Abstract
Background:
Crimean-Congo Hemorrhagic Fever (CCHF) is a feverous and hemorrhagic disease endemic in some parts of Iran and caused by an arbovirus related to Bunyaviridae family and Nairovirusgenus. The main virus reservoir in the nature is ticks, however small vertebrates and a wide range of domestic and wild animals are regarded as reservoir hosts. This study was conducted to determine the infection rate of CCHF virus in hard ticks of Sarpole-Zahab County, Kermanshah province, west of Iran.
Methods:
From total number of 851 collected ticks from 8 villages, 131 ticks were selected randomlyand investigated for detection of CCHF virus using RT-PCR.
Results:
The virus was found in 3.8% of the tested ticks. Hyalommaanatolicum, H. asiaticum and Rhipicephalus sanguineus species were found to have viral infection, with the highest infection rate (11.11%) in Rh. sanguineus.
Conclusion:
These findings provide epidemiological evidence for planning control strategies of the disease in the study area.
Keywords: Ixodidae, CCHFV, Kermanshah, Iran
Introduction
Crimean Congo Hemorrhagic Fever (CCHF) is a viral disease with approximate mortality rate of 30% in humans (Ergonul 2006). The disease can be transmitted via contact with blood or secretions of infected animals and tick bite or manipulation and squishing of CCHF infected ticks. Human to human transmission i.e. nosocomial infection is another main way for the disease transmission (Hoogstraal 1979, Charrel 2004, Appannanavar and Mishra 2011). CCHF disease has a worldwide dissemination and is considered to be an endemic disease in many countries in Asia, Africa and Europe continents (Charrel 2004, Appannanavar and Mishra 2011).
Until now CCHF virus has been detected in 31 species of several species of hard and soft ticks (Hoogstraal 1979, Linthicum and Bailey 1994, Papa et al. 2002). The disease was first reported in Iran during 1970 (Chumakov 1972), and now is considered as an endemic disease in many parts of the world. Previous studies confirmed CCHF cases. In Iran, many studies were conducted on disease carriers; however in 1978, the virus was separated from soft tick larvae, Ornithodoros lahorensis (Sureau 1980) for the first time. Since then, many studies were conducted in different regions of Iran to find the CCHF infection in ticks. Based on previous studies, CCHF infection was detected in 5 genera of soft and hard ticks including Hyalomma, Rhipicephalus, Dermacentor, Haemaphysalis and Ornithodoros (Shirani et al. 2004, Telmadarraiy et al. 2007). A diverse range of infection rates has been reported in these ticks from 0.2 to 33.3% (Shirani et al. 2004, Moradi et al. 2008, Nasiri 2008, Tahmasebi et al. 2010, Telmadarraiy et al. 2007, 2010, 2014, Salim-Abadi et al. 2011, Chinikar et al. 2012, Faghihi et al. 2015, Sarifinia 2012, Karimi 2013, Mehravaran et al. 2013, Champour et al. 2014).
Kermanshah Province contains a big population of nomads in west of the country, so it is an important region for the legal and illegal import/export of domestic animals from Iraq, the neighbor country at the borderline of Sarpole-Zahab City. Crimean-Congo hemorrhagic fever is reported from some parts of Iraq and seems to be an endemic disease there, where during 1979–1981, 63 cases of the disease were reported within number of 48 mortalities. Studies over 50% of Iraqi goat, sheep, and horse sera were positive for the presence of antibodies, in another sero-survey 29% of all animal breeders tested in Iraq were also reported to be positive for those antibodies (Defens Pest Managment Information Analysis Center Armed Forces Glen section Walter Reed Army Medical Center Washington 1999). According to the national monitory system of Iraq, 0–6 of CCHF cases were annually reported during 1998–2009, and in 2010, 11 confirmed cases of the disease with 36% mortality and 28 suspected cases with 4% mortality were reported (Majeed et al. 2012).
Presence of more than 140,000 livestock in the county makes it as a major area of animal husbandry in Kermanshah province. Adjacent plains and mountainous areas are the major locations for nomad migration, which have large herds of livestock. Sarpole-Zahab has a long borderline with Iraq; make it a suitable area for legal/illegal livestock export/import trades between two countries. So risk of the disease transmission exists in both sides of the borderlines of the two neighboring countries where it can be passed from one side to another side of the border, periodically. This study was aimed to investigate CCHF virus infection rate in ticks of domestic animals, as the main vectors of the disease.
