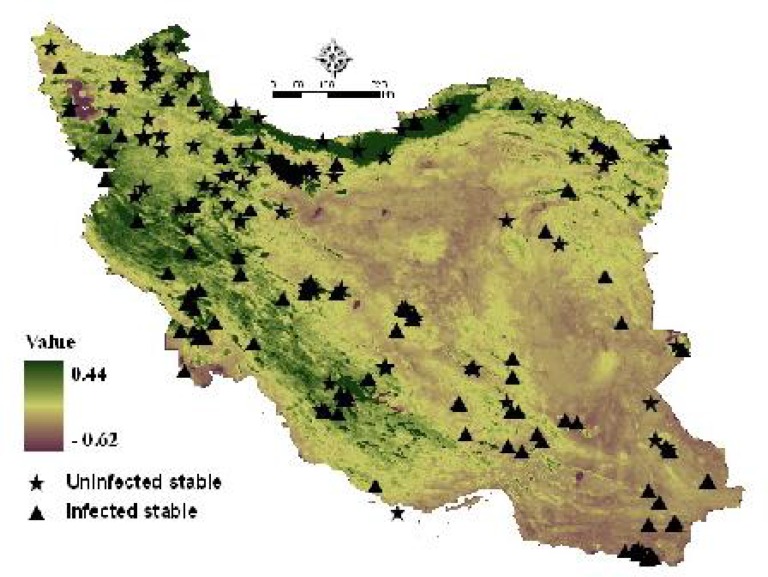
Fig. 5 shows annual mean NDVI in spring and distribution of the stables according to the normalized local NDVI differences.
Fig. 5.
Annual mean NDVI in spring in Iran and the geographic distribution of WNV infected and uninfected stables
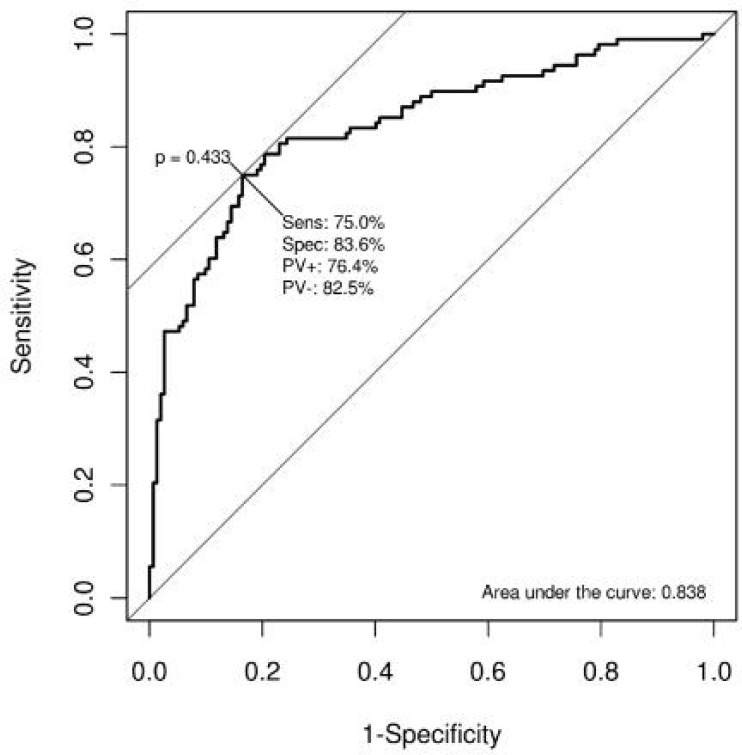
The ROC analysis resulted in an AUC of 0.838, indicating the model had relatively good discrimination ability. The model computes probabilities for a cell to have a high WNV circulation status. Computing sensitivity and specificity of this prediction for various probability cut-offs clearly showed that at a decision threshold of 0.4. The model had a sensitivity of 75 % and a specificity of 83.6 %. Corresponding positive and negative predictive values were 76.4 % and 82.5 %, respectively (Fig. 6).
Fig. 6.