Materials and Methods
Study area
Kermanshah province is located at the western region of Iran. Sarpole-Zahab County located at the western margin of the province, in coordinates of 34°27′40″ N and 45 ° 51′46″ E, with an area of about 1.271 square kilometers. Sarpole-Zahab has a relatively warm and semi-arid climate with the mild winters and hot summers. The county has mountainous, plain, and foothill topographic areas. In this study, eight villages of Sarzal, Ghalee Vari, Mela Kabob, Salman Tape, Berimov and, Anzal, Dare Balut and Sare Baghe Golin located in different geographical locations of the county were selected randomly (Table 1). Tick specimen collection from the livestock was conducted as described below during the years 2012–2013 (Fig. 1). Based on statistical analysis of available data, 131 tick specimens were selected to determine the CCHF infection rate in ticks of the area study.
Table 1.
Details of the tick species were collected and examined for the presence of CCHF virus genome, Sarpole-Zahab County, Western Iran
| Animal | Location | Hy. anatolicum (tested/+) | Hy. asiaticum (tested/+) | Hy. dromedarii (tested/+) | Hy. marginatum (tested/+) | Hy. detritum (tested/+) | Hy. sp (tested/+) | Rh. sanguineus (tested/+) | Rh. Bursa (tested/+) | Rh. Sp (tested/+) | Ha. sulcata (tested/+) | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sheep | SZ | 7/0 | 3/0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 1/0 | 0/0 | 0/0 | 12/0 |
| GV | 6/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/0 | 8/0 | 0/0 | 1/0 | 1/0 | 17/0 | |
| MK | 5/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 5/0 | |
| ST | 7/1 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 7/1 | |
| BV | 3/0 | 2/0 | 4/0 | 2/0 | 2/0 | 0/0 | 1/1 | 0/0 | 0/0 | 0/0 | 14/1 | |
| AN | 1/0 | 2/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 4/0 | |
| DB | 4/0 | 0/0 | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 5/0 | |
| SBG | 1/0 | 3/0 | 0/0 | 0/0 | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 6/0 | |
| Cow | SZ | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| GV | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2/0 | |
| MK | 7/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 7/0 | |
| ST | 3/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3/0 | |
| BV | 2/0 | 0/0 | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 4/0 | |
| AN | 9/1 | 4/1 | 5/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 18/2 | |
| DB | 6/0 | 4/0 | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 12/0 | |
| SBG | 1/1 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | |
| Goat | SZ | 3/0 | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 5/0 |
| GV | 0/0 | 3/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 3/0 | |
| MK | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/0 | |
| ST | 1/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/0 | |
| BV | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2/0 | |
| AN | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | |
| DB | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | |
| SBG | 2/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2/0 | |
| Total | 73/3 | 23/1 | 15/0 | 2/0 | 5/0 | 1/0 | 9/1 | 1/0 | 1/0 | 1/0 | 131/5 | |
SZ: Sarzal, GV: GhaleeVari, MK: Mela Kabob, ST: Salman Tape, BV: Berimovand, AN: Anzal, DB: DareBalut, SBG: SareBagheGolin.
Fig. 1.
Map of the Study area in Kermanshah Province, Iran
Sample collection and preparation
Tick specimens were collected seasonally based on the species diversity, type of host animals and geographic location of the area study. Samples were maintained individually in labeled tubes and transferred to the laboratory of School of Public Health, Tehran University of Medical Sciences in cool boxes. The specimens were identified to species level using the known morphologically keys (Hoogstraal 1979, Walker et al. 2003). 128 specimens of the identified ticks were selected in random and transferred in cold chain to the Arbovirus and Viral Hemorrhagic Fevers Laboratory (National Ref. Lab) at Pasture Institute of Iran for molecular detection of CCHF virus.
RNA Extraction and RT-PCR
For RNA extraction, each tick was washed twice with PBS 1X and then crushed by a mortar and pestle in 300 μl of PBS buffer. RNA extraction was performed using RNA easy mini kit (QIAGEN, Germany) based on the protocol recommended by the manufacturer. The extracted RNA was dissolved in RNase-free water and kept at −70 °C until use. RT-PCR reaction was performed using One-Step RT-PCR Kit (QIAGEN, Germany) based on the protocol. In each PCR reaction, 5μl of the extracted RNA and 1μl of each specific primer (Forward: 5′-TGGACACC TTCACAAACTC-3′ and Reverse: 5′-GAC AATTCCCTACACC-3′) were added to amplify the small segment (S-segment) of the virus (Chinikar et al. 2004). At next step, 5 μl of RT-PCR products were mixed with 1μ of loading buffer and the mixture was loaded on 1.5% agarose gel for electrophoresis. DNA bands were stained with ethidiumbromide and were visualized under UV trans illuminator (Chinikar et al. 2008, 2010).
Sequencing and sequence analysis
RT-PCR products were sequenced by ABI Genetic Analyzer 3130 machine using Big Dye Terminator V3.1 Cycle sequencing Kit and specific primers (Chinikar, Shah-Hosseini et al. 2013). The partial sequences around 500 bp of the S-segment were used for phylogenetic analysis (Table 2). The multiple alignments were performed using Clustal W, for seven sequences of current study and some sequences from Gen Bank. Phylogenetic tree was drawn by maximum-likelihood method with Kimura 2-parameter model using Mega 5.2 software. Bootstrap method with replications of 1000 was used for assessing confidence in phylogenetic tree results.
Table 2.
Details of sequence data used in this study
| Isolate Name or Accession Number | Country | City | Host isolated | Reference |
|---|---|---|---|---|
| CCHF14 | Iran | Sarpole-Zahab | Sheep | This study |
| CCHF16 | Iran | Sarpole-Zahab | Sheep | This study |
| CCHF18 | Iran | Sarpole-Zahab | Cow | This study |
| CCHF22 | Iran | Sarpole-Zahab | Cow | This study |
| CCHF24 | Iran | Sarpole-Zahab | Cow | This study |
| HM452305 | Afghanistan | NS | Human | Ölschläger et al. 2011 |
| AJ538198 | Pakistan | Karachi | Human | Hewson et al. 2004 |
| AY366379 | Iran | Sistan-Baluchistan | Human | Chinikar et al. 2004 |
| AY366378 | Iran | Sistan-Baluchistan | Human | Chinikar et al. 2004 |
| DQ446213 | Iran | NS | NS | Direct submission |
| DQ446212 | Iran | NS | NS | Direct submission |