Receiver operating curve of the multivariate logistic model of the presence of anti-WNV seropositive animals in Iranian stables, 2008–2009
Discussion
The evidence presented here reveals the geographic factors associated with WNV circulation in Iran, which is reported for the first time in the country. Four studied factors, temperature, distance to wetlands, local and regional differences NDVI were correlated to the WNV infection in equine.
Positive anomalies of the temperature in some southwestern province appear to have facilitated the mosquito abundance and, consequently, WNV infection in equine. We observe a north-south gradient of seropositivity following the gradient of annual average temperature. Annual mean temperature above 22 °C was notable in which all, except three, of the stables by this temperature were seropositive. These results are consistent with previous observations made in Egypt (Taylor et al. 1956). In southern provinces of the country, where the climate is arid, the infection reaches a high level. In Northern provinces, the situation is quite different: warm, wet summers are common, but droughts occasionally occur in late summer and early fall; and outbreaks of WNV can occur at that time. Temperature in Iran gets warmer from west to east and from north to south. Increase of temperature from west to east is due to concentration of the mountains at western part of the country while increase from north to south is because of approaching the equator and increasing of the solar radiation angle. Overall, northern and mountainous parts of the country have higher annual fluctuating and southern areas have relative stability (Alijani 1995).
Temperature influences the mosquito life cycle, its reproduction rates, development of eggs within the mosquito (gonotrophic cycle), the vector–host contact rate and consequently, virus transmission. Although, Patz and et al. (2000) suggested that higher temperatures may increase or reduce survival rate, depending on the vector, its behavior, ecology, and many other factors. Thus, the probability of transmission may or may not be increased by higher temperatures (Taylor et al. 1956, Patz et al. 2000), but it has been shown that even a small increase in temperatures can have a significant impact on transmission of WNV by mosquitoes (Kilpatrick et al. 2008). Effect of high temperature on increasing of mosquito abundance and vector competency, has also been experimentally proven (Dohm et al. 2002).
WNV is endemic in people living in the semi-arid regions, where the weather most of the year is decidedly hot and dry. Cases has been regularly reported in subdesertic areas around the Mediterranean basin: (i) along the Nile Valley (Egypt, 1950, 1994) (ii) along the Syrian-African Rift (Israel 1951, 1998–2000, Jordan 2003), (iii) in the Timimoun oasis in the Central Sahara (Algeria 1994) (Taylor et al. 1956, Murgue et al. 2001). The warmer condition of these area, along with directly effects on vector and host, cause the standing water with high rich organic materials attracting the mosquito and birds, consequently increase the interaction and circulation of WNV between them (Epstein and Defiippo 2001).
Beside the vectors, temperature often plays a significant role in bird life cycles, availability of food and habitat and also in the distribution of migratory birds (Greenberg and Marra 2005).
Precipitation and humidity could have a major influence on the distribution of mosquito species. Humidity increases vector flight and host-seeking behaviors and precipitation is necessary for the formation of mosquito breeding habitats (Shaman et al. 2002). However, we could not find a significant relation between WNV infection and annual mean humidity/rainfall. Evidences on relationship between WNV/mosquito abundance and precipitation and humidity are variable, some indicate a weak, some strong correlations (Soverow et al. 2009, Wan Norafikah et al. 2009).
Seropositivity was negatively correlated to the distance to wetlands, so, near to wetland can increase infection of the animals to the virus. This is consistent with the previous studies, which showed WNV infection is more strictly linked to the wetlands with abundant bird populations, especially migratory birds. Wetlands provide suitable breeding habitat for mosquitoes and birds and consider as a natural foci of WNV infections in Palearctic. Outbreaks in temperate area, especially in Europe, often have occurred in or near wetlands (Hubalek and Halouzka 1999, Jourdain et al. 2007). In 1998, WNV was isolated from horses suffering from neurologic disease and residing in a large wetland area in Italy. In Camargue (France), wetland variations identified as important risk factors in WNV spillover in horses. (Autorino et al. 2002, Pradier et al. 2014). Moreover, proximity to wetland increase mosquito abundance, as the first three stages of their life is aquatic dependent. Iranian wetlands serve as wintering or staging area for migratory birds coming from WNV endemic area and using the West Siberian-Caspian-East African and Central Siberian-Indus-South Asian flyways (UNDP 2004).