| AY366377 | Iran | Sistan-Baluchistan | Human | Chinikar et al. 2004 |
| AY366374 | Iran | Sistan-Baluchistan | Human | Chinikar et al. 2004 |
| AY366373 | Iran | Sistan-Baluchistan | Human | Chinikar et al. 2004 |
| DQ446214 | Iran | NS | NS | Direct submission |
| U88414 | NS | NS | NS | Direct submission |
| AY366376 | Iran | Sistan-Baluchistan | Human | Chinikar et al. 2004 |
| AY366375 | Iran | Qom | Human | Chinikar et al. 2004 |
| AF527810 | Pakistan | NS | NS | Direct submission |
| GU456725 | Iran | Hamedan | Tick | Chinikar et al. 2010 |
| GU456728 | Iran | Hamedan | Tick | Chinikar et al. 2010 |
| GU456724 | Iran | Hamedan | Tick | Chinikar et al. 2010 |
| GU456727 | Iran | Hamedan | Tick | Chinikar et al. 2010 |
| GU456726 | Iran | Hamedan | Tick | Chinikar et al. 2010 |
| AY905662 | Pakistan | Quetta | NS | Burt and Swanepoel 2005 |
| AY905663 | Pakistan | Quetta | NS | Burt and Swanepoel 2005 |
| AY905661 | Pakistan | Quetta | NS | Burt and Swanepoel 2005 |
NS: Not Stated
Results
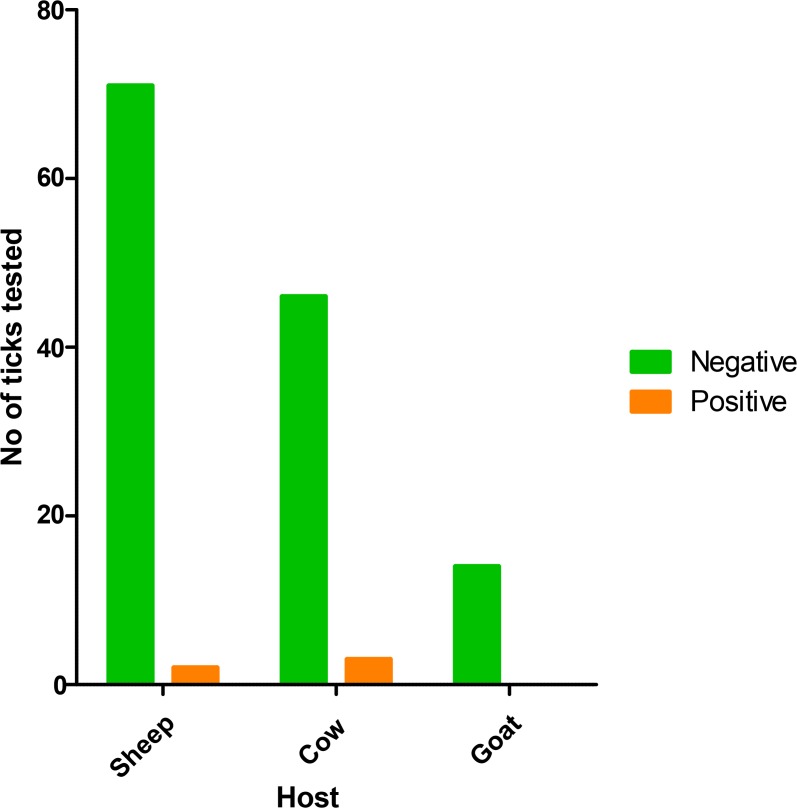
A total number of 851 ticks were collected and identified in this study. Tick infestation rate was accounted as much as 84.2%, 10.53% and 5.27%, in sheep, cows and goats respectively. Three genera of Ixodid ticks including 10 species Hyalommaanatolicum, Hy. asiaticum,Hy. dromedarii, Hy. marginatum, Hy. detritum, Hy. sp, Rhipicephalus sanguineus, Rh. bursa, Rh. sp and Haemaphysalissulcata were identified. A subsetof 131 ticks (15.4%) out of 851 ticks was examined to detect CCHF virus transcripts (Fig. 2). RT-PCR amplification of S-segment of CCHF virus produced a PCR band of 536bp (Table 1). The results of RTPCR showed an infection rate of 3.8% (n=5) among the tested specimens. The infected species were found to be Hy. anatolicum (4.1%), Hy. asiaticum (4.54%) and Rh. sanguineus (11.11%). The infected ticks were collected from cow and sheep. Molecular results showed CCHF virus genome in 6.38% (3/47) and 2.85% (2/70) of ticks from cowand sheep, respectively, while all ticks collected from goat were negative (Fig. 3).
Fig. 2.
RT-PCR products of CCHF S-segment (536 bp band) found in tick specimens collected in Sarpole-Zahab County, Kermanshah Province. Lad: 100 bp ladder, PC: positive control, NC: negative control, S1, S2, S3, S4, S5, S6, S7 and S9: negative samples; S8 and S10: positive samples.
Fig. 3.
Details of CCHF infected ticks and their animal hosts in Sarpole-Zahab County, Kermanshah, Western Iran
Phylogenetic analysis
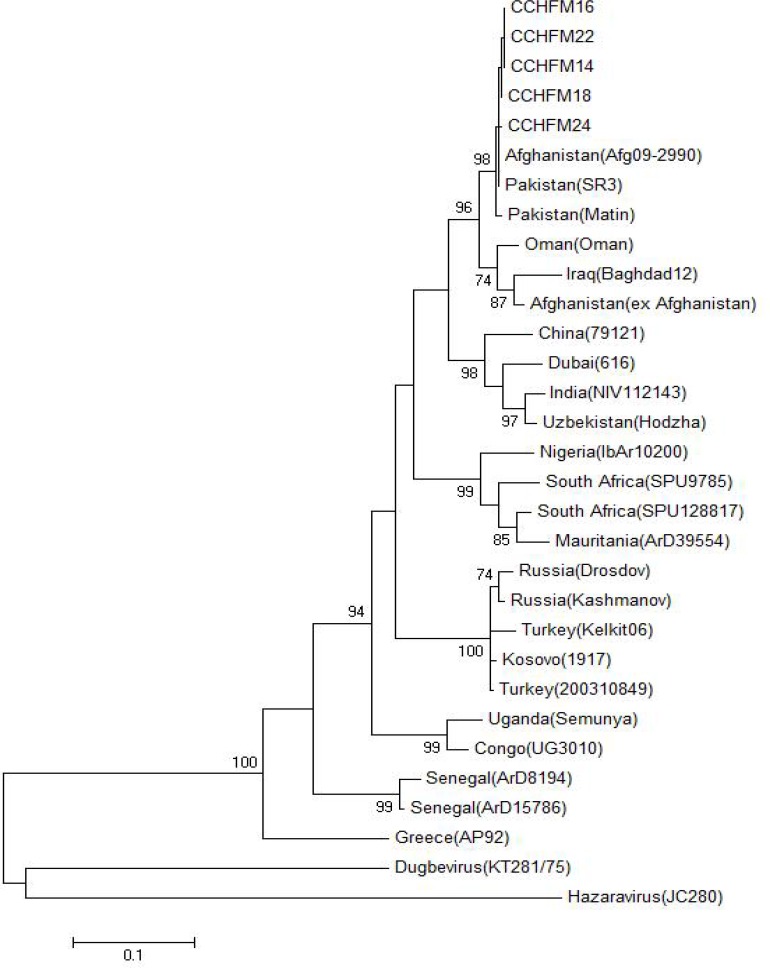
The Phylogenetic relationship of the isolated CCHF sequences from ticks of Sarpole-Zahab with genbank available sequences was drawn using Maximum Likelihood method and kimura2 parameter (Fig. 4). All the isolates were clustered in Asia I clade, closely to Matin and SR3 strains of Pakistan and newly released strain from Afghanistan (Ölschläger et al. 2011). Overall mean distance computation of this study isolates show just 0.3% divergence between them.
Fig. 4.
Maximum Likelihood phylogenetic tree retrieved from 500bp of CCHFV partial S-segment sequences obtained in this study (CCHFM14, CCHFM16, CCHFM18, CCHFM22 and CCHFM24) and the available data from Genbank. Only boot strap values more than 70% are shown.
Discussion
Our results in this study showed the presence of CCHF virus in different regions of Sarpol-e-Zahab City, Kermanshah Province. This infection was confirmed in Hy. anatolicum, Hy. asiaticum, R. sanguineus. Findings of this study are consistent with the results obtained other groups in different regions of the country (Tahmasebi et al. 2010, Telmadarraiy et al. 2010). Although Hyalomma ticks are the main carriers of the virus in Africa, Asia, Europe and the Middle East (Swanepoel et al. 1987, Whitehouse 2004, Ergönül 2006) the virus was also detected in other genera of both soft and hard ticks (Whitehouse 2004) as we found in R. sanguineus.
In other studies conducted in western part of Iran, CCHF virus infection was reported in Hy. marginatum, Hy. anatolicum, Hy. detritum, Hy. dromedarii, Hy. asiaticum, Haemaphysalispunctata, R. sanguineus, and R. bursa, (Moradiet al. 2008, Tahmasebi et al. 2010, Sharifinia 2012, Fakoorziba et al. 2012, Nasiri Unpublished data). According to our previous study conducted in Ardabil Province, northwest of Iran, viral infection was detected in Hy. schulzei, Hy. marginatum, Hy. aegyptium and R. bursa (Telmadarraiy et al. 2010). Studies conducted in west part of Iran showed that the genome of CCHF virus exists in different species of three genera of hard ticks including Hyalomma, Rhipicephalus and Haemaphysalis.
The infection rate of ticks in this study was 3.8%. This rate has been reported from 0.2 to 33.3% in previous studies in Iran (Shirani et al. 2004, Telmadarraiy et al. 2006, 2007, 2010, 2014, Moradi et al. 2008, Nasiri 2008, Tahmasebi et al. 2010, Salim-Abadi et al. 2011, Chinikaret al. 2012, Fakoorziba et al. 2012, Sarifinia 2012, Karimi 2013, Mehravaran et al. 2013, Champour et al. 2014). Ticks of Hyalomma genus showed an infection rate of 3.36%, while the species of this genus were found to be infected 1.57% to 20% to CCHF virus in other studies (Shiraniet al. 2004, Telmadarraiy et al. 2006, 2007, 2010, 2014, Moradi et al. 2008, Nasiri 2008, Tahmasebi et al. 2010, Salim-Abadi et al. 2011, Chinikar et al. 2012, Fakoorziba et al. 2012, Sarifinia 2012, Karimi 2013, Mehravaran et al. 2013, Champour et al. 2014). Although 9.09% of Rhipicephalus ticks were infected in this study, other groups reported the infection rate between 1.8% to 55% (Telmadarraiy et al. 2006, 2010, Moradiet al. 2008, Tahmasebi et al. 2010, Karimi 2013), therefore it can be concluded that although the Hyalomma ticks are usually introduced as the vectors of CCHF, this potential exists in other genera as well.