The strongest association was detected between seropositivity and NDVI, at seasonal and, especially, local differences. While this relation could not be found with annual mean value of NDVI. The local NDVI’s contrast during spring is a major risk factor of the transmission of West-Nile virus in Iran. During the early drought season, the stable seropositivity is increasing with the contrast between the local and regional NDVI environment of the stable. This so-called oasis effect is associated to the attractiveness of the local NDVI environment for WNV’s vectors (mosquitoes and birds). This oasis effect disappeared in autumn, and the local NDVI’s contrast becomes protector (negative impact). This oasis effect is consistent with the seasonal production of vegetation in spring, which is more important compared to the national average (Contrast spring-summer). The higher level of seropositivity of the stables can be related to the attraction of many birds to the resources around these stables (Bock et al. 2008). The extreme climatic situation of the southwestern provinces of Iran encourages birds to congregate around shrinking water sites, encouraging viral circulation among birds and mosquitoes, while heat accelerates viral maturation. Droughts and heat wave were found to induce WNV amplification by bringing the hosts and the vectors together. In opposition with the dogma that increasing precipitation predicts mosquito abundance, some authors hypothesize that wild populations should generate outbreaks under drought conditions. Standing water pools become richer in the organic material that Culex vectors needs to thrive and the mosquito predators, such as amphibians and dragonflies, are fewer in number (Epstein 2004). These findings are consistent with the hypothesis that spring= nesting (in an “oasis”, the birds will nest closer to the horses) and autumn = dispersal of juveniles (juveniles are going further if they leave from an “oasis”). These findings are also consistent with the fact that viral circulation is related to the presence of chicks, sedentary and still bare skin in altricial species (Marra et al. 2004).
High values of NDVI are related to higher photosynthetic activity and improved ecological condition. This suitable condition increases the main food supply, such as arthropods, for most migratory birds. Several studies have used NDVI for assessments of WNV infection foci and its vector habitat. Seasonal difference in NDVI was one the best predictive factors for determining mosquito distribution abundance in the model studied by Jacob et al (Jacob et al. 2009). Ward et al. investigated association between NDVI and cases of equine WNV encephalomyelitis. They found that the mean NDVI in biweekly periods with reported cases was significantly higher than the mean NDVI in periods without cases (Ward 2009).
Climatic condition of Iran varies throughout the country, resulting in a complex hydrological and vegetative landscape, with arid and semiarid areas in the center, southeast and southwest and temperate rain forest in the north. Although areas with high WNV infection rates, such as Khuzestan, have a dry climate, but their water sources are mainly from rivers originated from mountains and precipitations, which play an important role in producing oasis areas in these areas.
Although these results provide a new understanding of some ecological parameters effects on WNV circulation in Iran, additional work is needed. Arboviruses circulation is multifactorial and studies are needed to evaluate various mosquito species for their potential to transmit WNV in Iran. There are many studies on mosquito fauna in Iran, however there is no specific information about WNV vectors. WNV has been identified in numerous mosquito species, including members of the genera Culex, Aedes and Ochlerotatus, but mosquitoes belonging to the Culex species are the main vectors for WNV. Culex pipiens, the main vector of WNV in US, has spread in different ecological zones of Iran and its distribution is very similar to its climatic distribution in North, South America and Africa. The virus has been isolated from Cx. vishnui complex in Pakistan. There are some doubtful records of this mosquito in Iran; however, based on the record of this species in Pakistan, it seems that Cx. vishnui may occur in southeastern Iran (Zaim et al. 1985, Hubalek and Halouzka 1999, Vatandoost et al. 2004, Dehghan et al. 2010).
If we consider Iranian WNV vector within Culex spp, it is speculated that other than several species may be involved in WNV circulation in south and southwestern parts of Iran, where there is a high circulation of the virus. However, vector competency studies should be conducted on various species of mosquitoes in order to determine the ability of the mosquito species to transmit WNV and detection of the virus from mosquitoes.
Another study was conducted to determine WNV antibody and genome in different species of wild water birds captured from wetlands in Iran. Samples were collected from 26 different species, 15% of which were serologically positive, while no WNV viral RNA-positive samples were found in this study. The majority of positive birds were common coot (Fulica atra) (Fereidouni et al. 2011). They did not find any positive samples from Khuzestan province where the most important region for the virus circulation is. Moreover, they had a few samples (n=4) from this province, all of which were birds other than common coot, maybe due to lower abundance of common coot (Fulica atra) in this area. The area identified with high risk for WNV circulation may be used for entomological or epidemiological surveillance.
Conclusion
The model indicated that local NDVI’s contrast during spring is a major risk factor of the transmission of West-Nile virus in Iran. This so-called oasis effect consistent with the seasonal production of vegetation in spring, and is associated to the attractiveness of the local NDVI environment for WNV vectors and hosts. Further studies are needed to understand better the ecology of WNV in Iran. The reservoir hosts and vectors are the keys of circulation of the virus and will be the focus of the future work.
Acknowledgements
We thank all the personnel of IR of Iran Meteorological Organization (IRIMO), especially Dr Ghaemi. We also thank the personnel of Iran Environment Protection Organization, especially Ms Elahi-Rad and Mr Amini. The authors declare that there is no conflict of interests.
References
- Ahmadnejad F, Otarod V, Fallah MH, Lowenski S, Sedighi-Moghaddam R, Zavareh A, Durand B, Lecollinet S, Sabatier P. ( 2011) Spread of West Nile virus in Iran: a cross-sectional serosurvey in equines, 2008–2009. Epidemiol Infect. 139( 10): 1587– 1593. [DOI] [PubMed] [Google Scholar]
- Alijani B. ( 1995) Climate of Iran. Piame Noor University, Tehran. [Google Scholar]
- Autorino GL, Battisti A, Deubel V, Ferrari G, Forletta R, Giovannini A, Lelli R, Murri S, Scicluna MT. ( 2002) West Nile virus epidemic in horses, Tuscany region, Italy. Emerg Infect Dis. 8( 12): 1372– 1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari-Hamidian S. ( 2007) Checklist of Iranian mosquitoes (Diptera: Culicidae). J Vector Ecol. 32( 2): 235– 242. [DOI] [PubMed] [Google Scholar]
- Bagherzadeh-Karimi M, Rouhani-Rankouhi M. ( 2007) A guide to the Iranian wetlands registered in Ramsar Convention. Ruze No, Tehran. [Google Scholar]
- Bian L, Li L, Yan G. ( 2006) Combining global and local estimates for spatial distribution of mosquito larval habitats. GIsci Remote Sens. 43( 2): 128– 141. [Google Scholar]
- Bock CE, Jones ZF, Bock JH. ( 2008) The oasis effect: response of birds to exurban development in a southwestern savanna. Ecol Appl. 18( 5): 1093– 1106. [DOI] [PubMed] [Google Scholar]
- Bolling BG, Kennedy JH, Zimmerman EG. ( 2005) Seasonal dynamics of four potential West Nile vector species in north-central Texas. J Vector Ecol. 30 ( 2): 186– 194. [PubMed] [Google Scholar]
- Brown H, Duik-Wasser M, Andreadis T, Fish D. ( 2008) Remotely-sensed vegetation indices identify mosquito clusters of West Nile virus vectors in an urban landscape in the northeastern United States. Vector Borne Zoonotic Dis. 8( 2): 197– 206. [DOI] [PubMed] [Google Scholar]
- Chen CC, Jenkins E, Epp T, Waldner C, Curry PS, Soos C. ( 2013) Climate change and West Nile virus in a highly endemic region of North America. Int J Environ Res Public Health. 10( 7): 3052– 3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan H, Sadraei J, Moosa-Kazemi S. ( 2010) The morphological variations of Culex pipiens larvae (Diptera: Culicidae) in Yazd Province, central Iran. Iran J Arthropod Borne Dis. 4( 2): 42– 49. [PMC free article] [PubMed] [Google Scholar]