Comparison of results obtained from different regions of the county showed that ticks collected from central regions were more infected than southern and northern regions. The reason of such infection may be due to the condensation of livestock and the quality of breeding management including poor hygienic conditions of livestock breeding sites.
People of Sarpol-e-Zahab area work in high risk professions in close contact with ticks and animals’ tissue and blood. Therefore, it is instructed to prevent these people from being subjected to different ticks infected with the virus and or infected animals’ blood or tissue. This study indicates that CCHF must be considered as a critical health problem in health centers of Sarpole-Zahab as well as Kermanshah and other neighboring provinces, and appropriate strategies must be used for controlling carrier ticks.
Future research should be focused on the population of carriers and their infection rate, presence of the virus in domestic animal populations and also humans in other regions of the province in order to present a better picture of the dissemination and epidemiology of the virus in the province.
Conclusion
The prevalence of infected to CCHF was higher in plain region of the county rather than mountain region. Teaching and prevention programs are recommended to people who are in close contact with ticks and animals’ tissue and blood to prevent from infection. This study indicates that CCHF must be considered as a critical health problem in health centers of Sarpole-Zahab and other neighbouring county, and appropriate strategies must be used for controlling carrier ticks.
Acknowledgement
The authors appreciate very much for kind collaboration of all the staff of Veterinary Office of Sarpole-Zahab County. This study has been done by financial supports of Tehran University offered by Medical Sciences and this work is part of Project No. 23859.
References
- Appannaanvar SB, Mishra B. ( 2011) An update on Crimean Congo hemorrhagic Fever. J Glob Infect Dis. 3 ( 3): 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champour M, Chinikar S, Mohammadi G, Razmi G, Shah-Hosseini N, Khakifirouz S, Mostafavi E, Jalali T. ( 2016) Molecular epidemiology of Crimean-Congo hemorrhagic fever virus detected from ticks of one humped camels (Camelus dromedarius) population in northeastern Iran. J Parasit Dis. 40( 1): 110– 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrel R, Attoui H, Butenko A, Clegg J, Deubel V, Frolova T, Gould E, Gritsun T, Heinz F, Labuda M. ( 2004) Tick-borne virus diseases of human interest in Europe. Clin Microbiol Infect. 10( 12): 1040– 1055. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Persson SM, Johansson M, Bladh L, Goya M, Houshmand B, Mirazimi A, Plyusnin A, Lundkvist A, Nilsson M. ( 2004) Genetic analysis of Crimean-Congo Hemorrhagic Fever virus in Iran. J Med Virol. 73( 3): 404– 411. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Shayesteh M, Khakifirouz S, Jalali T, Rasi-Varaie FS, Rafigh M, Mostafavi E, Shah-Hosseini N. ( 2013) Nosocomial infection of Crimean–Congo Hemorrhagic fever in eastern Iran: case report. Travel Med Infect Dis. 11( 4): 252– 255. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Shah-Hosseini N, Bouzari S, Jalali T, Shokrgozar AM, Mostafavi E. ( 2013) New circulating genomic variant of Crimean-Congo Hemorrhagic fever virus in Iran. Arch Virol. 158( 5): 1085– 1088. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Ghiasi S, Hewson R, Moradi M, Haeri A. ( 2010) Crimean-Congo hemorrhagic fever in Iran and neighboring countries. J Clin Virol. 47( 2): 110– 114. [DOI] [PubMed] [Google Scholar]
- Chinikar S, Ghiasi SM, Naddaf S, Piazak N, Moradi M, Razavi MR, Afzali N, Haeri A, Mostafavizadeh K, Ataei B. ( 2012) Serological evaluation of Crimean-Congo hemorrhagic fever in humans with high-risk professions living in enzootic regions of Isfahan Province of Iran and genetic analysis of circulating strains. Vector Borne Zoonotic Dis. 12( 9): 733– 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinikar S, Goya M, Shirzadi M, Ghiasi S, Mirahmadi R, Haeri A, Moradi M, Afzali N, Rahpeyma M, Zeinali M. ( 2008) Surveillance and Laboratory Detection System of Crimean-Congo Haemorrhagic Fever in Iran. Transbound Emerg Dis. 55( 5–6): 200– 204. [DOI] [PubMed] [Google Scholar]
- Chumakov M. ( 1972) Detection of antibodies to CHF virus in wild and domestic animal blood sera from Iran and Africa. pp. 367– 368.