- Dohm DJ, O’Guinn ML, Turell MJ. ( 2002) Effect of environmental temperature on the ability of Culex pipiens (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 39( 1): 221– 225. [DOI] [PubMed] [Google Scholar]
- Epstein PR. ( 2004) Climate change and public health: emerging infectious diseases. Encyclopedia of Energy. 1: 381– 392. [Google Scholar]
- Epstein PR, Defiippo C. ( 2001) West Nile virus and drought. Global Change and Human health. 2( 2): 105– 107. [Google Scholar]
- Fereidouni SR, Ziegler U, Linke S, Niedrig M, Modirrousta H, Hoffmann B, Groschup MH. ( 2011) West Nile virus monitoring in migrating and resident water birds in Iran: are common coots the main reservoirs of the virus in wetlands? Vector Borne Zoonotic Dis. 11( 10): 1377– 1381. [DOI] [PubMed] [Google Scholar]
- Fortes PS, Platonov AE, Pereira LS. ( 2005) GISAREG- A GIS based irrigation scheduling simulation model to suport improved water use. Agr Water Manage. 77( 1): 159– 179. [Google Scholar]
- Gibbs SE, Wimberly MC, Madden M, Masour J, Yabsley MJ, Stallknecht DE. ( 2006) Factors affecting the geographic distribution of West Nile virus in Georgia, USA: 2002–2004. Vector Borne Zoonotic Dis. 6( 1): 73– 82. [DOI] [PubMed] [Google Scholar]
- Greenberg R, Marra PP. ( 2005) Birds of two worlds: the ecology and evolution of migration. JHU Press, Baltimore. [Google Scholar]
- Gubler DJ, Reiter P, Ebi KL, Yap W, Nasci R, Patz JA. ( 2001) Climate variability and change in the United States: potential impacts on vector- and rodent-borne diseases. Environ Health Perspect. 109 ( Suppl 2): 223– 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer GL, Anderson TK, Donovan DJ, Brawn JD, Krebs BL, Gardner AM, Ruiz MO, Brown WM, Kitron UD, Newman CM, Goldberg TL, Walker ED. ( 2014) Dispersal of adult Culex mosquitoes in an urban west nile virus hotspot: a mark-capture study incorporating stable isotope enrichment of natural larval habitats. PLoS Negl Trop Dis. 8( 3): e2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heshmati GA. ( 2007) Vegetation characteristics of four ecological zones of Iran. Int J Plant Prod. 2: 215– 224. [Google Scholar]
- Hubalek Z, Halouzka J. ( 1999) West Nile fever- a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 5( 5): 643– 650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob BJ, Gu W, Caamano EX, Novak RJ. ( 2009) Developing operational algorithms using linear and non-linear squares estimation in Python for the identification of Culex pipiens and Culex restuans in a mosquito abatement district (Cook County, Illinois, USA). Geospat Health. 3( 2): 157– 176. [DOI] [PubMed] [Google Scholar]
- Jourdain E, Gauthier-Clerc M, Bicout DJ, Sabatier P. ( 2007) Bird migration routes and risk for pathogen dispersion into western Mediterranean wetlands. Emerg Infect Dis. 13( 3): 365– 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. ( 2008) Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 4( 6): e1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney RM, Huang CY, Whiteman MC, Bowen RA, Langevin SA, Miller BR, Brault AC. ( 2006) Avian virulence and thermostable replication of the North American strain of West Nile virus. J Gen Virol. 87( Pt 12): 3611– 3622. [DOI] [PubMed] [Google Scholar]
- Marra PP, Griffing S, Caffrey C, Kilpatrick AM, McLean R, Brand C, Saito E, Dupuis AP, Kramer L, Novak R. ( 2004) West Nile virus and wildlife. Bio Science. 54( 5): 393– 402. [Google Scholar]
- Murgue B, Murri S, Triki H, Deubel V, Zeller HG. ( 2001) West Nile in the Mediterranean basin: 1950–2000. Ann N Y Acad Sci. 951: 117– 126. [DOI] [PubMed] [Google Scholar]