- Defens Pest Managment Information Anaiysis Center. Armed Forces Glen section Walter Reed Army Medical Center Washington ( 1999) Regional Disease Vector Ecology Profile the Middel East.
- Ergonul O. ( 2006) Crimean-Congo haemorrhagic fever. The Lancet Infect Dis. 6( 4): 203– 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihi F, Chinikar Sadegh, Telmadarraiy Zakkyeh, Bakhshi Hasan, Khakifirooz Sahar, Jalali Tahmineh, Nutifafa Gidiglo Godwin. ( 2015) Crimean-congo hemorrhagic fever: A seroepidemiological and molecular survey in north of Iran. J Entomol Zool Stud. 3( 1): 1– 4. [Google Scholar]
- Hoogstraal H. ( 1979) The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J Med Entomol. 15( 4): 307– 417. [DOI] [PubMed] [Google Scholar]
- Karimi F. ( 2013) Determination of viral infection rate of hard ticks to Crimean-Congo hemorrhagic fever (CCHF) and their distribution pattern using Geographic Information System (GIS) in the Kashan District, Isfahan Province. Master of Science Medical Entomology. Teheran University of Medical Sciences, School of public Health and Institute of Public Health Research Department of Medical Entomology and Vector Control. [Google Scholar]
- Linthicum KJ, Bailey CL. ( 1994) Ecology of Crimean-Congo hemorrhagic fever. Ecological dynamic of tick-borne zoonoses (Sonenshine, Mather, eds). pp. 392– 437. [Google Scholar]
- Majeed B, Dicker R, Nawar A, Badri S, Noah A, Muslem H. ( 2012) Morbidity and mortality of Crimean-Congo hemorrhagic fever in Iraq: cases reported to the National Surveillance System, 1990–2010. Trans R Soc Trop Med Hyg. 106( 8): 480– 483. [DOI] [PubMed] [Google Scholar]
- Mehravaran A, Moradi M, Telmadarraiy Z, Mostafavi E, Moradi AR, Khakifirouz S, Shah-Hosseini N, Varaie FSR, Jalali T, Hekmat S. ( 2013) Molecular detection of Crimean-Congo haemorrhagic fever (CCHF) virus in ticks from southeastern Iran. Ticks and Tick-borne Dis. pp. 35– 38. [DOI] [PubMed] [Google Scholar]
- Moradi A, Chinikar S, Oshaghi M, Vatandoost H, Holakoui-Naini K, Zahirnia A. ( 2008) Molecular detection of Crimean-Congo Hemorrhagic Fever (CCHF) virus in Ticks (Ixodidae, Argasidae) of Hamedan Province, Iran. Biochem Cell Arch. 8( 1): 119– 123. [Google Scholar]
- Nasiri A. ( 2009) Molecular study on tick infectivity to CCHF virus and serological investigation on sheep and human in Abdanan region, Ilam Province, Iran. Master of Science Medical Entomology. Teheran University of Medical Sciences, School of Public Health and Institute of Public Health Research, Department of Medical Entomology and Vector Control. [Google Scholar]
- Ölschläger S, Gabriel M, Schmidt-Chanasit J, Meyer M, Osborn E, Conger NG, Allan PF, Günther S. ( 2011) Complete sequence and phylogenetic characterisation of Crimean-Congo hemorrhagic fever virus from Afghanistan. J Clin Virol. 50( 1): 90– 92. [DOI] [PubMed] [Google Scholar]