- Patz JA, McGeehin MA, Bernard SM, Ebi KL, Epstein PR, Grambsch A, Gubler DJ, Reither P, Romieu I, Rose JB, Samet JM, Trtanj J. ( 2000) The potential health impacts of climate variability and change for the United States: executive summary of the report of the health sector of the U.S. National Assessment. Environ Health Perspect. 108 ( 4): 367– 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradier S, Sandoz A, Paul MC, Lefebvre G, Tran A, Maingault J, Lecollinet S, Leblond A. ( 2014) Importance of wetlands management for West Nile Virus circulation risk, Camargue, Southern France. Int J Environ Res Public Health. 11( 8): 7740– 7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter P. ( 2001) Climate change and mosquito-borne disease. Environ Health Perspect. 109( 1): 141– 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO, Chaves LF, Hamer GL, Sun T, Brown WM, Walker ED, Haramis L, Goldberg TL, Kitron UD. ( 2010) Local impact of temperature and precipitation on West Nile virus infection in Culex species mosquitoes in northeast Illinois, USA. Parasit Vectors. 3( 1): 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO, Tedesco C, McTighe TJ, Austin C, Kitron U. ( 2004) Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int J Health Geogr. 3( 1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi S. TR, Javadian E, Nadim A. ( 1976) The prevalence of human infection with West Nile virus in Iran. Iran J Publ Hlth. 5( 1): 8– 13. [Google Scholar]
- Shaman J, Day JF, Stieglitz M. ( 2002) Drought-induced amplification of Saint Louis encephalitis virus, Florida. Emerg Infect Dis. 8( 6): 575– 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soverow JE, Wellenius GA, Fisman DN, Mittleman MA. ( 2009) Infectious disease in a warming world: how weather influenced West Nile virus in the United States (2001–2005). Environ Health Perspect. 117( 7): 1049– 1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth NC, Mysterud A, Ottersen G, Hurrell JW, Chan KS, Lima M. ( 2002) Ecological effects of climate fluctuations. Science. 297( 5585): 1292– 1296. [DOI] [PubMed] [Google Scholar]
- Stow DA, Hope A, McGuire D, Verbyla D, Gamon J, Huemmrich F, Houston S, Racine C, et al. ( 2004) Remote sensing of vegetation and land-cover change in Arctic Tundra Ecosystems. Remote Sens Environ. 89( 3): 281– 308. [Google Scholar]
- Taylor RM, Work TH, Hurlbut HS, Rizk F. ( 1956) A study of the ecology of West Nile virus in Egypt. Am J Trop Med Hyg. 5( 4): 579– 620. [DOI] [PubMed] [Google Scholar]
- United Nations Development Programme (UNDP) ( 2004) Conservation of Iranian Wetlands. Available at: http://www.thegef.org/gef/sites/thegef.org/files/repository/Iran_Conservation_of_Iranian_Wetlands.pdf.
- Vatandoost H, Ezeddinloo L, Mahvi A, Abai M, Kia E, Mobedi I. ( 2004) Enhanced tolerance of house mosquito to different insecticides due to agricultural and household pesticides in sewage system of Tehran, Iran. Iran J Environ Health Sci Eng. 1( 1): 46– 50. [Google Scholar]
- Venkatesan M, Rasgon JL. ( 2010) Population genetic data suggest a role for mosquito-mediated dispersal of West Nile virus across the western United States. Mol Ecol. 19( 8): 1573– 1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Norafikah O, Chen CD, Soh HN, Lee HL, Nazni WA, Sofian-Azirun M. ( 2009) Surveillance of Aedes mosquitoes in a university campus in Kuala Lumpur, Malaysia. Trop Biomed. 26( 2): 206– 215. [PubMed] [Google Scholar]
- Ward MP. ( 2009) Equine West Nile virus disease occurrence and the Normalized Difference Vegetation Index. Prev Vet Med. 88( 3): 205– 212. [DOI] [PubMed] [Google Scholar]
- Zaim M, Manouchehri A, Yaghoobi-Ershadi M. ( 1985) Mosquito fauna of Iran 2-Culex. Iran J Publ Health. 14: 1– 12. [Google Scholar]