- Papa A, Bozovi B, Pavlidou V, Papadimitriou E, Pelemis M, Antoniadis A. ( 2002) Genetic detection and isolation of Crimean-Congo hemorrhagic fever virus, Kosovo. Emerg Infect Dis. 8( 8): 852– 4.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salim-Abadi Y, Chinikar S, Telmadarraiy Z, Vatandoost H, Moradi M, Oshaghi MA, Ghiasi-Seyed M. ( 2011) Crimean-Congo hemorrhagic fever: a molecular survey on hard ticks (Ixodidae) in Yazd province, Iran. Asian Pac J Trop Med. 4( 1): 61– 63. [DOI] [PubMed] [Google Scholar]
- Sharifinia N, Rafinejad J, Hanafi-Bojd AA, Biglarian A, Chinikar S, Baniardalani M, Sharifinia F, Karimi F. ( 2013) Knowledge and Attitudes of the Rural Population and Veterinary and Health Personnel Concerning Crimean-Congo Hemorrhagic Fever in Western Iran in 2012. Florida Entomologist. 96( 3): 922– 928. [Google Scholar]
- Shirani M, Asmar M, Chinikar S, Mirahmadi R, Piazak N, Mazaheri V. ( 2004) Detection of CCHF virus in soft ticks (Argasidae) by RT-PCR method. J Infec Dis Trop Med. 9 ( 24): 11– 15. [Google Scholar]
- Sureau P, Klein J, Casals J, Digoutte J, Salaun J, Piazak N, Calvo M. ( 1980) Isolation of Thogoto, Wad Medani, Wanowrie and Crimean-Congo haemorrhagic fever viruses from ticks of domestic animals in Iran. Annales de Virolo. 131( 2): 185– 200. [Google Scholar]
- Swanepoel R, Shepherd A, Leman P, Shepherd S, McGillivray G, Erasmus M, Searle L, Gill D. ( 1987) Epidemiologic and clinical features of Crimean-Congo hemorrhagic fever in southern Africa. Am J Trop Med Hyg. 36( 1): 120– 132. [DOI] [PubMed] [Google Scholar]
- Tahmasebi F, Ghiasi S, Mostafavi E, Moradi M, Piazak N, Mozafari A, Haeri A, Fooks A, Chinikar S. ( 2010) Molecular epidemiology of crimean-congo hemorrhagic fever virus genome isolated of ticks from Hamadan Province of Iran. J Vector Borne Dis. 47: 211– 216. [PubMed] [Google Scholar]
- Telmadarraiy Z, Davari A, Vatandoost H. ( 2006) Ruminant animal ticks and their role in CCHF transmission in Ghaen, south Khorasan Province, Iran during 2005. In ICOPA XI11th Inter Con Parasito pp. 6– 1. [Google Scholar]
- Telmadarraiy Z, Chinikar S, Vatandoost H, Holakoui K, Faghihi F, Zarei Z, Oshaghi M. ( 2007) P1044 Serology and immunological study on the infectivity of host animals and ticks (Ixodidae, Argasidae) to CCHF virus in Ardabil Province, Iran. International J Antimicrobial Agents. 29: S280. [Google Scholar]
- Telmadarraiy Z, Ghiasi SM, Moradi M, Vatandoost H, Eshraghian MR, Faghihi F, Zarei Z, Haeri A, Chinikar S. ( 2010) A survey of Crimean-Congo haemorrhagic fever in livestock and ticks in Ardabil Province, Iran during 2004–2005. Scand J Infect Dis. 42( 2): 137– 141. [DOI] [PubMed] [Google Scholar]
- Telmadarraiy Z, Saghafipour A, Farzinnia B, Chinikar S. ( 2014) Molecular Detection of Crimean-Congo Hemorrhagic Fever Virus in Ticks in Qom Province, Iran, 2011–2012. Iranian J Virolo. 6( 3): 13– 18. [Google Scholar]
- Walker AR, Bouattur A, Camicas J, Estrada-Pena A, Horak I, Latif A, Pegram R, Preston P. ( 2003) Ticks of domestic animals in Africa: A guide to identification of species. Bioscience reports Edinburgh. pp. 572– 577 [Google Scholar]
- Whitehouse CA. ( 2004) Crimean-Congo Hemorrhagic fever. Antiviral research. 64: 145– 60. [DOI] [PubMed] [Google Scholar]